Journal of Experimental Marine Biology and Ecology 255 (2000) 153–174
www.elsevier.nl / locate / jembe
Evaluating the impact of predation by fish on the assemblage
structure of fishes associated with seagrass (Heterozostera
tasmanica) (Martens ex Ascherson) den Hartog, and
unvegetated sand habitats
a,b ,* c a
Jeremy S. Hindell , Gregory P. Jenkins , Michael J. Keough
a
Department of Zoology, University of Melbourne, Parkville 3010, Australia
b
Queenscliff Marine Station, Queenscliff 3225, Australia
c
Marine and Freshwater Resources Institute, Queenscliff 3225, Australia
Received 22 November 1999; received in revised form 4 July 2000; accepted 1 September 2000
Abstract
The role of fish predation in structuring assemblages of fish over unvegetated sand and seagrass was examined using enclosure and exclusion cages to manipulate the abundance of predatory fish from November 1998 to January 1999. In our exclusion experiment, piscivorous fish were excluded from patches of unvegetated sand and seagrass to measure how they altered abundances of small fishes, i.e., fish ,10 cm in length. Habitats from which piscivorous fish were excluded contained more small fish than those with partial cages, which in turn contained more fish than uncaged areas. These patterns were consistent between unvegetated sand and seagrass areas, although the relative differences between predator treatments varied with habitat. Overall, small fish were more abundant in unvegetated sand than seagrass. Atherinids and syngnathids were the numerically dominant families of small fish and varied in complex ways amongst habitats and cage treatments. The abundance of atherinids varied inconsistently between cage treatments through time. Only during the final two sampling times did the abundance of atherinids vary significantly across cage treatments. Syngnathids were strongly associated with seagrass and were significantly more abundant in caged than uncaged habitats. In our enclosure experiment, five individuals of a single species of transient piscivorous fish, Western Australian salmon (Arripidae:
Arripis truttacea Cuvier), were enclosed in cages to provide an estimate of the potential for this
species to impact on small fish. The abundance of small fish varied significantly between cage treatments. Small fish were more abundant in enclosure cages and exclusion cages than uncaged areas; however, there was no difference in the abundance of small fish in enclosure cages and partial cages, and no difference between exclusion cages and partial cages. These patterns were
*Corresponding author. Queenscliff Marine Station, PO Box 138, Victoria 3225, Australia. Tel.: 1 61-3-5258-3686; fax: 161-3-5258-3632.
E-mail address: [email protected] (J.S. Hindell).
154 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
consistent amongst habitats. Atherinids and syngnathids were again the numerically dominant families of small fish; atherinids varied more with cage structure while syngnathids did not vary statistically between cages, blocks (locations within which a single replicate of each cage treatment was applied) or habitats. Dietary analysis of caged A. truttacea demonstrated the potential for this species to influence the assemblage structure of small fish through predation – atherinids were consumed more frequently in unvegetated sand than seagrass, and syngnathids were consumed only in seagrass, where they are most abundant. Observations of significant cage or predation effects depended strongly on the time at which sampling was undertaken. In the case of the atherinids, no predation or cage effects were observed during the first two sampling times, but cage effects and predation effects strongly influenced abundances of fish during the third and fourth sampling times, respectively. Our study suggests that transient piscivorous fish may be important in structuring assemblages of small fish in seagrass and unvegetated sand, and seagrass beds may provide a refuge to fishes. But the importance of habitat complexity and predation, in relation to the potentially confounding effects of cage structure, depends strongly on the time at which treatments are sampled, and the periodicity and multiplicity of sampling should be considered in future predation studies. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Arripis truttacea; Australia; Unvegetated sand; Caging experiment; Heterozostera tasmanica; Piscivory; Seagrass; Structural complexity; Temperate; Temporal variability
1. Introduction
Predation can be an important process structuring post-settlement assemblages of fish (Choat, 1982), and one of the most abundant predators are other fishes. Correlative studies often show that the abundance of piscivorous fish is negatively associated with the abundance of smaller (prey) fish (Hixon, 1991; Bailey, 1994; Connell and Kingsford, 1997, 1998). Dietary studies complement correlative analyses by demonstrating the importance of particular suites of small fish in the diets of predatory fishes (Hall et al., 1990; Kingsford, 1992; Edgar and Shaw, 1995a; Connell and Kingsford, 1997; Connell, 1998). In concert, correlative studies and dietary analyses imply that predatory fish are important determinants of the structure of small fish assemblages. However, few experimental studies have unequivocally concluded that fish predation is an important contributor to variability in small fish assemblage structure (Hixon, 1991).
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 155
is an important source of mortality, and predation efficiency, as a function of detection, selection, pursuit and capture of prey (Mattila, 1995), decreases with increasing habitat complexity (Choat, 1982; Stoner, 1982; Gotceitas et al., 1997). Thus, patterns in the abundance of fish across habitats which differ markedly in structure may reflect differential predation pressure, i.e., the structure of the vegetation and the type of habitat complexity it generates determines the intensity and nature of predator–prey interac-tions, and thereby affects the structuring capacity of predation (Mattila, 1995).
Seagrasses are a common form of biogenic habitat in marine and estuarine systems worldwide (Pollard, 1984; Bell and Pollard, 1989; Kemp, 1989), and compared with alternative, usually unvegetated habitat, generally contain higher numbers of predatory and other fishes (Heck et al., 1989; Edgar and Shaw, 1995a,b; Jenkins et al., 1997b). The high, but temporally variable (Heck et al., 1989), association of small fish with seagrass habitat has led to a paradigm which promotes the importance of seagrass beds in the provision of nursery habitat for juvenile fishes (Pollard, 1984; Bell and Pollard, 1989; Jenkins and Wheatley, 1998). Increased food availability (Bell and Pollard, 1989) and protection from environmental disturbance (Kemp, 1989; Edgar, 1990) are two popular theories why seagrass beds contain high abundances of small fish. But, it is also plausible that the structural complexity provided by aspects of the seagrass affects the efficacy or selectivity of predators (Levin et al., 1997), and provides juvenile fish with a refuge from predation (Orth et al., 1984; Orth, 1992).
