L
Journal of Experimental Marine Biology and Ecology, 241 (1999) 263–284
Effect of the burrowing crab Chasmagnathus granulata
(Dana) on the benthic community of a SW Atlantic coastal
lagoon
*
Florencia Botto , Oscar Iribarne
´
Departamento de Biologıa(FCEyN), Universidad Nacional de Mar del Plata, CC 573 Correo Central, 7600 Mar del Plata, Argentina
Received 19 March 1999; accepted 23 June 1999
Abstract
This study evaluated the effect of the burrowing crab Chasmagnathus granulata on the benthic community in mudflats of the Mar Chiquita coastal lagoon (Argentina, 378 459S, 578 269W). A significantly higher abundance of the polychaete Laeonereis acuta was found inside the C. granulata crab bed than outside. However, L. acuta abundance decreased in summer probably due to the greater activity of crabs. A series of exclusion and inclusion field experiments showed a combined effect of the non-burrowing crabs Cyrtograpsus angulatus and C. granulata on the polychaete L. acuta inside crab beds, and also, an effect of C. granulata on the polychaetes L. acuta and Heteromastus similis when added outside the crab bed. C. granulata also affected nematodes, even when experiments were of short duration. The effect of crab burrows on the small scale distribution of nematodes may be related to passive transport of organisms. Adults of both crab species, Cy. angulatus and C. granulata, also affected the settlement of Cy. angulatus. The juveniles of C. granulata showed no effect for any meiofaunal species. The results of this work showed that C. granulata plays an important role in determining the benthic community in mudflats of the Mar Chiquita coastal lagoon. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Benthos; Bioturbation; Burrowing crabs; Chasmagnathus granulata; Community; Mudflats
1. Introduction
There are several saltmarshes between southern Brazil and northern Patagonia (Argentina) on estuaries with large discharges and prevailing brackish conditions (Adam,
*Corresponding author.
E-mail address: [email protected] (F. Botto)
1990). There is no information on the ecological processes within these estuaries appropriate to predict the ecological impact of human alterations. The burrowing crab
Chasmagnathus granulata (Dana) is one of the most abundant macroinvertebrates (up to
40 mm carapace width) inhabiting these environments (Boschi, 1964). They are distributed in almost all the zones of the marsh intertidal; in the mudflat and in the
22
Spartina densiflora cordgrass habitats with a mean density of 20 crabs m (Spivak et al., 1994; Iribarne et al., 1997). This crab species is an important bioturbator in mudflats,
22 21
removing and processing up to 5.9 kg m day of sediment (Iribarne et al., 1997).
This reworking of the upper sediment layer, probably affects the sedimentary environ-ment and benthic organisms, especially during spring–autumn when crab activity is intense (pers. obs.).
Crabs are often important habitat modifiers (e.g., Montague, 1980; Bertness, 1985), significantly influencing microtopography (e.g., Bertness, 1985; Hall et al., 1991), sediment chemistry (Hoffman et al., 1984; Wolfrath, 1992) and drainage (Bertness, 1985). Sediment disturbance resulting from the foraging and burrowing activities of decapods may have direct and indirect impacts on macroinfauna and / or meiofaunal species (e.g., Hoffman et al., 1984; Thrush, 1988; DePatra and Levin, 1989; Warwick et al., 1990). Such sediment-mediated interactions are common in many infaunal com-munities of mudflats and creeks (Rhoads and Young, 1970; Aller and Dodge, 1974; Kneib, 1991; Wilson, 1991).
Burrowing organisms can also affect benthic community composition by altering recruitment patterns, increasing mortality of settling larvae by ingestion or suffocation (Woodin, 1976), or increasing survival by providing refuge (e.g., Tamaki and Ingole, 1993). Burrowing animals may also increase sediment transported through bedload (Aller and Dodge, 1974) affecting benthos indirectly by controlling food availability (Luckenbach et al., 1988; Miller and Sternberg, 1988), increasing mortality by abrasion (Miller, 1989) or predation (Grant, 1981), or directly favoring dispersion through passive transport (Palmer, 1988).
C. granulata, in bare mudflats of Mar Chiquita coastal lagoon (Argentina, 378459S,
578 269 W) is a deposit feeder that excavates and maintains semi-permanent open
burrows (Iribarne et al., 1997). These burrows are funnel shaped, with large entrances (up to 14 cm major axis length) and a surface sediment mound which is a product of feeding or burrow maintenance (Iribarne et al., 1997). C. granulata coexists with
Cyrtograpsus angulatus (Dana), another grapsid crab (up to 40 mm carapace width)
which is highly mobile and does not make permanent burrows in this environment (Spivak et al., 1994). While Cy. angulatus is mainly a subtidal species, C. granulata inhabits the upper intertidal zones, and the two species overlap only during high tides. Juveniles of Cy. angulatus are confined to protected microhabitats (like areas with stones, or in crevices of the tube building polychaete Ficopomatus enigmaticus; Spivak et al., 1994), while juveniles of C. granulata live in bare mudflats with adults (Spivak et al., 1994).
that fluctuate widely in abundance (Ieno and Bastida, 1998). The few studies of meiofauna in these environments showed also low diversity of ostracods (two species, Whatley et al., 1997) and nematodes (two species, Mammoli, 1992). No previous studies of other meiofaunal organisms exist for these environments.
Due to their behavior, abundance and extensive distribution C. granulata may play a determinant role in the intertidal mudflat communities of most SW Atlantic estuaries. Particularly, species that are sensitive to modifications of the structure, composition or chemistry of the sedimentary environment, may suffer higher mortality rate due to crab activities (e.g., Kneib, 1991; Billick and Case, 1994). Also burrows may enhance spatial heterogeneity, in a small scale affecting meiofauna distribution (e.g., Bell, 1980). Thus, the objective of this work is to determine abundance and distribution patterns of benthic organisms associated with areas inhabited by C. granulata, and experimentally evaluate the effect of these crabs on benthic organisms.
2. Materials and methods
2.1. Study area
The study was performed between January 1996 and April 1997 in the Mar Chiquita coastal lagoon, a body of brackish water (salinity range: 6 to 33‰), affected by low
amplitude (#1 m) tides and characterized by mudflats surrounded by large beds of
cordgrass (S. densiflora, Olivier et al., 1972a). The burrowing crab C. granulata is distributed in both, the S. densiflora areas and mudflats (Spivak et al., 1994; Iribarne et al., 1997). However, only the part of the population living in the open intertidal mudflats was studied.
