Remnant-like particle (RLP) cholesterol is an independent
cardiovascular disease risk factor in women: results from the
Framingham Heart Study
Judith R. McNamara
a,b,c,*, Paulesh K. Shah
b, Katsuyuki Nakajima
d,
L. Adrienne Cupples
e, Peter W.F. Wilson
f, Jose M. Ordovas
c, Ernst J. Schaefer
b,caLipid Research Laboratory,New England Medical Center,750Washington Street,Box216,Boston,MA02111,USA
bLipid Research Laboratory,Di6ision of Endocrinology,Diabetes,Metabolism,and Molecular Medicine,New England Medical Center,Boston, MA,USA
cLipid Metabolism Laboratory,Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts Uni6ersity,Boston,MA,USA dOtsuka America Pharmaceutical Incorporated,Rock
6ille,MD,USA
eDepartment of Epidemiology and Biostatistics,Boston Uni
6ersity School of Public Health,Boston,MA,USA
fNational Heart,Lung,and Blood Institute’s Framingham Heart Study,National Institutes of Health,Framingham,MA,USA
Received 9 December 1999; received in revised form 16 March 2000; accepted 23 March 2000
Abstract
Remnants of triglyceride-rich lipoproteins (TRL) of both intestinal and liver origin are considered to be atherogenic, but separation of remnant lipoproteins from other TRL is difficult. An assay has been developed that allows immunoseparation of remnant-like particles (RLP) and measurement of cholesterol (RLP-C) and triglyceride (RLP-TG). We measured RLP-C and RLP-TG in fast plasma samples obtained from 1567 women participating in cycle 4 of the Framingham heart study (FHS). When values from 83 women with cardiovascular disease (CVD) were compared with the values from 1484 women without disease, concentrations in women with CVD were found to be significantly higher for both RLP-C (0.21590.102 vs. 0.18690.162 mmol/l;
+15.6%;PB0.0001) and RLP-TG (0.31990.352 vs. 0.25190.716 mmol/l; +27.0%;PB0.0002). Logistic regression analysis revealed that RLP-C was significantly associated with prevalent CVD in women (PB0.002) after adjustment with other major risk factors. In conclusion, we have documented that RLP-C is an independent risk factor for CVD in women, and provides significantly more information than do triglycerides. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Lipoproteins; Very low density lipoproteins; Chylomicrons; Triglycerides; Apolipoprotein B; Cardiovascular disease
www.elsevier.com/locate/atherosclerosis
1. Introduction
Elevated serum triglyceride concentrations are fre-quently observed in patients with cardiovascular disease (CVD), and are significantly associated with CVD in most univariate analyses. Associations frequently do not remain significant, however, after adjustment with other CVD risk factors [1,2]. Triglyceride concentration repre-sents a measure of triglyceride-rich lipoproteins (TRL), but it does not distinguish among the various subspecies of TRL, which may have varying degrees of
atherogenic-ity. Partially hydrolyzed lipoprotein remnants of both intestinal (chylomicron) and hepatic (very low density lipoproteins (VLDL)) origin have been implicated as the subspecies that may be particularly important in poten-tiating CVD risk [3 – 9]. Studies have shown that these particles can remain in circulation for extended periods of time postprandially, even in individuals with normal fasting triglyceride concentrations [10 – 14].
Routine isolation and measurement of lipoprotein remnants has not been feasible, because of the difficulty in distinguishing among particles with similar apolipo-protein composition and overlapping size ranges. Al-though direct methods for isolation and measurement of cholesterol in low density lipoproteins (LDL), high density lipoproteins (HDL), and even lipoprotein(a) * Corresponding author. Tel.: +1-617-5563104; fax: +
1-617-5563103.
E-mail address: mcnamara –[email protected] (J.R. McNamara).
[Lp(a)] are available [15 – 20], assays for remnant lipo-protein isolation and measurement have not previously been available. An assay that separates remnant-like particles (RLP) has recently been developed, however [21,22], utilizing immunoseparation to isolate partially hydrolyzed, apolipoprotein (apo) E-enriched, RLP from other TRL.
The relationship between CVD and triglycerides is much stronger in women than in men in most of the studies [23,24], and so in the current study we have tried to assess the atherogenic potential of RLP in women by isolation and measurement of the cholesterol (RLP-C) and triglyceride (RLP-TG) concentrations in these parti-cles in plasma obtained from the female offspring partic-ipants of the Framingham heart study (FHS), allowing comparisons between individuals who have been previ-ously diagnosed with CVD, and those with no evidence of disease.
2. Methodology
2.1. Study subjects
Subjects for the study were female participants in exam cycle 4 (1987 – 1990) of the FHS offspring study, under an ongoing, approved protocol. In 1972 offspring of the original FHS cohort and their spouses, totaling 5124 subjects, were recruited to the Framingham offspring/ spouse study. With examination cycles, occurring every 3 – 4 years, the number of participants at exam 4 reflects the typical number (4000) attending exams since the second examination cycle, with no evidence that those who attend during any given cycle are different from those who do not, with the possible exception of geo-graphic distribution. Women at exam 4 ranged in age from 22 to 79 years (mean age, 52 years).