Previous studies suggest that broad-scale patterns in small fish assemblages may be influenced by variable larval supply (Jenkins and Black, 1994; Jenkins et al., 1997a) and, micro-habitat selection, not predation, is the proximate cause for variability in the abundance of fauna within seagrass beds (Bell et al., 1987; Edgar and Robertson, 1992; Levin et al., 1997). However, many of these studies have manipulated predatory fish
2
over relatively small (1 m ) spatial scales (Bell et al., 1987), which may reflect prey movements and behaviour more than predation effects (Englund and Olsson, 1996; Englund, 1997). Furthermore, while exclosure cages have been used extensively to manipulate abundances of predatory fish (Steele, 1998; Levin et al., 1997; Kennelly, 1991), few studies have used enclosure cages to assess the role of predatory fish in structuring assemblages of fish in structurally diverse habitats. A more thorough understanding of (a) the role of fish predation in structuring assemblages of small fish, and (b) the importance of seagrass beds in the provision of refuge from predation, will be gained by conducting carefully designed experiments which manipulate predator abundance using controlled enclosure and exclosure caging experiments over similar spatial and temporal scales in large plots of habitat (seagrass and unvegetated sand) that differ markedly in structural complexity.
156 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 2. Materials and methods
2.1. Study site
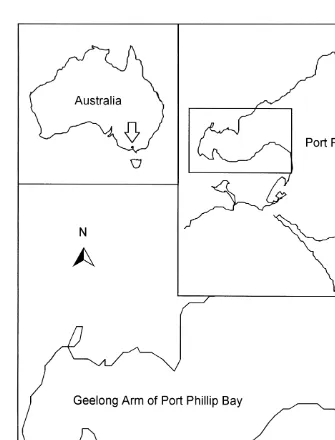
Our study was carried out at Grand Scenic, which is situated on the southern shore of the Geelong Arm of Port Phillip Bay (Fig. 1). The currents in this region of Port Phillip
21
Bay are weak, generally much less than 17 cm s (Rosenberg et al., 1992), and the area
is protected from the prevailing southerly winds. These largely sheltered conditions facilitate sedimentary processes that generate a substrate composed primarily of silty sand (Anon., 1973), rich in detritus (J. Hindell, pers. obs.). Tides in this area are semidiurnal and the range is less than 1 m (Jenkins et al., 1998). Grand Scenic contains large contiguous subtidal beds of Heterozostera tasmanica, whose distribution is broken by patches of unvegetated sand. A variety of algae and small patches of rocky reef occur sporadically throughout the beds of seagrass. One other species of seagrass, Zostera
muelleri, Irmisch ex Ascherson, also occurs in this region of Port Phillip Bay, but its
distribution is largely confined to the intertidal.
2.2. Exclusion experiment
2
In our exclusion experiment, predatory fish were excluded from 16-m plots of
unvegetated sand and seagrass to test whether predatory fish influence the assemblage structure of small fish, and whether any observed predator effects are independent of habitat complexity and time of sampling. A variety of predatory fishes, including pike-headed hardyheads, Kestratherina esox Klunzinger (Atherinidae), Arripis truttacea, yank flathead (Platycephalus speculator Klunzinger), and rock flathead (Platycephalus
laevigatus Cuvier), were potentially excluded using cages, but only K. esox and A.
truttacea have been found to consume the small, early post-settlement stages of fish
sampled in the cage treatments at this location (Hindell et al., 2000).
Exclusion cages were constructed from 2.1 m long galvanised steel starpickets,
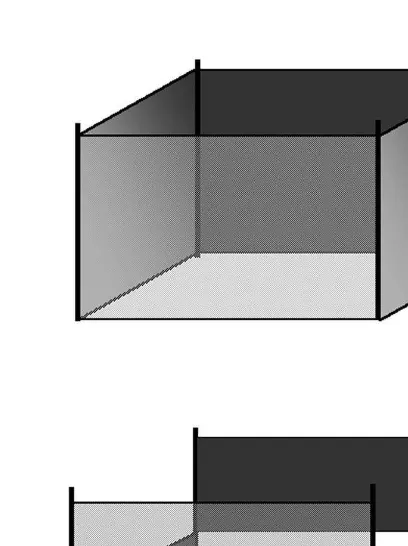
hammered into the substrate at each corner of a 43 4-m square plot. Black, 20-mm
polypropylene netting of 1.5 m height, was placed around the perimeter of the plot, 2
enclosing an area of 16 m (Fig. 2a). This mesh size was chosen because it is small enough to prevent the passage of predatory fish. The mesh was tightened at the top and bottom of each cage with 5 mm nylon cord, and the bottom line was weighted to prevent fish from swimming between the bottom of the cage walls and the surface of the substrate. The top of the cage walls exceeded the water level throughout the tidal range, however, the substrate within each cage treatment was always submerged. Partial cages were constructed from the same materials and in the same dimensions as exclusion cages, except only half of each wall of the cage was attached (Fig. 2b). Each partial cage provided the structure of a cage but did not change the abundance of predatory fishes inside partial enclosures relative to uncaged areas (J. Hindell unpublished data). These cages were used to assess the role of cage structure per se in altering fish assemblages.
Uncaged areas were unmanipulated 43 4-m plots of habitat.
Four locations (blocks) were selected randomly at Grand Scenic, i.e., by dividing this
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 157
Fig. 1. Location of study site, Grand Scenic, in the Geelong arm of Port Phillip Bay. Insets: location of Port Phillip Bay in Australia and location of study region in Port Phillip Bay.
158 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
Fig. 2. Design of (a) exclusion / enclosure cages and (b) cage controls used to manipulate the abundance of predatory fish.
experiment was arranged in a completely randomised block design with ¯10 m
between cage / habitat treatments within blocks, and ¯50 m between blocks.
Following construction, cages were left for 1 week before sampling. Each cage was cleaned weekly to reduce the build up of drift algae which interferes with water movement and may attenuate light (Virnstein, 1978). Cages remained in the field for 1 month. Little algae grew on cage walls and the seagrass did not appear to be influenced detrimentally by excessive sedimentation or overgrowth from epiphytes (J. Hindell, pers. obs.). Recent studies have shown that there is no difference in the particle size distribution, amounts of combustible organic matter, or composition of meiofauna between exclusion and partial cages (J. Hindell, pers. obs.).