2.2. Distribution patterns of benthic organisms associated with the presence of crabs
2.2.1. Sampling design
Macroinfauna samples were taken with a core 10 cm diameter and 10 cm depth, organisms were sieved through a 0.5-mm mesh screen. Organisms retained were
preserved in alcohol (70%) and sorted under a 203 dissection microscope. Meiofaunal
sub-samples (core size: 2 cm diameter 10 cm depth) were taken, sieved through a 0.44-mm mesh screen and then stained with bengal rose to facilitate sorting. Organisms retained were preserved with formalin (5%), identified to the major taxa, and counted
under 203 dissection microscope.
2.2.2. Relationship between the presence of the burrowing crab C. granulata, the
abundance of other benthic organisms and sediment characteristics
intertidal level [0.5 m over mean lower low water (MLLW)]. To evaluate vertical distribution of macroinfauna in each area, samples were divided in five depth layers of 2 cm in width each. To evaluate possible temporal variations over a year, these samples were taken in May, July, September, November (1996), January and March (1997).
Sediment samples (10 replicates per area; 10 cm diameter and 10 cm depth) were taken to evaluate water content, organic matter content and grain size. Water content was calculated as the difference between wet and dry weight (after drying at 708C during 72 h). Organic matter content was calculated as the percentage of ash free dry weight
(AFDW), ashes were obtained after incinerating sub-samples (10 g each, at 5508C
during 6 h). Grain size distribution was evaluated by sieve and sedimentation analysis following Carver (1971).
2.2.3. Small scale patterns in meiofaunal distribution associated with burrows of C.
granulata
To evaluate if there was any difference in the spatial distribution of meiofauna related to the presence of burrows of C. granulata, meiofaunal samples were taken at three distances from the burrow: edge, 5 cm and 10 cm from the burrow opening. Given that crabs make sediment mounds on one side of the burrow entrance (Iribarne et al., 1997) which may affect meiofaunal distribution, samples at each distance were repeated on the side of the mound and on the opposite side. These samples were taken from 10 burrows of similar shape located at the same intertidal level (approximately 0.5 m over MLLW).
2.3. Experiments
2.3.1. Experimental designs
All experiments described below were performed as follows: inclusion and exclusion treatment plots of adult crabs were performed with completely closed wire cages
(50350 cm area, with a mesh size of 5 mm). Cages had 60 cm length sides which were
pushed into the sediment to a depth of 40 cm with 20 cm protruding above the surface. This design was performed to avoid crabs passing through their burrows. In inclusion treatments six individuals of the corresponding species (three males and three females) within a size range of 30 to 35 mm were included. In exclusion treatment, crabs were extracted using dead fish tied at the end of a cord as bait. In all cases treatments had 10 replicates and were randomly distributed and separated each other by 10 m.
2.3.2. Discriminating the effect of crabs on benthic organisms inside crab beds A field experiment was performed during summer 1996 to discriminate the effect of
C. granulata from the effect of other predators on benthic organisms. This experiment
lasted 80 days (starting on 15 February) to ensure the presence of all predators in the area. This experiment was run on the open mudflat with the following treatments: (1) exclusion of crabs, (2) inclusion of C. granulata; (3) inclusion of Cy. angulatus; (4)
exclusion of big fishes and birds with ceilings of 50350 cm standing 5 cm over the
sediment by PVC pipes, (5) control for cage effect (border without ceilings), and (6)
control (50350 cm previously marked areas without any manipulation). Birds excluded
sandpipers (Calidris spp.) and yellowlegs (Tringa spp.). Fishes were croaker
(Micro-pogonias furnieri ) and mullet (Mugil platanus). At the end of the experiment,
macroinfauna and meiofauna samples were taken from the center of each plot.
2.3.3. Effect of C. granulata on infaunal organisms living at different depths in
sediment
Given that the previous experiment showed the effect of this species on benthic fauna, an experiment was performed during summer 1997 to evaluate if this effect varies with sediment depths. The experiment had two treatments: (1) exclusion and (2) inclusion of
C. granulata. The experiment ran from 18 January to 10 February (1997). Samples of
macroinfauna and meiofauna were taken from the center of each treatment and were divided into two layers (5 cm each) to evaluate if effect differs between depth.
2.3.4. Effect of crabs on recruitment of Cy. angulatus
Given that recruitment of the crab Cy. angulatus occurs mainly at the end of the summer–early autumn (Luppi et al., 1994), an experiment similar to the previous ones was performed to evaluate the effect of C. granulata and Cy. angulatus on crab recruitment. The experiment had a duration of 40 days (starting on 2 March and included the recruitment period) and consisted of three treatments: (1) inclusion of C. granulata; (2) inclusion of Cy. angulatus; and (3) exclusion of crabs.
2.3.5. Inclusion of crabs in an area not inhabited by crabs
Previous experiments were performed in areas inhabited (and therefore disturbed) by the burrowing crab C. granulata. However, areas not inhabited by crabs present the opportunity to evaluate their actual effect on the environment. Therefore, an experiment was performed in an intertidal zone close to crab beds (distant 200 m), with the same tidal regime, and similar sediment characteristics, but not disturbed by crabs. This experiment consisted of a C. granulata inclusion treatment and control treatment (similar closed cages without crabs). Macroinfauna and meiofauna samples were taken after 40 days (when placement of crabs in the new habitat was evident) and samples were divided into two depth layers (5 cm each).
2.3.6. Effect of juvenile crabs on abundance of meiofaunal organisms
A field experiment was performed to evaluate the effect of juveniles of C. granulata on meiofaunal organisms. The experiment consisted of three treatments: (1) inclusion of crabs of 12 to 15 mm carapace width, (2) inclusion of crabs of 7 to 10 mm carapace width, and (3) control. All treatments consisted of PVC pipes (10 cm diameter 15 cm depth), covered at the top with a plastic net (1 mm mesh size) and inserted 12 cm inside the substrate. Four crabs were included in the two first treatments while no crabs were added in controls. Treatments were arranged in the intertidal zone inhabited by adults of
22
this species. Cores were homogeneously perforated all around (0.5 perforations cm ) to
2.4. Statistical analysis
A fixed factor MANOVA was performed to evaluate differences in polychaete abundance between areas and among months and depth layers in Section 2.2.2. Percentage of water content and AFDW were compared between areas with t-tests (Zar, 1984) and differences in grain size distribution between areas was evaluated with a Kolmogorov–Smirnov two-sample test (Conover, 1980).