Information concerning smoking status, height, weight, and systolic and diastolic blood pressure (SBP and DBP, respectively) was collected at the time of examination [25]. Women reporting cigarette use within the year prior to the exam were classified as current smokers; those who reported smoking at some time in the past, but not within the previous year, were classified as former smokers; the remaining were classified as never having smoked. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as SBP \140 or DBP\90 mmHg or intake of anti-hypertensive medica-tion. Diabetes was defined as fasting blood glucose
\6.85 mmol/l (125 mg/dl) or intake of hypoglycemic medication. Diagnosis of CVD included history of angina pectoris, myocardial infarction, stroke, or tran-sient ischemic attack using previously published methods [25]. All informations were reviewed by a panel of physicians to determine the diagnosis.
Of the 2043 women who attended exam 4, plasma aliquots and complete data were available for 1614. We compared with these 1614 women who had RLP mea-surements performed and those 429 who were not, and found that those who measured and reported upon here were 1 – 2 years older, 30% more likely to have hyperten-sion, 50% more likely to be on beta blockers, twice as likely to be treated with cholesterol-lowering drugs, and at 37% greater odds of having CVD. The women included in the study had slightly higher total cholesterol levels (5.37 vs. 5.25 mmol/l) and were more likely to be on hormone replacement therapy (12 vs. 8%). However, we found no other differences in lipid levels (LDL-C, HDL-C, or triglyceride), and no differences in the rates of smoking or diabetes. Thus, subjects measured for RLP in this study were at somewhat greater atherogenic risk than those who were not.
Statistical analyses were performed with and without the inclusion of subjects taking medications known to affect lipids (47 women), resulting in final study sets 1567 or 1614 women. Results obtained from women with documented CVD (83/92) were compared with those from women without disease (1484/1522).
2.2. Lipoprotein analyses
Blood was drawn from each subject after a 12 h, overnight fast into tubes containing EDTA (final concen-tration, 0.15%). Plasma was separated by centrifugation (2500 rpm, 4°C, 20 min). Plasma lipid and lipoprotein concentrations (total cholesterol, triglycerides, and HDL-C) were measured fresh, using standard enzymatic methods, essentially as previously described [26]. LDL-C was calculated according to the formula of Friedewald et al. [27], except when triglycerides were \4.5 mmol/l (400 mg/dl). In those cases, which included 19 of the 1567 women (1.3%), LDL-C values were omitted from the data set.
2.3. RLP analyses
and fewer triglyceride and apo C molecules than those particles bound by the antibody. These unbound parti-cles represent approximately 10 – 13% of TRL in nor-molipidemic samples, but as much as 65% of TRL in familial dysbetalipoproteinemic (type III) samples [21]. More recently, in in vitro studies by Doi et al., the unbound RLP were shown to inhibit vasorelaxation, while bound (non-RLP) TRL produced no inhibition [32].
RLP-C and RLP-TG concentrations were measured in the FHS plasma aliquots that had been frozen at −80°C until the time of analysis. Plasma was incu-bated with the gel for 2 h, after which the gel, contain-ing the bound (non-RLP) lipoproteins, was precipitated with low-speed centrifugation (5 min, 135×g). RLP-C and RLP-TG were then measured in unbound super-nates on an Abbott Spectrum chemistry analyzer (Ab-bott Diagnostics, Irving, TX), using two-reagent enzymatic, colorimetric assays containing a sensitive chromophore (Kyowa Medex, Tokyo, Japan). Precision studies have yielded among-run RLP-C imprecision for two levels of RLP control over 20 runs of 9.1% at 7 mg/dl (0.18 mmol/l) and 7.3% at 24 mg/dl (0.62 mmol/ l). Among-run RLP-TG imprecision for the same con-trols was 8.3% at 22 mg/dl (0.25 mmol/l), and 5.0% at 109 mg/dl (1.23 mmol/l) [25].
2.4. Statistical analysis
Those subjects with prevalent CVD were compared with those with no evidence of CVD on a variety of known risk factors, as well as with C and RLP-TG. Student’s t-test was used to compare with the mean values of continuous measures, and a x2 statistic was calculated for categorical factors. For continuous measures, which were highly skewed, we compared those with CVD and those without, using log trans-formed values, although we report the untranstrans-formed means and standard deviations for each group. To
further evaluate the relationship between CVD and RLP-C and RLP-TG, we established quartiles for each measure and computed age-adjusted rates for each quartile, using direct standardization and 10-year age groups (20 – 29, 30 – 39, etc.). The age distribution pro-vided the standard distribution for these comparisons, and the Mantel extension test was employed to calcu-late a test of linear trend in these rates across quartiles. To adjust for known risk factors for CVD, we used logistic regression analysis with the presence or absence of prevalent CVD as the outcome. The covariates that we considered were age, definite hypertension (SBP
\140 or DBP \90 mmHg or on hypertensive treat-ment), use of beta blockers, smoking status (current or former or never), prevalent diabetes, LDL-C, HDL-C, and hormone replacement therapy.