2.3. Sampling small fish
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 159
same day during low tide using a modified beach seine net (4 m wide31.5 m high31.5
m deep31-mm mesh). In this study, a pole was attached to each side of the net to aid in
hauling and foam floats and lead weights were attached to the top and bottom of the net, respectively. The net was drawn between two steel posts inside and at one end of the cage, and hauled through to the opposite side of the cage by two people, one person holding each pole. Because pilot studies showed that roughly 90% of all small fish were captured in the first haul of this type of net, regardless of habitat, only a single haul of the net was conducted in each experimental arena. Captured fish were anaesthetised in benzocaine and preserved in 70% ethanol. This sampling procedure was repeated once weekly for four consecutive weeks. In the laboratory, fish were counted and identified to species (Gomon et al., 1994).
2.4. Enclosure experiment
In our second experiment, enclosure cages, each containing juvenile Arripis truttacea were added to the experimental design used in our exclosure experiment and the experiment was re-conducted using reconstructed cage treatments. Juvenile A. truttacea, whose total length at the study location ranges between 10 and 15 cm (J. Hindell, pers. obs.), are transient and gregarious, and commonly occur in shallow water over mosaics of seagrass and rocky reef interspersed with patches of unvegetated sand. A. truttacea are perennial in this type of habitat, and at the time of year our study was conducted, they feed voraciously on early post-settlement fishes, particularly atherinids (Hindell et al., 2000). During winter and early spring, when small fishes are generally less abundant, juvenile A. truttacea consume a range of pelagic invertebrates, the most common of which are crustaceans of the order Mysidacea (J. Hindell, pers. obs.). A. truttacea was chosen to enclose in cages because they are robust to handling stress, easy to catch and maintain, and previous research has shown that their abundances are negatively related to local abundances of juvenile atherinids and sillaginids (Hindell et al., 2000).
The structure and dimensions of enclosure cages were identical to the exclusion cages. In our enclosure experiment, each cage type (exclusion cage, enclosure cage, partial cage and uncaged) was applied to haphazardly chosen unvegetated sand and seagrass plots within each of four blocks. The block component of the enclosure experiment incorporated both temporal (amongst weeks) and spatial (position along the shore) variability, and each block of the experiment (orthogonal combination of cage and habitat) was conducted independently and successively.
After the construction of each block, five juvenile Arripis truttacea (each ,15 cm
total length), approximately the ambient field density of this species at this location (J. Hindell, pers. obs), were added to each enclosure cage. A. truttacea were captured 2 days before being placed in enclosure cages and were maintained in 300-l flowing-seawater aquaria at the Queenscliff Marine Station. After 2 days of confinement, the A.
truttacea, as well as the small fish assemblage, in each combination of habitat and cage,
within a block, were sampled using the net and methods described earlier. Short periods of enclosure of predatory fish were chosen to mimic the temporal patchiness of A.
truttacea in the field. All fish were anaesthetised and preserved in 70% ethanol. Small
160
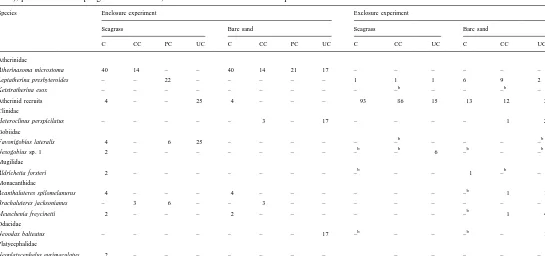
Percent abundance of small fishes in each regime of cage (C, exclusion cage; CC, cage control; PC, enclosure cage; UC, uncaged) and habitat (seagrass, unvegetated
a
sand), pooled across sampling times and blocks, for the enclosure and exclusion experiments
Species Enclosure experiment Exclosure experiment
Seagrass Bare sand Seagrass Bare sand
J.
All data are rounded to whole numbers.
b
162 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
The stomach of each re-captured Arripis truttacea was excised and the gut contents were categorised and counted. The dietary composition of A. truttacea was described using percent frequency of occurrence (F ), percent mass (M ), and percent abundance (N ) (Hyslop, 1980).
2.5. Statistical analysis
The exclusion experiment was analysed as a repeated measures (time), three factor (block, habitat and cage) randomised blocks design. Habitat and cage were treated as fixed factors. Block was treated as a random factor and time was the repeated factor.
Raw data were log(x11) transformed where the assumptions of homogeneity of
variances and normality were not met. The assumption of sphericity was checked by the
Greenhouse-Geisser (G-G) epsilon value (e). The potential for sphericity to influence
our results was controlled by using the G-G adjusted probability (P) values, however, where the adjusted P value did not alter the significance of the un-adjusted P value, the un-adjusted P value was used. A priori tests were used to determine how the levels of the cage effect varied. Where the number of a priori tests exceeded the degrees of
freedom (df) for the effect being tested, the significance level (a) was adjusted to control
for the experimentwise Type I error rate by dividing the significance level for that test (0.05) by the number of comparisons in excess of the df for the effect being tested. This
gave a critical level ofa/(no. planned comparisons2df). Where interactions were found
between a main effect and time, separate one factor ANOVAs and a priori tests were conducted for each time to determine where the levels of the interacting main effect varied.
The enclosure experiment was analysed as a three-factor (block, habitat, cage) randomised blocks design. Tests for assumptions and comparisons of main effects were carried out as described for our exclosure experiment. All analyses were carried out
using SYSTAT statistical software (Wilkinson et al., 1992).
3. Results
3.1. Exclusion experiment
A diverse assemblage of small fish from 10 families and 22 species was sampled throughout our study (Table 1). Small fish were influenced both by manipulating predatory fish using cages, and by the habitat within which this manipulation was conducted, but habitat and caging acted independently (Table 2).