In Section 2.2.3 differences in mean abundance of meiofaunal organisms among distances from crab burrows and in both directions were evaluated with a two-fixed factors analysis of variance (ANOVA) and a posteriori Tukey multiple comparison test (Zar, 1984).
In all experiments, treatment effect was evaluated independently for each species with
t-tests or ANOVAs (Zar, 1984) and abundances were then compared between depth
layers with paired t-tests (Zar, 1984), taking each sample as a pair. When assumptions were not met, transformations were performed or, when the problem persisted the non-parametric Mann–Whitney or Kruskall–Wallis test (Conover, 1980) were used. A Tukey test (Zar, 1984) or a non-parametric multiple comparison test (Conover, 1980) was performed after ANOVA or Kruskall–Wallis, respectively.
In Sections 2.3.2, 2.3.3 and 2.3.5, abundance of benthic organisms in experiments were also evaluated with non-metric multi-dimensional scaling ordination (MDS), using the Bray–Curtis similarity measure (Warwick and Clarke, 1991). Then, the significance of differences between treatments were evaluated with one-way ‘‘analysis of similarity’’ (ANOSIM) following Clarke (1993).
3. Results
3.1. Distribution patterns of benthic organisms associated with the presence of crabs
3.1.1. Relationship between the presence of the burrowing crab C. granulata, the
abundance of polychaetes and sediment characteristics
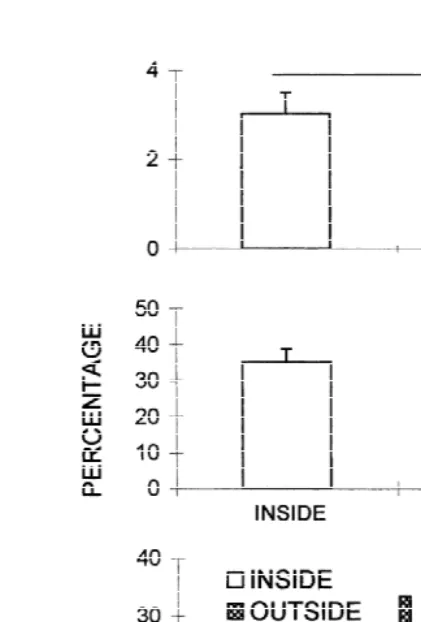
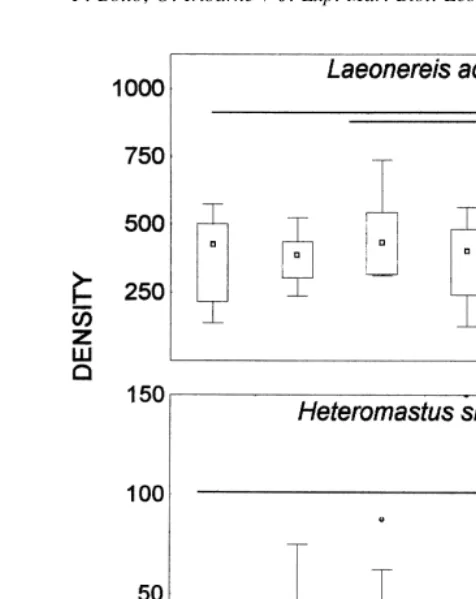
The only species found in all sampling periods was the polychaete L. acuta (Treadwell). The MANOVA showed effects of months and areas but did not show effects of depth (Table 1; Fig. 1). Also, interactions were found between month and depth and area and month and all three factors (Table 1). A posteriori Tukey test showed that polychaetes were more abundant in May, July, September and November than in the other months inside the crab bed (Fig. 1). However, outside crab bed, L. acuta density remained constant all year around (Fig. 1). In May and July abundance of polychaetes were greater in the upper layers inside crab beds (Fig. 2). In September samples were taken after a period of low rainfall and sediment was much dryer than in the other sampling periods. In this month, abundance increased in the deepest layers inside the crab bed (Fig. 2). Outside crab bed no differences among depth layers were found in any month sampled.
The analysis of sediment characteristics showed no differences in organic matter
22
Fig. 1. Density (ind m ) of the polychaete L. acuta in different months of the year inside (IN) and outside (OUT) crab bed. Lines connect no significant differences (P,0.05; Tukey test) among months at each area (lines outside graph) and between areas for each month (lines inside graph). Here and thereafter box plots are constructed with limits of boxes being the 75th and 25th percentile, lines represent 10th and 90th percentiles, lines or points inside boxes are medians, circles are outliers (StatSoft, 1998).
content was significantly greater inside the area inhabited by crabs than outside (t55.1,
df58, P,0.05; Fig. 3B). Grain size distribution differed between areas (T50.42,
n1526, n2533; P,0.05; Fig. 3C). Sediment in the area inhabited by crabs was
mainly silt (80% silt, 16% sand) and outside crab beds, it was mainly sand (58% sand and 40% silt).