To evaluate the relationship between CVD and RLP, we performed several analyses with variable definitions of the remnant variables, (1) using the actual measured RLP-C or RLP-TG concentration as a continuous vari-able on a log scale; (2) a set of three measures indicat-ing quartiles 2, 3 and 4; and (3) usindicat-ing only the quartile 4 as a dichotomous measure. These various definitions were used to evaluate whether the relationship between CVD and the lipid measures was linear on a log odd scale, as using the actual measurement assumes. In some analyses, National Cholesterol Education Pro-gram (NCEP) cutpoint measures were also used for LDL-C, HDL-C, and triglyceride, in order to compare with the effects of elevated remnant lipoproteins to that of other elevated lipids as stipulated by NCEP guideli-nes. Evaluation of triglycerides, using the quartile 4 as a dichotomous measure, was also used.
3. Results
Comparisons were performed between the 1484 FHS offspring women with no evidence of CVD and the 83
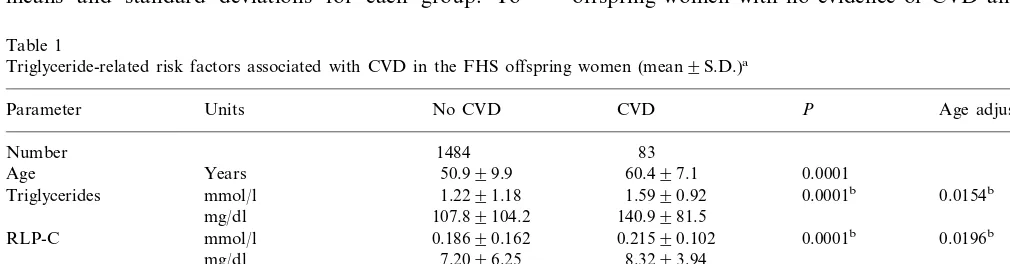
Table 1
Triglyceride-related risk factors associated with CVD in the FHS offspring women (mean9S.D.)a
Age adjustedP P
No CVD
Parameter Units CVD
Number 1484 83
0.0001
Age Years 50.999.9 60.497.1
1.5990.92 0.0001b
Triglycerides mmol/l 1.2291.18 0.0154b
140.9981.5 107.89104.2
mg/dl
0.21590.102 0.0001b
RLP-C mmol/l 0.18690.162 0.0196b
8.3293.94 mg/dl 7.2096.25
0.0002b
0.31990.352 0.0174b
0.25190.716
RLP-TG mmol/l
28.2931.1 mg/dl 22.2963.3
0.001 51.8%
23.5% RLP-C \75th percentile
24.0% 43.2% 0.001
RLP-TG \75th percentile
aBMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; RLP-C, remnant-like particle cholesterol; RLP-TG,
remnant-like particle triglyceride.
women diagnosed with prevalent CVD. Prior to per-forming statistical analyses, standard log transformation was imposed on the non-linear variables, C, RLP-TG, and triglyceride (Table 1). However, while triglyce-ride was made linear by the transformation, RLP-C and RLP-TG remained non-linear, indicating a threshold effect. In addition, the relationship between RLP and age-adjusted rates for CVD was also non-linear (Table 2). It was therefore decided that statistical analyses would be performed using dichotomous cut-points for all lipid variables. Since cutpoints based on the approx-imate, 75th percentile of the population are used as part of the NCEP Adult Treatment Panel recommendations, and are also generally used by physicians to determine the need for treatment; it appears to be a reasonable approach for those measures, and avoids the problems of non-linearity associated with RLP measures. There-fore, NCEP cutpoints were used for LDL-C []4.1 mmol/l (160 mg/dl)], HDL-C [B0.90 mmol/l (35 mg/ dl)], and TG [\2.25 mmol/l (200 mg/dl)]; 75th percen-tile cutpoints were used to discriminate RLP-C and RLP-TG. Since the NCEP cutpoint for triglycerides is higher than the 75th percentile for the FHS women, the 75th percentile for triglycerides was also used in a separate analysis. Comparisons to either exclude or include individuals on lipid-lowering medication, with adjustment for medication effects, were virtually
identi-cal, and therefore only results from comparisons where those individuals were excluded are provided.