There was significant variability in the abundance of small fish amongst cage types (Table 2 and Fig. 3). The abundance of fish in uncaged areas was significantly lower
than either exclusion cages (P50.001) or cage controls (P50.012). Cage controls
contained significantly fewer fish than exclusion cages (P50.034). The abundance of
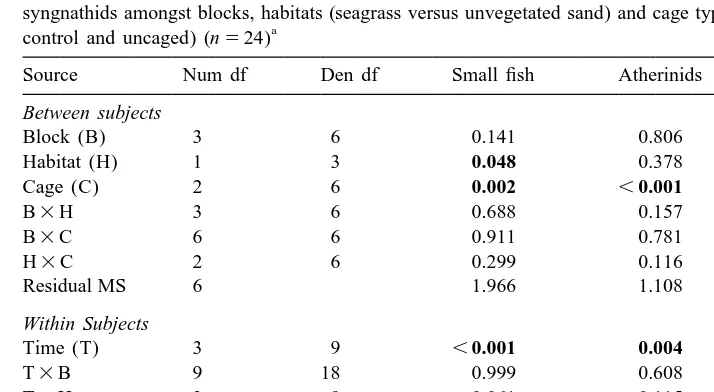
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 163 Table 2
Three factor repeated measures analysis of variance comparing the numbers of small fish (total), atherinids and syngnathids amongst blocks, habitats (seagrass versus unvegetated sand) and cage types (exclusion cage, cage
a
control and uncaged) (n524)
Source Num df Den df Small fish Atherinids Syngnathids Between subjects
Residual MS 6 1.966 1.108 1.017
Within Subjects
Residual MS 18 0.646 1.634 0.444
a
The data table shows, for each group of small fish analysed, the probabilities associated with each of the terms in the model (Source) and the Residual MS. This information allows full reconstruction of the original ANOVA table. Data were log(x11) transformed prior to analysis.
(Table 2 and Fig. 3). Although the main effects (block, habitat and cage) acted independently, there were near significant interactions between time and habitat, time
and cage, and time and the habitat3block interaction (Table 2 and Fig. 3).
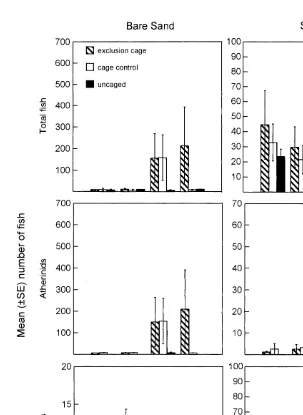
Atherinidae and Syngnathidae were the numerically dominant families of small fish in this experiment (Table 1); therefore, these two families of fish were analysed separately. Despite a trend suggesting otherwise, the abundance of atherinids did not vary between habitats (Table 2 and Fig. 3). Additionally, the abundance of atherinids varied inconsistently between cage treatments through time (Table 2 and Fig. 3). During the first and second sampling times, the abundance of atherinids did not vary between cage
treatments (df , MS50.761, P50.427) and (df , MS50.876, P50.378),
respec-2,18 2,18
tively. During the third sampling time, the abundance of atherinid recruits at Grand Scenic increased substantially (J. Hindell, pers. obs.). This event corresponded with significant variability in the abundance of atherinids, now predominantly juvenile fish,
across cage treatments for the third and fourth sampling times (df , MS514.319,
2,18
P,0.001) and (df , MS513.194, P,0.001), respectively. In the third sampling
2,18
time the abundance of atherinids did not vary significantly between exclusion cages and
cage controls (P50.748), however, uncaged areas contained significantly fewer
atherinids than exclusion cages (P,0.001) and cage controls (P,0.001). In the final
sampling time exclusion cages contained significantly more atherinids than cage controls
(P50.001) and uncaged areas (P,0.001). Cage controls and uncaged areas contained
164 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
Fig. 3. Mean abundance of small fish, atherinids and syngnathids in unvegetated and seagrass habitats during each sampling time in each caging treatment (exclusion cage, cage control and uncaged).
Syngnathids showed a strong association with patches of seagrass; significantly more syngnathids occurred in seagrass than unvegetated sand (Table 2 and Fig. 3). Syngnathids also varied significantly between cage treatments (Table 2), but this result was driven primarily by the much higher abundance of syngnathids in exclusion cages
compared to uncaged areas (P50.015), particularly in seagrass (Fig. 3). The abundance
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 165
cages (P50.294) or uncaged areas (P50.069), nor across times (Table 2, Fig. 3).
While the abundance of syngnathids varied with the position along the shore (block) (Table 2), we were more interested in the variance component and the subsequent reduction in residual variation associated with the block effect, than specific differences between groups. Therefore, no further analysis was conducted for this effect.
3.2. Enclosure experiment
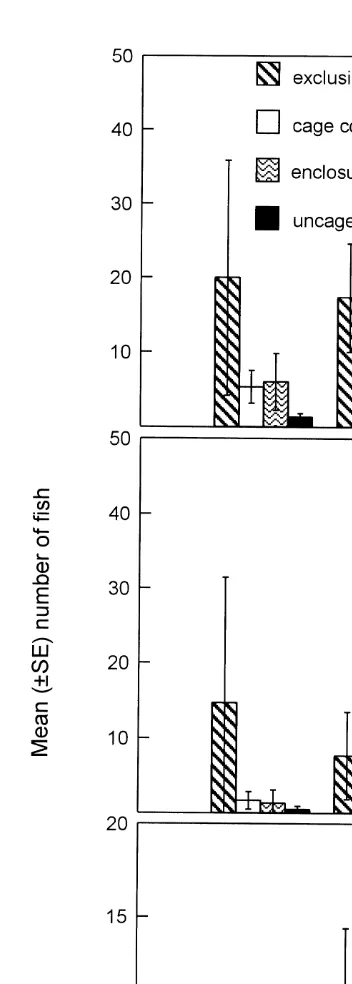
The abundance of small fish did not vary between habitat types or blocks (Table 3 and Fig. 4a). However, the abundance of small fish did vary across cage treatments (Table
3); uncaged areas contained significantly fewer fish than either exclusion cages (P5
0.001), cage controls (P50.005) and predator enclosures (P50.012) (Fig. 4a). Small
fish did not vary between cage controls and predator enclosures (P50.464); however,
there were significantly more small fish sampled from exclusion cages than predator
enclosures (P50.021). The abundance of small fish almost varied significantly between
cage controls and exclusion cages (P50.058). When the level of significance was
adjusted to control for the experimentwise Type I error rate, only the difference in the abundance of small fish between exclusion cages and cages containing predators became non-significant.
