A DNA-binding activity for the promoter of the gene encoding C
4phosphoenolpyruvate carboxylase is modulated by phosphorylation
during greening of the
Sorghum
leaf
Szecherezada Katarzyna Rydz
a, Jose Luis Prieto
a, Anna Maria Rychter
b,
Jean Vidal
a,*
aInstitut de Biotechnologie des Plantes,UMR CNRS8618,Uni6ersite´ de Paris-Sud,baˆtiment630,91405Orsay Cedex,France bInstitute of Experimental Plant Biology,Uni6ersity of Warsaw,Pawinskiego5a,02-106Warsaw,Poland
Received 11 February 2000; received in revised form 26 April 2000; accepted 26 June 2000
Abstract
Electrophoresis mobility shift assay (EMSA) identified nuclear proteins with binding activity to a 430 bp promoter fragment of theSorghumC4phosphoenolpyruvate carboxylase gene (S6C4). The DNA binding activities (two main retarded bands; PC1 and PC2) were high in nuclear extracts from etiolated leaves, decreased during greening and became very low or null in nuclear extracts from green leaves. This process was found to be mediated by phytochrome and was apparently irreversible since the DNA-binding activities were not restored in green plants kept in continuous darkness. The AT-rich region of the promoter fragment was identified to be the interaction domain of PC2. The detection of PC2 with EMSA was markedly reduced by preincubation of nuclear protein extracts with Mg-ATP or Mg-GTP and restored in the presence of a general protein serine/threonine-kinase inhibitor, K252a. The results suggested that the PC2 binding activity was modulated by phosphorylation during the greening process of theSorghumleaf. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:C4phosphoenolpyruvate carboxylase; Gene promoter; Binding activities; Phosphorylation;Sorghum6ulgare
www.elsevier.com/locate/plantsci
1. Introduction
Phosphoenolpyruvate carboxylase (PEPC, EC 4.1.1.31) is an essential enzyme of photosynthetic CO2fixation in C4plants [1,2]. InSorghum, PEPC
is nuclear encoded by a small multigenic family [3]. We have previously reported the cloning and sequencing of three PEPC genes, S6C3, S6C3RI
and S6C4 from a Sorghum genomic library, in-cluding their 5% flanking sequences and 3%
untrans-lated regions. S6C4 is highly and specifically expressed in a phytochrome-mediated light depen-dent and tissue specific (mesophyll cells) manner [4 – 6] during leaf greening. This was correlated with the accumulation of specific mRNA and C4
PEPC in the mesophyll-cell cytoplasm [7]. More-over, it has been shown that the light induction of C4 PEPC gene expression during the greening
process of etiolated maize leaves relies on light-de-pendent developmental changes [8].
Various cis-elements and the corresponding
trans-acting factors of light regulated
photosyn-thetic gene promoters have been identified [9]. Leaf-specific protein factors, MNF1, MNF2a, MNF2b and PEP1 have been shown to interact with the maize C4 PEPC gene promoter, among
which MNF1 and PEP1 were presumed to act as positive transcriptional effectors [10,11] and MNF2a as a negative transcriptional effector [12,13]. Subsequently, two proteins, MNB1a and MNB1b, which bind the MNF1 box in the maize C4 PEPC gene promoter have been cloned and
sequenced [12 – 15]. MNB1a markedly enhances this promoter activity in vivo [9].
* Corresponding author. Tel.: +33-1-69336344; fax: + 33-1-69336423.
E-mail address:[email protected] (J. Vidal).
From the data summarized above, it is evident that no clear picture has emerged till now concern-ing the regulatory mechanisms that control tran-scription of the C4PEPC genes. However, there is
growing evidence that light-dependent genes are regulated via transduction cascades and protein phosphorylation events [16 – 20]. In this report, we describe the identification in EMSA and in vitro phosphorylation in nuclear extracts of a protein factor, PC2, which binds to an AT-rich domain of
S6C4 promoter.