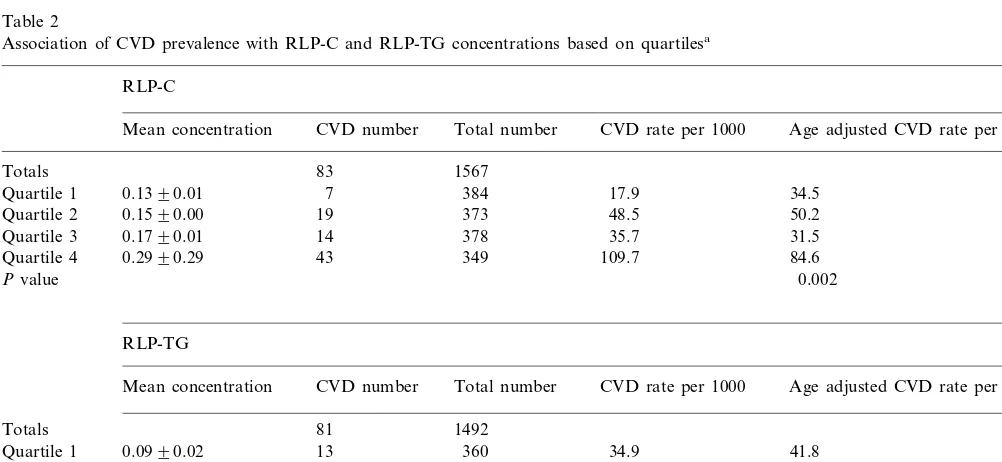
Comparisons to RLP-C, RLP-TG, and triglyceride concentrations for FHS offspring women with and without diagnosed CVD are shown in Table 1. All three measures were significantly higher in women with CVD than in those without disease. The percentage of women with CVD who had RLP-C and RLP-TG concentrations above the 75th percentile of the population [28] was also significantly higher. Other parameters that were signifi-cantly higher (PB0.01) in women with prevalent CVD were age, BMI, SPB, glucose, total and LDL-C, and triglyceride, while HDL-C was significantly lower; mea-sures other than RLP-C and RLP-TG have been previ-ously reported [33,34]. After age-adjustment, significant differences (PB0.05) remained for RLP-C, RLP-TG, BMI, SBP, triglycerides, and HDL-C. Histories of beta blocker use, hypertension, and diabetes also occurred significantly more frequently in those with prevalent CVD than in those without diagnosed disease (PB0.01), but a history of cigarette smoking was not significant. Prevalence rates for CVD increased dramatically in the upper quartile of remnant lipoprotein levels among women (Table 2), with an age-adjusted estimate of 84.6 per 1000 women in the upper quartile of RLP-C com-pared with the estimates of 31.5 – 50.2 per 1000 for the lower three quartiles, and 77.3 per 1000 for RLP-TG versus 38.3 – 47.2 per 1000. These results suggest that
Table 2
Association of CVD prevalence with RLP-C and RLP-TG concentrations based on quartilesa
RLP-C
Total number CVD number
Mean concentration CVD rate per 1000 Age adjusted CVD rate per 1000
83 1567
Totals
0.1390.01
Quartile 1 7 384 17.9 34.5
0.1590.00
Quartile 2 19 373 48.5 50.2
31.5 35.7
378 14
0.1790.01 Quartile 3
43 349 109.7 84.6
Quartile 4 0.2990.29
0.002 Pvalue
RLP-TG
Mean concentration CVD number Total number CVD rate per 1000 Age adjusted CVD rate per 1000
81
Totals 1492
13 360
Quartile 1 0.0990.02 34.9 41.8
0.1490.01 14
Quartile 2 359 37.5 38.3
50.9 354
19 0.1990.02
Quartile 3 47.2
0.5991.35 35
Quartile 4 338 93.8 77.3
Pvalue 0.021
aRLP-C, remnant-like particle cholesterol; RLP-TG, remnant-like particle triglyceride; mean concentration, mean plasma concentration
Table 3
Univariate correlations of RLP-C and RLP-TG with CVD risk factorsa
aRLP-C, remnant-like particle cholesterol; RLP-TG, remnant-like
particle triglyceride; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
bMeasures are not normally distributed and were log-transformed
for statistical analysis.
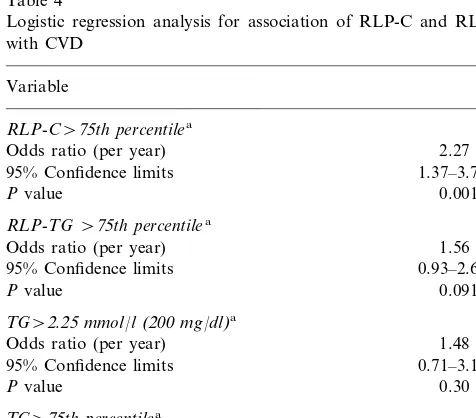
Results for logistic regression analyses are presented in Table 4. Since the age-adjusted prevalence rates indicate a non-linear relationship between RLP and CVD, we report analyses for the dichotomous measure representing the upper gender-specific quartile. The re-sults indicated that RLP-C levels in the upper quartile were significantly associated with prevalent CVD (P= 0.0015), with an odds ratio (OR) of 2.3 (95% confidence limits, 1.4 – 3.8), after adjustment for age, hypertension, smoking, diabetes, LDL-C, HDL-C, b blockers, and replacement hormones. In comparable models after ad-justment for the same covariates, the association for RLP-TG with CVD was weaker (OR=1.6, 95% confi-dence limits; 0.9 – 2.6, P=0.09), while the association for total triglycerides with CVD was not statistically significant, either as the NCEP cutpoint, or as the 75th percentile. We did not evaluate the relationship of RLP-C, RLP-TG, and total triglycerides in the same model because these variables are highly correlated.