As in the exclusion experiment, syngnathids and atherinids were the numerically dominant families of small fish in our enclosure experiment (Table 1), and separate analyses were conducted for each of these families.
The atherinids showed similar patterns to the total fish in that the abundance of atherinids did not vary with block or habitat (Table 3 and Fig. 4b), but varied significantly across cage treatments (Fig. 4b). Exclusion cages contained significantly
more atherinids than either cage controls (P50.029), predator enclosures (P50.018) or
uncaged areas (P50.007). There was no significant difference in the abundance of
atherinids between uncaged areas and predator enclosures (P50.465) or cage controls
(P50.292), nor between cage controls and predator enclosures (P50.722). After the
significance level was adjusted to control for Type I errors (P50.016), only a
Table 3
Three factor analysis of variance comparing the numbers of small fish (total), atherinids and syngnathids amongst blocks, habitats (seagrass, unvegetated sand) and cage types (exclusion cage, cage control, enclosure
a
cage and uncaged) (n524)
Source Num df Den df Small fish Atherinids Syngnathids
Block (B) 2 6 0.587 0.084 0.121
Habitat (H) 1 2 0.212 0.783 0.121
Cage (C) 3 6 0.003 0.029 0.096
B3H 2 6 0.696 0.796 0.553
B3C 6 6 0.818 0.798 0.359
H3C 3 6 0.932 0.989 0.953
Residual MS 6 0.762 0.943 0.436
a
166 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 167 Table 4
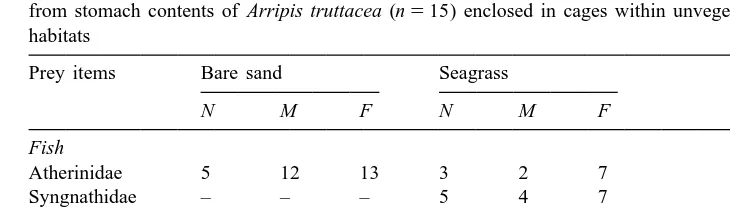
The percent abundance (N ), percent mass (M ), and percent frequency of occurrence (F ) of dietary categories from stomach contents of Arripis truttacea (n515) enclosed in cages within unvegetated sand and seagrass habitats
Prey items Bare sand Seagrass
N M F N M F
Fish
Atherinidae 5 12 13 3 2 7
Syngnathidae – – – 5 4 7
a
Unknown fish 3 – 13 – – –
Other
Crustaceans 92 87 60 89 93 40
Polychaetes 3 2 7 – – –
a
Value ,0.5.
significant difference in the abundance of small fish between exclusion cages and predator cages existed, despite trends which suggest otherwise (Fig. 4b).
The syngnathids in the enclosure experiment displayed similar patterns to those seen for syngnathids in the exclosure experiment (Figs. 3 and 4c). Despite the trends (Fig. 4c), the abundance of syngnathids did not vary statistically across cages, blocks or habitats (Table 3 and Fig. 4c).
3.3. Dietary analysis of enclosed Arripis truttacea
In seagrass habitats, 40% of Arripis truttacea contained no food compared with 53% of A. truttacea enclosed over unvegetated sand. Of the A. truttacea with stomachs containing prey, crustaceans were the most common dietary item, and represented 92 and 87% abundance (N ) and percent mass (M ), respectively. Teleost prey, which included atherinids, syngnathids and unknown fish remains (Table 4), appeared to be a more important component in the diets of A. truttacea enclosed over unvegetated sand than seagrass (Table 4). While atherinids were consumed in both unvegetated sand and seagrass, syngnathids were consumed only in seagrass, the habitat within which they occur most commonly.
4. Discussion
168 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
structure at the end of an experiment, even though selecting the appropriate duration of an experiment is crucial to the nature of impacts observed (Minello, 1993), and therefore, may ‘lose’ information about how effects may change through time. In our study, the time at which our experiment was sampled had a pronounced effect on not only whether differences between cage treatments consistent with predation effects were observed, but whether these differences were confounded by cage effects. For example, during the first two sampling times in the exclusion experiment, the mean numbers of atherinids were similar between cage treatments, and therefore fish were responding neither to the structure of the cage treatments nor predation. At the third sampling time, uncaged areas contained significantly fewer fish than exclusion cages or cage controls, and this suggested that atherinids were responding to the cage structure rather than predation. At the final sampling time, exclusion cages contained more fish than either cage controls or uncaged areas, which contained similar numbers of fish, and this clearly demonstrated that predation by fish, not some artefact associated with the structure of the cage, was important in determining the abundances of atherinids. Clearly, sampling the same experimental units at short time intervals (several days) strongly influences the relative variability in the numbers of fish between caging treatments. However, it is unclear whether these results represent temporal variability in predation and cage effects, or whether they simply reflect an initial prey response to structure, with a later-emerging predation effect. Replicated experiments that evaluate the consistency of results from caging studies over variable lengths of time, and over the same temporal scales at different times, are needed to separate these alternatives. Irrespective of how the numbers of fish varied between cage treatments in our study, this work clearly demonstrates that a strong effect of piscivorous fishes can be measured after 4 weeks, and this effect depends on the habitat within which it was measured.
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 169
partial and enclosure cages, both of which contained similar numbers of small fish, than uncaged areas. And there was a trend for more fish to be associated with exclusion cages than either enclosure cages or cage controls. These data suggest that either predation pressure is similar inside enclosure and partial cages, or the various effects of these two treatments balance.