2. Materials and methods
2.1. Plant material
Sorghum plants (S. 6ulgare Pers. cv. Tamaran)
were grown in vermiculite watered with Hoagland’s nutrient solution under 16 h of white light (700mE m−2 s−1) and an 8 h dark cycle for 11 days, or maintained for the same period in complete darkness. For red/far-red light experi-ments, etiolated 8-day-old plants were irradiated by red or far-red light pulses using white lamps (Philips TL15) wrapped in a red rhodoid filter (lmax, 660 nm) or a Kodak filter (Wratten 88A; lmax, 730 nm), respectively. The light intensity was 2 mE m−2 s−1 at the leaf level. The plants were treated with red and far-red light in 3 modes; 10 min of red light every 3 h for 3 days; 20 min of far-red light every 3 h for 3 days; 10 min of red light followed by 20 min of far-red light every 3 h for 3 days.
2.2. Preparation of nuclei
This was performed according to the procedure described in [21]. Experiments were carried out at
4°C. Sorghum leaves (100 g) were powdered in
liquid nitrogen with a mortar and pestle and ho-mogenized in 100 ml of extraction buffer contain-ing 20 mM Tris – HCl pH 7.8, 250 mM sucrose, 5 mM MgCl2, 5 mM KCl, 40% (v/v) glycerol, 0.25%
(v/v) Triton X-100 and 5 mM 2-mercaptoethanol. The brei was filtered through a 30mm nylon filter, and centrifuged at 1000×g for 10 min in a swing-ing bucket rotor. The pellet was resuspended in 45 ml of extraction buffer without Triton X-100 and recentrifuged. The nuclei-enriched pellet was used directly for the extraction of nuclear proteins.
2.3. Preparation of nuclear protein extracts
Extraction of nuclear proteins was performed by a modification of the method described in [16]. All the steps were carried out at 4°C. The nuclear pellet (108 of nuclei) was resuspended in 1 ml of
lysis buffer containing 50 mM HEPES – NaOH pH 8.0, 1 mM EDTA, 0.3% (v/v) Triton X-100, 1 mM ascorbic acid, 10 mM DTT, 1 mM PMSF, 1 mM benzamidine and 1 mM leupeptin. Incubation was carried out on ice for 20 min, and then the lysate was ultracentrifuged at 170 000×g for 15 min. The chromatin-containing pellet was resuspended in 0.25 ml of high salt buffer containing 50 mM HEPES – NaOH pH 8.0, 400 mM NaCl, 5 mM MgCl2, 1 mM ascorbic acid, 1 mM PMSF, 1 mM
benzamidine, 1 mM leupeptin and 10 mM DTT. The extraction of nuclear proteins was performed on ice for 1 h, and then the insoluble material was eliminated by ultracentrifugation (170 000×g, 15 min). The nuclear protein extracts were aliquoted, frozen in liquid nitrogen, and stored at −80°C. Protein concentration was determined according to [22].
For red/far-red light experiments, extraction of chromatine-bound proteins was performed at 4°C as per the method described by [23]. Leaf tissue (100 g) were homogenized at maximal speed of a Polytron homogenizer for 4×20 s in 100 ml of extraction buffer (15 mM HEPES – NaOH pH 8.0, 110 mM KCl, 5 mM MgCl2, 1 mM ascorbic acid,
10 mM DTT, 1 mM PMSF, 1 mM benzamidine, 1 mM leupeptin). The homogenate was filtered through one layer of Miracloth and then 0.1 vol of 4 M (NH4)2SO4 was added to the filtrate. After
incubation for 30 min at 4°C, the chromatin frac-tion was pelleted by ultracentrifugation at 170 000×g, 15 min. Proteins were salted out of the supernatant by the addition of 0.3 g ml−1
(NH4)2SO4 and incubated for 30 min at 4°C.
2.4. Determination of C4 PEPC acti6ity in protein
extracts from Sorghum lea6es
Leaf tissue (200 mg) was harvested and immedi-ately extracted in 500 ml of extraction medium (100 mM Tris – HCl pH 8, 10 mM MgCl2, 1 mM
EDTA, 10% (v/v) glycerol, 10 mM DTT, 2% (w/v) insoluble PVP and some washed sand) in a pre-cooled mortar (0°C). The homogenate was cen-trifuged for 5 min at maximal speed in an Eppendorf centrifuge. C4PEPC activity was
deter-mined spectrophotometrically from an aliquot of the supernatant in a 1 ml assay mixture containing 100 mM HEPES – KOH pH 8, 5 mM phospho-enolpyruvate, 5 mM MgCl2, 5 mM NaHCO3, 0.2
mM NADH and 5 U of commercial NAD – MDH. The decrease in absorbance was recorded at 340 nm and 30°C. One C4 PEPC unit was defined as
that amount of enzyme that catalyzed the trans-formation of 1 mmol of substrate per min under the described experimental conditions.