There were 839 postmenopausal women in the study, representing 56.5% of the total number of women. Within the postmenopausal group, there were 67 with established CVD. Therefore, 8.0% of postmenopausal women had CVD, but 80.7% of the 83 women with CVD were postmenopausal. Results of logistic regres-sion analysis for postmenopausal women were similar to those for the entire female cohort (RLP-C \75th percentile,P=0.0024; RLP-TG \75th percentile,P= 0.12).
Age and use of beta blockers were the two most significant variables associated with CVD; current smoking was also significant. Introduction of beta blockers into the model also accounted for a significant decrease in magnitude of the association of RLP-C with prevalent CVD, a phenomenon we have previously observed in comparisons to other potential risk factors, i.e. b blockers alter lipoprotein composition [35,36]. Since b blockers are commonly prescribed for CVD patients, they represent an important confounder, fre-quently overlooked in statistical evaluations of CVD associations. However, after adjustment for age and beta blocker use, as well as other known risk factors, RLP-C was still highly significant.
4. Discussion
Cardiovascular disease remains the leading cause of death and disability in USA [37]. Because many pa-tients die suddenly of CVD, outside the hospital, the identification of individuals at high risk continues to be important for prevention. Major CVD risk factors identified by the NCEP Adult Treatment Panel include age, gender, hypertension, cigarette smoking, diabetes mellitus, family history of premature CVD, increased LDL-C, and decreased HDL-C [37].
Table 4
Logistic regression analysis for association of RLP-C and RLP-TG with CVD
Variable
RLP-C\75th percentilea
Odds ratio (per year) 2.27
95% Confidence limits 1.37–3.77
0.0015 Pvalue
RLP-TG\75th percentilea
Odds ratio (per year) 1.56
95% Confidence limits 0.93–2.60
Pvalue 0.091
TG\2.25mmol/l(200mg/dl)a
Odds ratio (per year) 1.48
95% Confidence limits 0.71–3.10
Pvalue 0.30
TG\75th percentilea
Odds ratio (per year) 1.37
95% Confidence limits 0.82–2.31
Pvalue 0.23
aAfter adjustment for age, hypertension, diabetes, smoking, beta
blocker use, LDL-C, and HDL-C.
the primary risk of remnant lipoproteins lie in the upper quartile.
Potential additional lipoprotein risk factors that have been proposed include increased Lp(a) [36,38 – 40], the presence of small dense LDL [41 – 45], triglycerides [23,24,44 – 51], and RLP-C and RLP-TG concentrations [50,52 – 54]. Difficulties with total triglyceride measure-ment as a risk factor include biologic variability and differences in triglyceride content among TRL subspe-cies. These sources of variability may account for the lack of consistent finding for triglycerides as an inde-pendent CVD risk factor [23,24,45 – 51]. Significant as-sociations between triglycerides and CVD have been observed more frequently in women, however, than in men [23,24,47]. LDL particle size also has been not shown clearly to be an independent risk factor [42 – 45]. TRL remnants have been proposed as a CVD risk factor for many years [3], but they have been difficult to isolate due to their size heterogeneity, which overlaps with other TRL, and the fact that they contain the same lipid and apolipoprotein components. Methods of separation have previously been limited to ultracen-trifugation and chromatography. Separation methods based on size and density have necessarily resulted in a heterogeneous population of particles in most cases. In addition, the procedures required to isolate remnants by these methods generally precluded large studies.
Previous work by us and by others using the im-munoseparation method have shown associations be-tween RLP concentrations and CVD risk factors [50,52 – 55]. Preliminary comparisons to a combined group of CVD patients from FHS and New England Medical Center indicated that RLP-C and RLP-TG were significantly higher in male and female CVD patients than in FHS controls [50]. Studies by Takeichi et al. have shown significantly higher levels of RLP-C and RLP-TG in victims of sudden cardiac death than in individuals who died from sudden non-cardiac causes of death [52,54]. A small prospective study in patients with coronary artery disease has also shown that pa-tients in the highest tertile for RLP-C had the greatest number of secondary events over a 3-year period [55]. In addition, studies involving individuals who are at particular risk for CVD, namely those with diabetes mellitus and those with type III hyperlipoproteinemia, have shown similar increases in RLP-C and RLP-TG concentrations [53,56 – 58].