3.1.2. Small scale patterns in meiofaunal distribution associated with burrows of C.
granulata
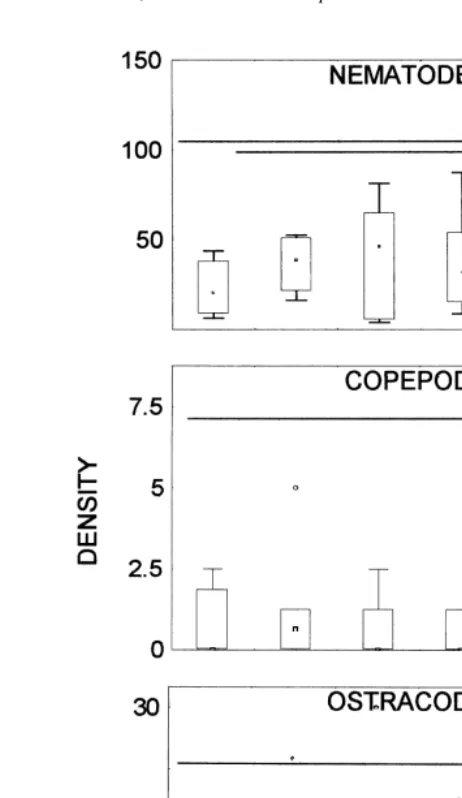
In this sampling site, the most abundant organisms recorded were nematodes. The two-way fixed factor ANOVA showed only interaction effects (data were log
trans-formed to meet the assumptions of homocedasticity; direction: F54.5, df51, 42,
Table 1
Results of MANOVA analysis (*5P,0.05)
Source of variation df MS F
Effect
Month 5 96.028 13.478*
Area 1 498.719 69.989*
Depth 4 12.291 1.7255
Month–area 5 84.738 11.892*
Month–depth 20 21.110 2.962*
Area–depth 4 14.369 2.016
Month–area–depth 20 19.484 2.734*
22
Fig. 2. Density (ind m ) of the polychaete L. acuta in each depth layer inside (IN) and outside (OUT) crab bed and in each sampling month: May (A), July (B), September (C), November (D), January (E) and March (F).
P.0.05; distance: F53.51, df52, 42, P.0.05; interaction: F514.4, df52, 42,
P,0.05; Fig. 4). Interaction was important indicating that distance has different effects depending on if we consider mound or no mound direction. The multiple mean comparison Tukey test performed taking both factors jointly (Neter et al., 1990), showed that in the mound direction the lower abundance was at the edge of the burrow and the maximum was at 10 cm. However, in the opposite direction, the lower abundance was at 5 cm, and it increased at the edge of the burrow (Fig. 4). The abundance of ostracods was lower, and no effect was found (direction: F52.44, df51, 42, P.0.05; distance:
F52.08, df52, 42, P.0.05; interaction: F50.8, df52, 42, P.0.05; Fig. 4).
Copepods and amphipods were not present in this sampling.
3.2. Experiments
3.2.1. Discriminating the effect of crabs on benthic organisms inside crab beds The benthic organisms found in the experimental sites were: nematodes, ostracods, copepods, the polychaetes L. acuta, Nephtys fluviatilis (Monro) and H. similis (Soath-ern), the amphipod Corophium insidiosus and a nemertean non-identified species.
Fig. 3. Sediment characteristics INSIDE and OUTSIDE crab beds. (A) Percentage of AFDW (mean11 SD), (B) percentage of water content (mean11 SD); and (C) grain size distribution. Lines connect no significant differences (P.0.05).
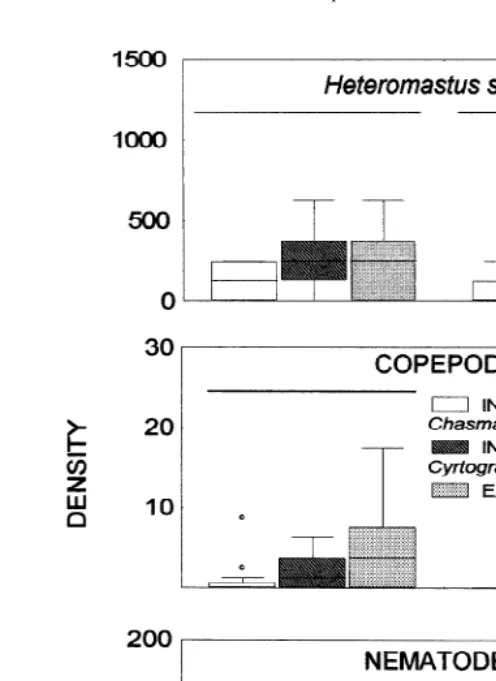
acuta (Kruskall–Wallis test; H511.22, df55, 60; P,0.05), increasing the density in the total exclusion treatment compared with the control and the exclusion of fishes and birds (Fig. 5A). Differences among treatments were also found for nematodes (Kruskall– Wallis test; H512.25, df55, 60; P,0.05; Fig. 6A), where differences were due to an increase of this group in the exclusion treatment in comparison with the treatment with enhanced density of C. granulata. No differences existed among treatments for H.
similis (Kruskall–Wallis test; H52.45, df55, 60, P.0.05; Fig. 5B), copepods
(Kruskall–Wallis test; H54.7, df55, 60, P.0.05; Fig. 6B) ostracods (Kruskall–
22
Wallis test; H54.9, df55, 60, P.0.05; Fig. 6C), N. fluviatilis (x56.4 ind m ,
SD527.9, Kruskall–Wallis test: H53.1, df55, 60, P.0.05), Co. insidiosus (x518
22
ind m , SD545, Kruskall–Wallis test: H52.11, df55, 60, P.0.05) or the
22
nemertean non-identified species (x522.7 ind m , SD548.9, Kruskall–Wallis test:
22
Fig. 4. Density of nematodes and ostracods (ind cm ) at different distances from the burrow, in the mound direction (mound) and in the opposite one (no mound). Equal letters indicate no significant differences (P.0.05).
MDS ordination of samples showed no treatment effect on the benthic community
structure (Fig. 7) and this was confirmed by ANOSIM (R50.09; P.0.01).
3.2.2. Effect of C. granulata on infaunal organisms living at different depths in
sediment
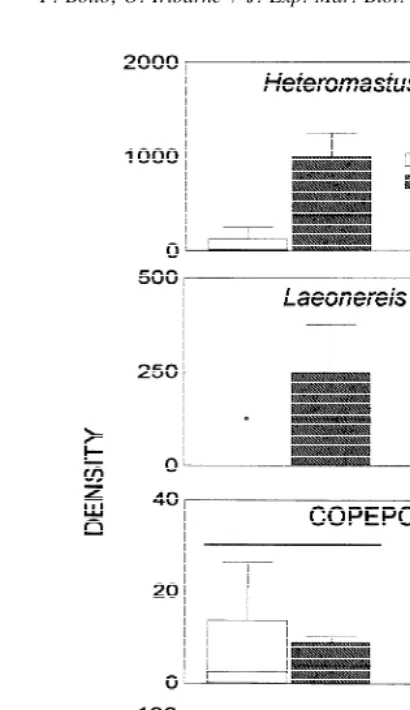
The most abundant organisms found during this experiment were nematodes and the polychaete L. acuta. When each species was evaluated, no effect was found on the
abundance of L. acuta at any depth layer [upper layer: data were transformed; x9 5log
(x11), t51.6, df516; lower layer: Mann–Whitney U544.5, n159, n2510; P.