Recent experiments using video recorders to quantify local abundances of predatory fish have shown that the design of the cage controls used in this study neither influences the ambient densities or movement of Arripis truttacea, nor attracts juvenile fishes (J. Hindell, unpublished data). Therefore, the predation pressure inside partial cages is likely to be similar to that exerted in areas without any form of cage structure, and the structure per se of cage treatments does not appear to be important in attracting juvenile fishes. Furthermore, enclosed A. truttacea were observed to swim and feed uninhibited — they consumed similar categories of prey to those published elsewhere (Robertson, 1982; Hoedt and Dimmlich, 1994). Given that predation impact is similar in enclosures and partial cages, we contend that partial cages may be a form of intermediate protection for small fish, and differences between partial and exclusion cages are more likely to reflect predation and not simply a linear increase in fish attracted to additional artificial structure per se.
170 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
Further data are required which (a) evaluate how the predation pressure imposed by confined fish differs from that imposed by uncaged fish, and (b) establish the importance of varying levels of structure in determining fish abundances. As Virnstein (1980) commented, a single line of evidence by itself is weak and, therefore, a pluralistic approach to caging experiments, i.e., exclusion and enclosure cages in concert with other measures of cage effects, potentially provides researchers with a rigorous evaluation of the importance of predation versus cage effects. On the basis of the syngnathid data and the results from the enclosure / exclusion experiments, the patterns in fish abundances between cage treatments can be interpreted as representing discernible contributions by both cage and predation effects.
Biomass of vegetation is positively associated with the abundance and diversity of animals (Pollard, 1984; Bell and Westoby, 1986a,b; Edgar, 1990; Edgar and Robertson, 1992; Connolly, 1994). In marine environments, fish faunas associated with seagrass are often more diverse and abundant than those in nearby unvegetated soft sediments (Pollard, 1984). While food resources, level of physical structure, number of mi-crohabitats, reduction of environmental disturbance and stabilisation of sediments may help to explain these patterns (Lewis, 1984), the mediation of predation by aspects of the seagrass may also be important in determining fish abundance (Orth et al., 1984). If predation by fish is important in structuring assemblages of small fish, and structural complexity mediates this predation, then we can expect predation pressure to be greatest in areas where structural complexity is low, such as unvegetated sand. When predators are excluded from habitats which differ in structural complexity, the effect of excluding predatory fish should be less in seagrass, whose structural complexity interferes with foraging by fish. And if predation produced the pattern of greater fish density in seagrass than unvegetated sand habitats, then one would predict a greater effect of predators and a larger relative change in prey abundances in unvegetated sand than seagrass (as shown by Summerson and Peterson, 1984). Our results suggest that patterns are at least partially explained by predation. Small fish under pressure from fish predation (no cage) are more abundant in seagrass than unvegetated sand. When predators are excluded, the relative increase in small fish is greater over unvegetated sand than seagrass, suggesting that the structural complexity generated by aspects of the seagrass may be mediating predation. However, regardless of habitat, the numbers of fish varied between cage treatments in qualitatively similar ways. These results suggest therefore, that while predation influences abundances of fish in both seagrass and unvegetated sand habitats, it is relatively more important in habitats without any form of refuge. Therefore, we consider that a substantial portion of the variability in fish abundance between seagrass and unvegetated sand may be related to differential predation pressure between these two habitats.
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 171
suggested by Lewis (1984), provide a refuge from predation. Bologna and Heck (1999) found that scallops experience highest predation pressure in the areas favourable to growth, and fish may alter their foraging patterns amongst structurally variable habitats in the presence of predators (Gotceitas and Brown, 1993). Whether our results were due to the selection of habitats which either offer refuge or have low numbers of predators, or whether they were the result of direct mortality, i.e., being eaten, is not known. Theoretically, either alternative is possible — Levin et al. (1997) showed that predatory fish can directly alter the population structure of fish by consuming small individuals, while Jordan et al. (1996) demonstrated that behaviourally mediated predator avoidance modifies the habitat use in pinfish. Dietary analysis of caged Arripis truttacea and extensive dietary data on predatory fish at Grand Scenic (Hindell et al., 2000) demonstrate the potential for predation, but further research is required to separate the importance of direct predation versus anti-predator behaviour.
5. Conclusion
Our study cautiously suggests that piscivorous fish influence the numbers of fish within and between seagrass and unvegetated habitats, and therefore, predation by fish may be a significant contributor to the widely documented variability in fish abundances observed between vegetated and unvegetated habitats. The changes in abundance of small fish in areas of seagrass and unvegetated sand where predatory fish were excluded suggest that habitat complexity may mediate predation. However, the relative impor-tance of predation versus antipredator behaviour in generating patterns in assemblages of small fish is equivocal, and further research is required to determine how patterns are generated. The importance of cage versus predation effects may be evaluated by manipulating predatory fish with a combination of enclosure, exclosure and partial cages. Additional information about how cage effects per se influence abundances of fish may be gleaned from descriptions of how the numbers of fish that associate positively with habitat complexity vary in relation to different levels of artificial structure. Because, at least in our experiment, whether or not predation and / or cage effects are observed depends on the time at which the treatments in an experiment are measured, multiple sampling of experimental treatments will enhance our understanding of the dynamic nature of predation impacts in estuarine mosaics of unvegetated sand and seagrass habitats.
Acknowledgements
This manuscript was greatly improved by comments from S. Connell, C. Styan, J. Carey, and two anonymous reviewers. Thanks also to M. Hendricks, R. Watson, L. McGrath and M. Wheatley for assistance in the field and at the research station. This research was funded by a Melbourne Research Scholarship and was conducted using the
172 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 References
Anon., 1973. Environmental study of Port Phillip Bay. Report on phase one, 1968–1971. Melbourne and Metropolitan Board of Works, and Fisheries and Wildlife Department of Victoria, Melbourne.
Bailey, K.M., 1994. Predation on juvenile flatfish and recruitment variability. Neth. J. Sea Res. 32 (2), 175–189.
Bell, J.D., Pollard, D.A., 1989. Ecology of fish assemblages and fisheries associated with seagrasses. In: Larkum, A.W.D., McComb, A.J., Shepherd, S. (Eds.), Biology of Seagrasses: A Treatise on the Biology of Seagrasses with Special Reference to the Australian Region. Elsevier, Amsterdam, pp. 565–609. Bell, J.D., Westoby, M., 1986a. Abundance of macrofauna in dense seagrass is due to habitat preference, not
predation. Oecologia 68, 205–209.