The current study included approximately 1500 well-characterized FHS female participants. All CVD diag-noses were carefully adjudicated, and all subjects were free-living at the time of exam, eliminating potential confounders associated with hospitalization [59,60]. Since this was a prevalence study, the likelihood exists that the most severely affected had been placed on drug therapy, while others had made significant changes to modify their risk factors. Those individuals with LDL-C concentrations above, or HDL-LDL-C concentrations be-low, the NCEP cut points, and not on therapy, would
have been relatively few, thereby minimizing lipo-protein differences between individuals with and with-out prevalent CVD. The fact that RLP-C was still significant in women, however, even under these cir-cumstances, indicates a potentially very important ana-lyte for assessing risk.
From the results of the study, it appears that mea-surement of RLP-C may be of particular benefit to women, as part of a risk assessment panel. Risk factors in women have previously been shown to differ some-what from those in men [23,61,62]. Diabetes mellitus, for example, is associated with a higher relative risk of CVD in women than in men; diabetes is also associated with increased triglycerides; and triglycerides have been shown to be a stronger CVD risk factor in women, than in men [23,61,62]. Postmenopausal status is also associ-ated with increased triglycerides and with increased risk of CVD. In the current FHS prevalence analysis of exam 4, postmenopausal women represented 56.5% of women, but represented 80.7% of women with CVD. And while triglyceride \2.25 mmol/l (200 mg/dl) or
\75th percentile was not independently significant when placed in the model, RLP-C was highly signifi-cant in women as a whole and also in the post-menopausal subgroup. And while we cannot exclude colinearity in these results among RLP and triglyce-rides, we have observed that the OR for RLP-C in women was greater than for either RLP-TG or total triglyceride. This suggests that RLP-C had a more directed association with CVD than do the other mea-sures, neither of which is statistically significant.
In conclusion, we have documented that RLP-C is an independent risk factor for CVD in women, and pro-vides significantly more information than do triglycerides.
Acknowledgements
Supported by contracts from Otsuka America Phar-maceutical Incorporated, Rockville, MD, and the Na-tional Heart, Lung, and Blood Institute’s Framingham Heart Study, National Institutes of Health, Bethesda, MD (NIH/NHLBI Contract N01-HC-38038). Pre-sented in part at the 69th Scientific Sessions of the American Heart Association, New Orleans, LA, November 11, 1996.
References
[1] Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoproteins as a protective factor against coronary heart disease. Am J Med 1977;62:707 – 14.
[3] Zilversmit DB. A proposal linking atherogenesis to the interac-tion of endothelial lipoprotein lipase with triglyceride-rich lipo-proteins. Circ Res 1973;33:633 – 8.
[4] Tatami R, Mabuchi H, Ueda K, et al. Intermediate-density lipoprotein and cholesterol-rich very low density lipoprotein in angiographically determined coronary artery disease. Circulation 1981;64:1174 – 84.
[5] Havel RJ. The role of the liver in atherosclerosis. Arteriosclerosis 1985;5:569 – 80.
[6] Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation 1993;88:2762 – 70.
[7] Zilversmit DB. Atherogenic nature of triglycerides, postprandial lipemia, and triglyceride-rich remnant lipoproteins. Clin Chem 1995;41:153 – 8.
[8] Davignon J, Cohn JS. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis 1996;124(Suppl.):S57 – 64. [9] Havel RJ. Triglyceride-rich lipoproteins. In: Rifai N, Warnick
GR, Dominiczak MH, editors. Handbook of Lipoprotein Test-ing, third ed. Washington, DC: AACC Press, 1997:115 – 26. [10] Weintraub MS, Eisenberg S, Breslow JL. Different patterns of
postprandial lipoprotein metabolism in normal, type IIa, type III, and type IV hyperlipoproteinemic individuals: effects of treatment with cholestyramine and gemfibrozil. J Clin Invest 1987;79:1110 – 9.
[11] Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res 1988;29:469 – 79.
[12] Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Plasma apolipoprotein changes in the triglyceride-rich lipo-protein fraction of human subjects fed a fat-rich meal. J Lipid Res 1988;29:925 – 36.
[13] Cohn JS, McNamara JR, Krasinski SD, Russell RM, Schaefer EJ. Role of triglyceride-rich lipoproteins from the liver and intestine in the etiology of postprandial peaks in plasma triglyce-ride concentration. Metabolism 1989;38:484 – 90.
[14] Cohn JS, Johnson EJ, Millar JS, et al. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postpran-dial increases in the plasma concentration of TRL triglycerides and retinyl esters. J Lipid Res 1993;34:2033 – 40.