0.05; Fig. 8A). No difference was found in abundance of this polychaete between both depth layers [transformed data x9 5log (x11), t51.98, df518, P.0.05; Fig. 8A). However, nematodes significantly increased in abundance in the upper layer (data were
log transformed, t52.4, df518, P,0.05), while no difference were found between
Fig. 5. Results on macroinfauna of the experiment to discriminate the effect of crabs. Box plots show the
22
abundance (ind m ) of the polychaetes (A) L. acuta and (B) H. similis in each treatment: control (CON), birds and fishes exclusion (B&F EXC), C. granulata inclusion (CG INC), Cy. angulatus inclusion (CA INC), cage control (CAGE CON), and total exclusion (EXC). Lines connect no significant differences (P.0.05).
22
differences in abundance between treatments were: ostracods (x50.7 ind cm , SD5
22
0.8, t51.67, df517; P.0.05); copepods (x51.5 ind cm , SD51.34, t50.75,
22
df517; P.0.05); N. fluviatilis (x56.36 ind m , SD528.48, tc51, df59; P.
22
0.05) and Co. insidiosus (x525.4 ind m , SD566.6, tc50.8, df512.7; P.0.05).
22
Also, no treatment effect was found for H. similis (x595.5 ind m , SD548.3,
22
tc51.3, df518; P.0.05) and the nemertean non-identified species (x59.1 ind m ,
SD56.6, tc51.9, df59; P.0.05), which were both present in the lower layers.
MDS and ANOSIM showed significant grouping of treatments (stress50.001; R5
0.39 P,0.01; Fig. 9), indicating differences between treatments in the community
structure.
3.2.3. Effect of crabs on recruitment of Cy. angulatus
Juveniles of the crab Cy. angulatus (carapace width: x55.82 mm, SD51.35, n518)
22
22
Fig. 6. Results on meiofauna of the experiment to discriminate the effect of crabs. Density (ind cm ) of (A) nematodes, (B) copepods and (C) ostracods in each experimental treatment: C. granulata inclusion (CG INC), birds and fishes exclusion (B&F EXC), control (CON), cage control (CAGE CON), Cy. angulatus inclusion (CA INC), and total exclusion (EXC). Lines connect no significant differences (P.0.05).
n510). These crabs made superficial burrows that covered the entire control areas. The most abundant infaunal organisms in this period were H. similis, copepods and nematodes. No differences among treatments were found for H. similis at any depth layer (ANOVA: superficial layer: F52.56, df52, 17; deep layer: F54.36, df52, 17;
P.0.05; Fig. 10A). Abundance of this polychaete was the same in both depth layers
Fig. 7. MDS ordination of replicates of the different treatments in the experiment to discriminate the effect of crabs from other predators. C. granulata inclusion (CG INC), birds and fishes exclusion (B&F EXC), control (CON), cage control (CAGE CON), Cy. angulatus inclusion (CA INC), and total exclusion (EXC).
and no significant differences in abundance among treatments were found (log
transformed data: ANOVA F52.59, df52, 24, P.0.05; Fig. 10B). Nematodes
decreased significantly in the upper layer when Cy. angulatus crabs were included (log
transformed data, ANOVA: F54.01, df52, 20, P,0.05, Fig. 10C). However, no
treatment effect was found at the lower layer (ANOVA: F50.75, df52, 20, P.0.05).
Nematodes were more abundant in the superficial layer (t52.76, df524, P,0.05; Fig. 10C). Other organisms found in this experiment in low abundance with no differences
22
between treatments were the polychaete Neanthes succinea (x546.7 ind m , SD5
22
78.3; Kruskall–Wallis test: H50.75 ind m , df52, 30, P.0.05), L. acuta (x525.47
22
ind m , SD577.7; Kruskall–Wallis: H50.67, df52, 30, P.0.05), Co. insidiosus
22 22
(x511 ind cm , SD522; Kruskall–Wallis: H54 ind m , df52, 30, P.0.05) and
22
the nemertean non-identified species (x521.2 ind m , SD548.2; Kruskall–Wallis:
H54.5, df52, 30, P.0.05).
3.2.4. Inclusion of crabs in an area not inhabited by crabs
The most abundant infauna in this experiment were the polychaetes H. similis and L.
acuta, nematodes and copepods. Both polychaete species were found only in the
superficial layer and significantly decreased in abundance in the inclusion treatment (H.
similis: log transformed data t5 22.46, df512; Fig. 11A; L. acuta: t5 22.34,
df514; P,0.05; Fig. 11B). Copepods were also found only in the upper layer, but no
significant differences between treatments were found (log transformed data: t50.86,
df512, P.0.05; Fig. 11C). Nematodes were found in both depth layers but were more
Fig. 8. Results of the experiment to evaluate crab effect on organisms living at two depths in sediment.
22 22
Density of (A) the polychaete L. acuta (ind m ) and (B) nematodes (ind cm ), in exclusion and inclusion treatments and at both depth layers. Lines connect no significant differences (P.0.05).
22 22
Fig. 10. Density of (A) the polychaete H. similis (ind m ), (B) copepods (ind cm ) and (C) nematodes (ind
22
cm ), in the three treatments, and at both depth layers. Lines connect no significant differences.
no differences in abundance between treatments at any layer (upper layer: t5 20.35,
df512; lower layer: t50.84, df512; P.0.05, Fig. 11D). Other organisms found in
22
low density without differences between treatments were ostracods (x50.13 ind cm ,
22
SD522; t50.6, df512, P.0.05) Co. insidiosus (x527.2 ind m , SD554.2;
22
t50.61, df512, P.0.05) and the nemertean non-identified species (x527.2 ind m ,
SD554.2; t50.6, df512, P.0.05).
MDS ordination did not show grouping of treatments (stress50.00001; ANOSIM:
R50.09, P.0.01; Fig. 12).