Bell, J.D., Westoby, M., 1986b. Variation in seagrass height and density over a wide spatial scale: effects on common fish and decapods. J. Exp. Mar. Biol. Ecol. 104, 275–295.
Bell, J.D., Westoby, M., Steffe, A.S., 1987. Fish larvae settling in seagrass: do they discriminate between beds of different leaf density? J. Exp. Mar. Biol. Ecol. 111, 133–144.
Bologna, P.A.X., Heck, K.L., 1999. Differential predation and growth rates of bay scallops within a seagrass habitat. J. Exp. Mar. Biol. Ecol. 239, 299–314.
Chesson, P., 1998. Spatial scales in the study of reef fishes: a theoretical perspective. Aust. J. Ecol. 23, 209–215.
Choat, J.H., 1982. Fish feeding and the structure of benthic communities in temperate waters. Annu. Rev. Ecol. Syst. 13, 423–449.
Connell, S.D., 1997. Exclusion of predatory fish on a coral reef: the anticipation, pre-emption and evaluation of some caging artefacts. J. Exp. Mar. Biol. Ecol. 213, 181–198.
Connell, S.D., 1998. Patterns of piscivory by resident predatory fish at One Tree Reef, Great Barrier Reef. Mar. Freshwater Res. 49, 25–30.
Connell, S.D., Kingsford, M.J., 1997. The utility of descriptive information for assessing the impact of coral reef piscivores on their prey. Proc. 8th Int. Coral Reef Symp. 1, 999–1004.
Connell, S.D., Kingsford, M.J., 1998. Spatial, temporal and habitat-related variation in the abundance of large predatory fish at One Tree Reef, Australia. Coral Reefs 17, 49–57.
Connolly, R.M., 1994. Removal of seagrass canopy: effects on small fish and their prey. J. Exp. Mar. Biol. Ecol. 184, 99–110.
Doherty, P.J., Sale, P.F., 1985. Predation on juvenile coral reef fishes: an exclusion experiment. Coral Reefs 4, 225–234.
Edgar, G.J., 1990. The influence of plant structure on the species richness, biomass and secondary production of macrofaunal assemblages associated with Western Australian seagrass. J. Exp. Mar. Biol. Ecol. 137, 215–240.
Edgar, G.J., Robertson, A.I., 1992. The influence of seagrass structure on the distribution and abundance of mobile epifauna: pattern and process in a Western Australian Amphibolis bed. J. Exp. Mar. Biol. Ecol. 160, 13–31.
Edgar, G.J., Shaw, C., 1995a. The production and trophic ecology of shallow-water fish assemblages in southern Australia. II. Diets of fishes and trophic relationships between fishes and benthos at Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 194, 83–106.
Edgar, G.J., Shaw, C., 1995b. The production and trophic ecology of shallow-water fish assemblages in southern Australia. I. Species richness, size-structure and production of fishes in Western Port, Victoria. J. Exp. Mar. Biol. Ecol. 194, 53–81.
Englund, G., 1997. Importance of spatial scale and prey movements in predator caging experiments. Ecology 78 (8), 2316–2325.
Englund, G., Olsson, T., 1996. Treatment effects in a stream fish enclosure experiment: influence of predation rate and prey movements. OIKOS 77, 519–528.
Gibson, R.N., Robb, L., Burrows, M.T., Ansell, A.D., 1996. Tidal, diel and longer term changes in the distribution of fishes on a Scottish sandy beach. Mar. Ecol. Prog. Ser. 130, 1–17.
J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174 173 Gotceitas, V., Colgan, P., 1989. Predator foraging success and habitat complexity: quantitative test of the
threshold hypothesis. Oecologia 80, 158–166.
Gotceitas, V., Brown, J.A., 1993. Substrate selection by juvenile Atlantic cod (Gadus morhau): effects of predation risk. Oecologia 93, 31–37.
Gotceitas, V., Fraser, S., Brown, J.A., 1997. Use of eelgrass beds (Zostera marina) by juvenile Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 54, 1306–1319.
Hall, S.J., Raffaelli, D., Basford, D.J., Robertson, M.R., Fryer, R., 1990. The feeding relationships of the larger fish species in a Scottish sea loch. J. Fish Biol. 37, 775–791.
Hamer, P.A., Jenkins, G.P., 1996. Larval supply and short term recruitment of a temperate zone demersal fish, the King George whiting, Sillaginodes punctata Cuvier, to an embayment in south-eastern Australia. J. Exp. Mar. Biol. Ecol. 208, 197–214.
Heck, K.L., Crowder, L.B., 1991. Habitat structure and predator-prey interactions in vegetated aquatic systems. In: Bell, S.S., McCoy, E.D., Mushinsky, H.R. (Eds.), Habitat Structure: the Physical Arrangement of Objects in Space. Chapman and Hall, London, pp. 281–299.
Heck, K.L., Able, K.W., Fahay, M.P., Roman, C.T., 1989. Fishes and decapod crustaceans of Cape Cod eelgrass meadows: species composition, seasonal abundance patterns and comparison with unvegetated substrates. Estuaries 12, 59–65.
Hindell, J.S., Jenkins, G.P., Keough, M.J., 2000. Variability in the abundances of fish associated with seagrass habitats in relation to the diets of predatory fishes. Mar. Biol. 136, 725–737.
Hixon, M.A., 1991. Predation as a process structuring coral reef fish communities. In: Sale, P.F. (Ed.), The Ecology of Fishes on Coral Reefs. Academic Press, San Diego, CA, p. 754.
Hoedt, F.E., Dimmlich, W.F., 1994. Diet of subadult Australian Salmon, Arripis truttacea, in Western Port, Victoria. Aust. J. Mar. Freshwat. Res. 45, 617–623.
Hyslop, E.J., 1980. Stomach contents analysis: a review of methods and their application. J. Fish Biol. 17, 411–429.