[15] McNamara JR, Cole TG, Contois JH, Ferguson CA, Ordovas JM, Schaefer EJ. Immunoseparation method for measuring low-density lipoprotein cholesterol directly from serum evaluated. Clin Chem 1995;41:232 – 40.
[16] Jialal I, Hirany SV, Devaraj S, Sherwood TA. Comparison of an immunoseparation method for direct measurement of LDL cholesterol withb-quantification (ultracentrifugation). Am J Clin Pathol 1995;104:76 – 81.
[17] Pisani T, Gebski CP, Leary ET, Warnick GR, Ollington JF. Accurate direct determination of low-density lipoprotein terol using an immunoseparation reagent and enzymatic choles-terol assay. Arch Pathol Lab Med 1995;119:1127 – 35.
[18] Burstein M, Samaille J. Sur un dosage rapide du cholesterol lie aux a- et aux B-lipoproteins du serum. Clin Chim Acta 1960;5:609 – 11.
[19] Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+
precipitation procedure for quantitation of high-density-lipo-protein cholesterol. Clin Chem 1982;28:1379 – 88.
[20] Seman LJ, Jenner JL, McNamara JR, Schaefer EJ. Quantitation of plasma lipoprotein(a) by cholesterol assay of lectin-bound lipoprotein(a). Clin Chem 1994;40:400 – 3.
[21] Campos E, Nakajima K, Tanaka A, Havel RJ. Properties of an apolipoprotein E-enriched fraction of triglyceride-rich lipo-proteins isolated from human blood plasma with a monoclonal antibody to apolipoprotein B-100. J Lipid Res 1992;33:369 – 80. [22] Nakajima K, Saito T, Tamura A, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo
B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta 1993;223:53 – 71.
[23] Castelli WP. The triglyceride issue: a view from Framingham. Am Heart J 1986;112:432 – 7.
[24] Hokanson JE, Austin MA. Plasma triglyceride level as a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213 – 9.
[25] Wilson PWF, Christianson JC, Anderson K, Kannel WB. Im-pact of national guidelines for cholesterol risk factor screening: the Framingham offspring study. J Am Med Assoc 1989;262:41 – 4.
[26] McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta 1987;166:1 – 8.
[27] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499 – 502.
[28] McNamara JR, Shah PK, Nakajima K, Cupples LA, Wilson PWF, Ordovas JM, Schaefer EJ. Remnant lipoprotein choles-terol and triglyceride: reference ranges from the Framingham Heart Study. Clin Chem 1998;44:1224 – 32.
[29] Leary ET, Wang T, Baker DJ, et al. Evaluation of an im-munoseparation method for quantitative measurement of rem-nant-like particle-cholesterol in serum and plasma. Clin Chem 1998;44:2490 – 8.
[30] Mjos OD, Faergeman O, Hamilton RL, Havel RJ. Characteriza-tion of remnants produced during the metabolism of triglyceride-rich lipoproteins of blood plasma and intestinal lymph in the rat. J Clin Invest 1975;56:603 – 15.
[31] Pagnan A, Havel RJ, Kane JP, Kotite L. Characterization of human very low density lipoproteins containing two elec-trophoretic populations: double prebeta lipoproteinemia and primary disbetalipoproteinemia. J Lipid Res 1977;18:613 – 22. [32] Doi H, Kugiyama K, Ohgushi M, et al. Membrane active lipids
in remnant lipoproteins cause impairment of endothelial-depen-dent vasorelaxation. Arterioscler Thromb Vasc Biol 1999;19:1918 – 24.
[33] Contois JH, McNamara JR, Lammi-Keefe CJ, Wilson PWF, Schaefer EJ. Reference intervals for plasma apolipoprotein A-I as determined with a commercially available immunoturbidomet-ric assay: results from the Framingham offspring study. Clin Chem 1996;42:507 – 14.
[34] Contois JH, McNamara JR, Lammi-Keefe CJ, Wilson PWF, Schaefer EJ. Reference intervals for plasma apolipoprotein B as determined with a commercially available immunoturbidometric assay: results from the Framingham offspring study. Clin Chem 1996;42:515 – 23.
[35] Wolinsky H. The effects of beta-adrenergic blocking agents on blood lipid levels. Clin Cardiol 1987;10:561 – 6.
[36] Genest JJ, Jr, McNamara JR, Ordovas JM, et al. Lipoprotein cholesterol, apolipoprotein A-I and B and lipoprotein(a) abnor-malities in men with premature coronary artery disease. J Am Coll Cardiol 1992;19:792 – 802.
[37] Expert panel. National Cholesterol Education Program. Second report of the expert panel on detection, evaluation, and treat-ment of high blood cholesterol in adults. (Adult Treattreat-ment Panel II). Circulation 1994;89:1333 – 445.
[38] Schaefer EJ, Lamon-Fava S, Jenner JL, et al. Lipoprotein(a) levels and risk of coronary heart disease in men. The Lipid Research Clinics Coronary Primary Prevention Trial. JAMA 1994;271:999 – 1003.