3.2.5. Effect of juvenile crabs on abundance of meiofaunal organisms
22 22 22
Fig. 11. Density of (A) H. similis (ind m ), (B) L. acuta (ind m ), (C) copepods (ind cm ) and (D)
22
nematodes (ind cm ), in INCLUSION and CONTROL treatments outside crab bed, and at both depth layers. Lines connect no significant differences.
[t55.04, df59, P,0.05; data were transformed by x9 5log (x11) to meet normali-ty]. However, no effect of juvenile crabs was found at any layer (upper layer: ANOVA:
F52.96, df52, 27; lower layer: log transformed data, ANOVA: F50.44, df52, 27;
P.0.05; Fig. 13). Copepods and ostracods were also found in this experiment, but due
to their low density no statistical analysis was performed.
4. Discussion
Fig. 12. MDS of experiment samples of INCLUSION of C. granulata and CONTROL treatment in an area uninhabited by crabs.
polychaete species was affected by the activity of the crab C. granulata. This crab also affected nematode abundance in the superficial layer and their distribution on a small scale around burrows. The recruitment of the crab Cy. angulatus was also affected by adults of the same species and of C. granulata. When adults of C. granulata were added in an area previously not inhabited by crabs, the abundance of the polychaetes L. acuta and H. similis decreased. Juveniles of C. granulata did not affect meiofauna.
Many studies have shown that large scale patterns in abundance are often correlated
22
with changes in sediment grain size and sediment sorting (Logbottom, 1970; Posey, 1986), principally affecting the distribution of some deposit feeders (Whitchlach, 1981; Taghon, 1982). Deposit feeders, moreover, are known to strongly influence the physical characteristics of the environment, particularly through active burrowing (Rhoads and Young, 1970; Posey, 1986; Levinton, 1989). The most common effects are an increase in water content (specially in sediments with high contents of silt and clay; Rhoads and Young, 1970) and the redistribution of sediment particles (Levinton, 1989). These common effects of deposit feeders on sediment, suggest that sediment characteristics found in our study could be in part caused by the burrowing activity of C. granulata, especially considering that these crabs rework large amounts of sediment (Iribarne et al., 1997). Previous studies showed an enhanced content of organic matter associated with these organisms (Rhoads and Young, 1970), but we found no increases in organic matter content inside crab beds. However, burrows of C. granulata, in this environment, work as traps for detritus (Iribarne et al., 1997), which suggest that organic matter may concentrate inside burrows and decrease at the surface.
The effect of C. granulata on sediment, therefore, may determine the distribution of other deposit feeders. Trophic group amensalism hypothesis predicts patterns of infaunal distribution where trophic modes are separate (Rhoads and Young, 1970; Posey, 1990). Although no studies exist in this environment about trophic modes of most organisms found, sediment observed in the gut of the polychaete L. acuta (F.B., pers. obs.) suggests that these organisms deposit feed in mudflats. This polychaete showed a larger abundance inside crab beds, probably favored by reworked sediment. Although organic matter content did not vary, availability may be lower outside crab beds due to larger
grain size, since most deposit feeders collect particles greater than 100 mm with
difficulty (Levinton, 1989).
Although crabs could benefit the polychaete L. acuta by making sediment environ-ment more suitable for them, manipulative experienviron-ments showed that polychaetes are negatively affected by crab activity. Also the fact that polychaete abundance greatly decreased during summer may be in part caused by the increase of crab activity during this period. In the experiment to discriminate the effect of crabs, the polychaete L. acuta increased its density in the exclusion treatment, compared with the control and the exclusion of birds and fishes treatment, indicating that vertebrates did not affect their abundance. No effect was found when each crab species was included, indicating that probably the effect was due to a combined effect of both crab species. In the other experiments inside crab beds, no effect was found for C. granulata on L. acuta possibly because the density of this polychaete was too low to enable effect to be evaluated. However, crabs added to an undisturbed sediment greatly decreased the abundance of L.
acuta. The same pattern occurs with the polychaete H. similis which was not affected by crabs inside crab beds.
Local studies on stomach contents of Cy. angulatus showed that this species feeds on worms and detritus (Olivier et al., 1972b), thus, this crab is probably preying on L.
acuta. Several previous studies have reported predation on polychaetes by crabs (e.g.,
polychaetes (Brenchley, 1981; Posey et al., 1991). The different effects inside or outside crab beds may be interpreted as the result of greater bioturbation when crabs are excavating new burrows (Posey et al., 1991). Outside the crab bed, sediments were more compact, probably reducing mobility of polychaetes and thus making them more susceptible to sediment disturbances associated with burrow digging (Tamaki, 1988).
Samples of meiofauna collected at different distances from burrow entrances of C.
granulata showed that meiofaunal distribution patterns are influenced by the presence of
burrow structures. Posey (1986) suggested that small scale patterns in species abundance in soft sediments could be the result of current velocity directly affecting larval transport and settlement. Meiofaunal animals although lacking pelagic larval stages are themselves so small that even as adults they might disperse transported while suspended in the water column (Gerlach, 1977; Palmer, 1988). Nematodes are highly susceptible to being passively transported (DePatra and Levin, 1989) and given that depressions in surface sediments enhance the deposition of suspended material by decreasing shear stress (DePatra and Levin, 1989), depressions like burrow entrances may increase the deposition of nematodes (DePatra and Levin, 1989; Sun and Fleeger, 1994; Iribarne et al., 1997). The low abundance of nematodes in the mound direction could be due to the continuous deposition of sediments on this side by crabs which may be enhancing the suspension of nematodes and their transport to other sites or just affecting their survival. As suggested by DePatra and Levin (1989) low densities of nematodes may also be a response to decreased food supply.