Jenkins, G.P., Black, K.P., 1994. Temporal variability in settlement of a coastal fish (Sillaginodes punctata) determined by low-frequency hydrodynamics. Limnol. Oceanogr. 39 (7), 1744–1754.
Jenkins, G.P., Wheatley, M.J., 1998. The influence of habitat structure on nearshore fish assemblages in a southern Australian embayment: comparison of shallow seagrass, reef algal and unvegetated sand habitats, with emphasis on their importance to recruitment. J. Exp. Mar. Biol. Ecol. 221, 147–172.
Jenkins, G.P., Black, K.P., Wheatley, M.J., Hatton, D.N., 1997a. Temporal and spatial variability in recruitment of a temperate, seagrass-associated fish is largely determined by physical processes in the pre- and post-settlement phases. Mar. Ecol. Prog. Ser. 148, 23–35.
Jenkins, G.P., May, H.M.A., Wheatley, M.J., Holloway, M.G., 1997b. Comparison of fish assemblages associated with seagrass and adjacent unvegetated habitats of Port Phillip Bay and Corner Inlet, Victoria, Australia, with emphasis on commercial species. Estuar. Coast. Shelf Sci. 44, 569–588.
Jenkins, G.P., Welsford, D.C., Keough, M.J., Hamer, P.A., 1998. Diurnal and tidal vertical migration of pre-settlement King George whiting Sillaginodes punctata in relation to feeding and vertical distribution of prey in a temperate bay. Mar. Ecol. Prog. Ser. 170, 239–248.
Jordan, F., Bartolini, M., Nelson, C., Patterson, P.E., Soulen, H.L., 1996. Risk of predation affects habitat selection by the pinfish Lagodon rhomboides (Linnaeus). J. Exp. Mar. Biol. Ecol. 208, 45–56.
Kemp, W.M., 1989. Estuarine Seagrasses. In: Day, J.W., Hall, C.A.S., Kemp, W.M., Yanez-Arancibia, A. (Eds.), Estuarine Ecology. Wiley, New York, pp. 226–253.
Kennelly, S.J., 1991. Caging experiments to examine the effects of fishes on understorey species in a sublittoral kelp community. J. Exp. Mar. Biol. Ecol. 147, 207–230.
Kingsford, M.J., 1992. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 11, 193–198.
Kingsford, M.J., 1998. Reef fishes. In: Kingsford, M.J., Battershill, C. (Eds.), Studying Temperate Marine Environments: a Handbook for Ecologists. Canterbury University Press, Christchurch, pp. 132–165. Laprise, R., Blaber, S.J.M., 1992. Predation by Moses perch, Lutjanus russelli, and blue-spotted trevally,
Caranx bucculentus, on juvenile brown tiger prawn, Penaeus esculentus: effects of habitat structure and time of day. J. Fish Biol. 40, 627–635.
174 J.S. Hindell et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 153 –174
Levin, P., Petrik, R., Malone, J., 1997. Interactive effects of habitat selection, food supply and predation on recruitment of an estuarine fish. Oecologia 112, 55–63.
Lewis, F.G., 1984. Distribution of macrobenthic crustaceans associated Thalassia, Halodule and unvegetated sand substrata. Mar. Ecol. Prog. Ser. 19, 101–113.
Mattila, J., 1995. Does habitat complexity give refuge against fish predation? Some evidence from two field experiments. In: Eleftheriou, A., Ansell, A., Smith, C.J. (Eds.), Biology and Ecology of Shallow Coastal Waters. Olsen and Olsen, Fredensborg, pp. 261–268.
Minello, T.J., 1993. Chronographic tethering: a technique for measuring prey survival time and testing predation pressure in aquatic habitats. Mar. Ecol. Prog. Ser. 101, 99–104.
Orth, R.J., 1992. A perspective on plant–animal interactions in seagrasses: physical and biological deter-minants influencing plant and animal abundance. In: John, D.M., Hawkins, S.J., Price, J.H. (Eds.), Plant–Animal Interactions in the Marine Benthos. Association Special Volumes, Vol. 46. Clarendon Press, Oxford, pp. 147–164.
Orth, R.J., Heck, K.L., von Montfrans, J., 1984. Faunal communities in seagrass beds: A review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7 (4a), 339–350.
Pollard, D.A., 1984. A review of ecological studies on seagrass-fish communities, with particular reference to recent studies in Australia. Aquat. Bot. 18, 3–42.
Robertson, A.I., 1980. The structure and organisation of an eelgrass fish fauna. Oecologia 47, 76–82. Robertson, A.I., 1982. Population dynamics and feeding ecology of juvenile Australian salmon (Arripis trutta)
in Western Port, Victoria. Aust. J. Mar. Freshwater Res. 33, 369–375.
Rosenberg, M., Hodgkinson, R., Black, K., Colman, R., 1992. In: Hydrodynamics of Port Phillip Bay. Field collection and data reduction. Victorian Institute of Marine Sciences, Melbourne.
Sale, P.F., 1998. Appropriate spatial scales for studies of reef-fish ecology. Aust. J. Ecol. 23, 202–208. Sogard, S.M., Able, K.W., 1994. Diel variation in immigration of fishes and decapod crustaceans to artificial
seagrass habitat. Estuaries 17 (3), 622–630.
Steele, M.A., 1998. The relative importance of predation and competition in two reef fishes. Oecologia 115, 222–232.
Stoner, A.W., 1982. The influence of benthic macrophytes on the foraging behaviour of pinfish, Lagodon rhomboides (Linnaeus). J. Exp. Mar. Biol. Ecol. 58, 271–284.
Summerson, H.C., Peterson, C.H., 1984. Role of predation in organising benthic communities of a temperate-zone seagrass bed. Mar. Ecol. Prog. Ser. 15, 63–77.
Virnstein, R.W., 1978. Predator caging experiments in soft sediments: caution advised. In: Wiley, M.L. (Ed.), Estuarine Interactions. Academic Press, New York, pp. 261–273.
Virnstein, R.W., 1980. Measuring effects of predation on benthic communities in soft sediments. In: Kennedy, V.S. (Ed.), Estuarine Perspectives. Academic Press, New York, pp. 281–290.