[40] Nguyen TT, Ellefson RD, Hodge DO, Bailey KR, Kottke TE, Abu-Lebdeh HS. Predictive value of electrophoretically detected lipoprotein(a) for coronary heart disease and cerebrovascular disease in a community-based cohort of 9936 men and women. Circulation 1997;96:1390 – 7.
[41] McNamara JR, Campos H, Ordovas JM, Peterson J, Wilson PWF, Schaefer EJ. Effect of gender, age, and lipid status on low density lipoprotein subfraction distribution: results of the Fram-ingham offspring study. Arteriosclerosis 1987;7:483 – 90. [42] Campos H, Genest JJ, Jr, Blijlevens E, et al. Low density
lipoprotein particle size and coronary artery disease. Arterioscler Thromb 1992;12:187 – 95.
[43] Coresh J, Kwiterovich PO, Jr. Small, dense low-density lipo-protein particles and coronary heart disease risk. A clear associ-ation with uncertain implicassoci-ations. JAMA 1996;276:914 – 5 Editorial.
[44] Chapman MJ, Bruckert E. The atherogenic role of triglycerides and small, dense low density lipoproteins: impact of ciprofibrate therapy. Atherosclerosis 1996;124(Suppl.):S21 – 8.
[45] Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA 1996;276:882 – 8.
[46] Criqui MH, Heiss G, Cohn R, et al. Plasma triglyceride level and mortality from coronary heart disease. New Engl J Med 1993;328:1220 – 5.
[47] Wilson P, Larson G, Castelli W. Triglycerides, HDL-cholesterol and coronary disease: a Framingham update on the interactions. Can J Cardiol 1994;10(Suppl. B):5B – 9B.
[48] Burchfiel CM, Laws A, Benfante R, et al. Combined effects of HDL cholesterol, triglyceride, and total cholesterol concentra-tions on the 18-year risk of atherosclerotic disease. Circulation 1995;92:1430 – 6.
[49] Lamarche B, Despres J-P, Moorjani S, Cantin B, Dagenais GR, Lupien P-J. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Quebec cardiovascu-lar study. Atherosclerosis 1996;119:235 – 45.
[50] McNamara JR, Shah PK, Nelson SM, Nakajima K, Wilson PWF, Schaefer EJ. Lipoprotein remnant cholesterol and
triglyce-ride values in coronary cases and Framingham controls. Circula-tion 1996;94:1 – 94.
[51] Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an 8-year follow-up in the Copenhagen Male Study. Circulation 1998;97:1029 – 36. [52] Takeichi S, Nakajima Y, Osawa M, et al. The possible role of
remnant-like particles as a risk factor for sudden cardiac death. Int J Legal Med 1997;110:213 – 9.
[53] Devaraj S, Vega G, Lange R, Grundy SM, Jialal I. Remnant-like particle cholesterol levels in patients with dysbetalipoproteinemia or coronary artery disease. Am J Med 1998;104:445 – 50. [54] Takeichi S, Yukawa N, Nakajima Y, et al. Association of
plasma triglyceride-rich lipoprotein remnants with coronary atherosclerosis in cases of sudden cardiac death. Atherosclerosis 1999;142:309 – 15.
[55] Kugiyama K, Doi H, Takazoe K, et al. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation 1999;99:2858 – 60.
[56] Shimizu H, Mori M, Saito T. An increase of serum remnant-like particles in non-insulin-dependent diabetic patients with microal-buminuria. Clin Chim Acta 1993;221:191 – 6.
[57] Nakajima K, Saito T, Tamura A, et al. A new approach for the detection of type III hyperlipoproteinemia by RLP-cholesterol assay. J Atheroscler Thromb 1994;1:30 – 6.
[58] Marcoux C, Tremblay M, Nakajima K, Davignon J, Cohn JS. Characterization of remnant-like particles isolated by im-munoaffinity gel from the plasma of type III and type IV hyperlipoproteinemic patients. J Lipid Res 1999;40:636 – 47. [59] Genest JJ, Corbett HM, McNamara JR, Schaefer MM, Salem
DN, Schaefer EJ. Effect of hospitalization on high-density lipo-protein cholesterol in patients undergoing elective coronary an-giography. Am J Cardiol 1988;61:998 – 1000.
[60] Genest JJ, McNamara JR, Ordovas JM, et al. Effect of elective hospitalization on plasma lipoprotein cholesterol and apolipo-proteins A-I, B and Lp(a). Am J Cardiol 1990;65:677 – 9. [61] Kannel WB, Wilson PWF. Risk factors that attenuate the female
coronary disease advantage. Arch Intern Med 1995;155:57 – 61. [62] Kuller LH, Meilahn EN. Risk factors for cardiovascular disease
among women. Curr Opin Lipidology 1996;7:203 – 8.