Another important result is the effect of both crab species C. granulata and Cy.
angulatus on settlement of Cy. angulatus. Juveniles of Cy. angulatus in this environment are confined to areas with refuges, like areas with stones, or in crevices of Ficopomatus
enigmaticus, a tube building polychaete (Spivak et al., 1994). This pattern of distribution could be the result of active habitat selection by megalopae, or differential survivorship. Several field and laboratory studies have shown that megalopae are capable of selecting settling sites on the bases of their physical or chemical conditions (Castro, 1978; Jensen, 1991; Boudreau et al., 1993; Fernandez et al., 1993). A recent study in this environment showed that megalopae of Cy. angulatus due to their swimming ability under flow velocities, are able to maneuver and select settlement sites (Valero et al., 1997). In the present study, experimental cages may have been detected as refuges, favoring settlement inside them. However, no larvae survived when adult crabs were present, indicating that predation by C. granulata and cannibalism are important regulating factors. Cannibalism was also demonstrated under laboratory conditions (Luppi et al., 1995), and as with other crab species (Fernandez et al., 1993; Moksnes et al., 1997) may be important in the regulation of settlement success.
these evidences suggest that C. granulata, mainly due to their bioturbation activities, may be considered an important species on SW Atlantic marshes. Thus, we suggest that this species should be considered in any marsh conservation endeavor.
Acknowledgements
We gratefully acknowledge G. Palomo, J. Valero, J. Gutierrez and L. Lucifora for field assistance and M. Kittlein for advice in statistical analysis. We also thank two anonymous reviewers for their helpful comments. This project was supported by the
´
Universidad Nacional de Mar del Plata (051 / 94), Fundacion Antorchas (13016 / 1-00012) and the International Foundation for Science (No. A / 2501-1). F.B. was
´ supported by a scholarship from the Consejo Nacional de Investigaciones Cientıficas y
´
Tecnicas (CONICET).
References
Adam, P., 1990. Saltmarsh ecology. In: Cambridge Studies in Ecology, Cambridge University Press, Cambridge.
Aller, R.C., Dodge, R.E., 1974. Animal sediment relations in a tropical lagoon Discovery Bay, Jamaica. J. Mar. Res. 32, 209–232.
Bell, S.S., 1980. Meiofauna–macrofauna interaction in a high salt marsh habitat. Ecol. Monogr. 50, 487–505. Bertness, M.D., 1985. Fiddler crab regulation of Spartina alterniflora production on a New England salt
marsh. Ecology 66, 1042–1055.
Billick, I., Case, T.J., 1994. Higher order interaction in ecological communities: what are they and how can they be detected? Ecology 75, 1529–1543.
´ ´
Boschi, E.E., 1964. Los crustaceos Decapodos Brachyura del litoral Bonaerense. Bol. Inst. Biol. Mar. (Mar del Plata, Argentina) 6, 1–99.
Botto, F., Iribarne, O., Martinez, M.M., Delhey, K., Carrete, M., 1998. The effect of migratory shorebirds on the benthic species of three southwestern Atlantic Argentinean estuaries. Estuaries 21, 700–710. Boudreau, B., Bourget, E., Simard, Y., 1993. Effect of age, injury and predator odors on settlement and shelter
selection by lobster Homarus americanus postlarvae. Mar. Ecol. Prog. Ser. 93, 119–129.
Brenchley, G.A., 1981. Disturbance and community structure: an experimental study of bioturbation in marine soft-bottom environments. Mar. Res. 39, 167–790.
Carver, R.E., 1971. Procedures in Sedimentary Petrology, Wiley Interscience, New York.
Castro, P., 1978. Settlement and habitat selection in the larvae of Echinoecus pentagonus (A. Milne Edwards), a brachyuran crab symbiotic with sea urchin. J. Exp. Mar. Biol. Ecol. 37, 259–270.
Clarke, K.R., 1993. Non-parametric multivariate analyses of changes in community structure. Austr. J. Ecol. 18, 117–143.
Conover, W.J., 1980. Practical Nonparametric Statistics, 2nd ed., Wiley, New York.
DePatra, K., Levin, L.A., 1989. Evidence of the passive deposition of meiofauna into fiddler crab burrows. J. Exp. Mar. Biol. Ecol. 125, 173–192.
´
Fernandez, M., Armstrong, D., Iribarne, O., 1993. First cohort of the young-of-the-year dungeness crab,
Cancer magister, reduces abundance of subsequent cohorts in intertidal shell habitat. Can. J. Fish. Aquat.
Sci. 50, 2100–2105.
Gerlach, S.A., 1977. Means of meiofauna dispersal. Mikrofauna Meeresboden 61, 89–103.
Hall, S.J., Basford, D.J., Robertson, M.R., Raffaelli, D.G., Tuck, I., 1991. Patterns of recolonization and the importance of pit-digging by the crab Cancer pagurus in a subtidal sand habitat. Mar. Ecol. Prog. Ser. 72, 93–102.
Hoffman, J., Katz, J., Bertness, M., 1984. Fiddler crab regulation of meiofauna abundance in saltmarsh habitats. J. Exp. Mar. Biol. Ecol. 82, 161–174.
Ieno, E.N., Bastida, R.O., 1998. Spatial and temporal patterns in coastal macrobenthos of Samborombon Bay, Argentina: a case study of very low diversity. Estuaries 21, 690–699.
Iribarne, O., Bortolus, A., Botto, F., 1997. Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Mar. Ecol. Prog. Ser. 155, 132–145.
Jensen, G., 1991. Competence, settling behavior and postsettlement aggregation by porcelain crab megalopae (Anomura porcellanidae). J. Exp. Mar. Biol. Ecol. 153, 49–61.
Kneib, R.T., 1991. Indirect effects in experimental studies of marine soft sediment communities. Am. Zool. 31, 874–885.
Kneib, R.T., A Weeks, C., 1990. Intertidal distribution and feeding habits of the mud crab, Eurytium limosum. Estuaries 4, 462–468.
Levinton, J.S., 1989. Deposit feeding and coastal oceanography. In: Lopez, G., Taghon, G., Levinton, J. (Eds.), Ecology of Marine Deposit Feeders Lecture. Notes On Coastal and Estuarine Studies, Springer, New York, pp. 1–23.
Logbottom, M.R., 1970. The distribution of Arenicola marina (L) with particular reference to the effects of particle size and organic matter of the sediments. J. Exp. Mar. Biol. Ecol. 5, 138–157.
Luckenbach, M.W., Huggett, D.V., Zobrist, E.C., 1988. Sediment transport, biotic modifications and selection of grain size in a surface deposit-feeder. Estuaries 11, 134–139.
Luppi, T., Spivak, E., Anger, K., 1994. La coexistencia de dos especies de cangrejo en el ecosistema del cangrejal: estudio comparativo del asentamiento y el reclutamiento. II Taller sobre cangrejos y cangrejales, Mar del Plata, Argentina, p. 5, Abstracts.
Luppi, T., Spivak, E., Anger, K., 1995. Canibalismo y depredacion en Cyrtograpsus angulatus y
Chasmag-nathus granulata. In: Congreso Latinoamericano de Ciencias del Mar, Mar del Plata, p. 177, Abstracts.
Mammoli, G.A., 1992. Taxonomia de los nematodes libres de la albufera Mar Chiquita. Tesis de Licenciatura. Universidad Nacional de Mar del Plata, Mar del Plata.
Miller, D.C., 1989. Abrasion effects on microbes in sandy sediments. Mar. Ecol. Prog. Ser. 55, 73–82. Miller, D.C., Sternberg, R.W., 1988. Field measurements of the fluid and sediment-dynamic environment of a
benthic deposit feeder. J. Mar. Res. 46, 771–796.
Moksnes, P.O., Lipcius, R.N., Pihl, L., van Montfrans, J., 1997. Cannibal–prey dynamics in young juveniles and postlarvae of the blue crab. J. Exp. Mar. Biol. Ecol. 215, 157–187.
Montague, C.L., 1980. A natural history of temperate Western Atlantic fiddler crabs (genus Uca) with reference to their impact on the salt marsh. Cont. Mar. Sci. 23, 25–55.
Neter, J., Wasserman, W., Kutner, M.H., 1990. Applied Linear Models, Regression, Analysis of Variance and Experimental Designs, 3rd ed., R.D. Irwin, Boston, MA.
´
Olivier, S.R., Escofet, A., Penchaszadeh, P., Orensanz, J.M., 1972a. Estudios ecologicos de la region estuarial ´
de Mar Chiquita (Buenos Aires, Argentina). I: Las comunidades bentonicas. Ann. Soc. Cient. Argentina 193, 237–262.
´
Olivier, S.R., Escofet, A., Penchaszadeh, P., Orensanz, J.M., 1972. Estudios ecologicos de la region estuarial ´
de Mar Chiquita (Buenos Aires, Argentina). II. Relaciones troficas. Ann. Soc. Cient. Argentina 194, 84–104.
Palmer, M.A., 1988. Dispersal of marine meiofauna: a review and conceptual model explaining passive transport and active emergence with implications for recruitment. Mar. Ecol. Prog. Ser. 48, 81–91. Posey, M.H., 1986. Changes in a benthic community associated with dense beds of a burrowing deposit-feeder,
Callianassa californiensis. Mar. Ecol. Prog. Ser. 31, 15–22.
Posey, M.H., 1990. Functional approaches to soft-substrate communities: how useful are they? Rev. Aquat. Sci. 39, 343–356.
Posey, M.H., Dumbauld, B.R., Armstrong, D.A., 1991. Effects of a burrowing mud shrimp, Upogebia
Rhoads, D.C., Young, D.K., 1970. The influence of deposit feeding organisms on sediment stability and community trophic structure. J. Mar. Res. 28, 150–178.
Spivak, E., Anger, K., Luppi, T., Bas, C., Ismael, D., 1994. Distribution and habitat preferences of two grapsid crab species in Mar Chiquita Lagoon (Province of Buenos Aires, Argentina). Helgolander Meeresunters. 48, 59–78.
StatSoft Inc, 1998. STATISTICA for Windows, Statsoft, Tulsa, OK.
Sun, B., Fleeger, J.W., 1994. Field experiments on the colonization of meiofauna into sediment depressions. Mar. Ecol. Prog. Ser. 110, 167–175.
Taghon, G.L., 1982. Optimal foraging by deposit feeding invertebrates: roles of particle size and organic coating. Oecologia 52, 295–304.
Tamaki, A., 1988. Effects of bioturbation activity of the ghost shrimp Callianassa japonica Ortmann on migration of a mobile polychaete. J. Exp. Mar. Biol. Ecol. 120, 81–95.
Tamaki, A., Ingole, B., 1993. Distribution of juvenile and adult ghost shrimps Callianassa japonica Ortmann (Thalassinidea), on an intertidal sand flat: intraspecific facilitation as s possible pattern-generating factor. J. Crust. Biol. 13, 175–183.
Thrush, S.F., 1988. The comparison of macrobenthic recolonization pattern near and away from crab burrows on a sublittoral sand flat. J. Mar. Res. 46, 669–681.
Valero, J., Luppi, T., Iribarne, O., 1997. Estudio experimental de natacion de megalopas de cangrejos estuariales: implicancias en el asentamiento. In: XVIII Reunion Argentina de Ecologia, Buenos Aires. Virnstein, R.W., 1977. The importance of predation by crabs and fishes on benthic fauna of Chesapeake Bay.
Ecology 58, 1199–1217.
Warwick, R.M., Clarke, K.R., 1991. A comparison of some methods for analyzing changes in benthic community structure. J. Mar. Biol. Assoc. UK 71, 225–244.
Warwick, R.M., Clarke, K.R., Gee, J.M., 1990. The effect of disturbance by soldier crabs, Mictyris platycheles H. Milne Edwards, on meiobenthic community structure. J. Exp. Mar. Biol. Ecol. 135, 19–33.
Whatley, R., Moguilevsky, A., Toy, N., Chadwick, J., Feijo Ramos, M.I., 1997. Ostracoda from the South West Atlantic. Part II. The littoral fauna from between Tierra del Fuego and the Rio de la Plata. Rev. Esp. Micropaleon. 29, 5–83.
Whitchlach, R.B., 1981. Animal sediment relationships in intertidal marine benthic habitats: some determinants of deposit-feeding species diversity. J. Exp. Mar. Biol. Ecol. 53, 31–45.
Wilson, W.H., 1991. Competition and predation in marine soft-sediment communities. Annu. Rev. Ecol. Syst. 21, 221–241.
Wolfrath, B., 1992. Burrowing of the fiddler crab Uca tangeri in the Ria Formosa in Portugal and its influence on sediment structure. Mar. Ecol. Prog. Ser. 85, 237–243.
Woodin, S.A., 1976. Adult larval interactions in dense infaunal assemblages: patterns of abundance. J. Mar. Res. 34, 25–41.