2.5. DNA probes
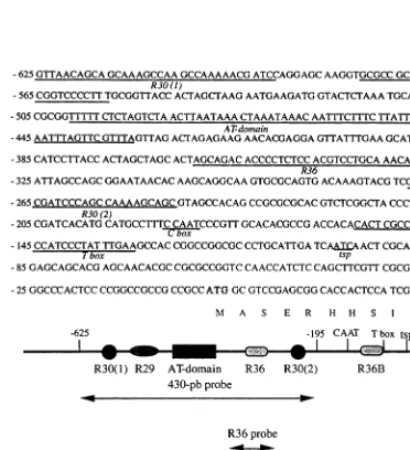
A 430-bp fragment of the S6C4 promoter
(−625 to −195 bp from the translation start site) was cut out from the 0.6-GUS-pSK+ plasmid by HindIII/SphI digestion. Probes shorter than 430 bp were prepared by PCR with the 0.6-GUS-pSK+ plasmid as template. Four DNA fragments were synthesized: R36 (−392 to −285 bp), R29R30 (−625 to −520 bp), ATC (−532 to −406 bp) and ATL (−573 to −406 bp) (Fig. 1). The probes were 3%-end labeled with a DIG Gel Shift Kit (Boehringer Mannheim) according to the manufacturer’s instructions and used in EMSA (see below).
2.6. EMSA
A typical binding reaction (20 ml) contained 2 mg of nuclear proteins or 20mg of leaf proteins, 15 fmol of DNA probe, 0.25 M NaCl, 10 mg double-stranded poly (dI-dC) (Pharmacia), 30 mM HEPES – NaOH pH 8.0, 3 mM MgCl2, 6 mM
DTT, 0.6 mM ascorbic acid, 0.6 mM PMSF, 0.6 mM benzamidine and 0.6 mM leupeptin. After incubation for 15 min at 25°C, 5 ml of loading buffer (60% 0.25×TBE, 40% (v/v) glycerol) was added and the samples were loaded onto a 1.8% agarose gel in 0.25×TBE [24]. Electrophoresis was carried out for 90 min at 8 V cm−1. DNA was
blotted onto a positively charged nylon membrane (Amersham) by overnight capillary transfer using 0.25×TBE buffer. The membrane was soaked in 10× SSC (1.5 M NaCl, 0.15 M sodium citrate pH 7.0), and the DNA was fixed to the nylon mem-brane by UV crosslinking at 120 mJ for 40 s. The chemiluminescent detection of probe signals (DIG Gel Shift Kit) was performed according to the manufacturer’s instructions.
2.7. Phosphorylation assays
Phosphorylation of nuclear protein extracts was performed in vitro as described in [16] with the following modifications. Protein extracts (2 mg) were preincubated with 15 mM NaF for 15 min on ice. ATP or GTP (2 mM) was added to the assay (total volume, 12.5ml) containing the preincubated nuclear protein extract, 15 mM NaF, 5 mM MgCl2, 400 mM NaCl, 10 mM DTT, 1 mM
ascorbic acid, 1 mM PMSF, 1 mM benzamidine, 1
Fig. 2. EMSA with theS6C4 promoter fragment (430-bp) and
nuclear protein extracts from etiolatedSorghum leaves. Nu-clear protein (2mg) and 15 fmol of the DNA probe were used in each assay. Lane 1, free probe; lane 2, probe with nuclear protein extract from etiolated leaves; lane 3, nuclear protein extract from etiolated leaves were preincubated at 50°C in the presence of 10mg of proteinase K for 20 min, prior to EMSA; lane 4, nuclear protein extract from etiolated leaves were boiled for 3 min prior to EMSA. The free probe and the DNA-protein complexes are indicated as FP, PC1 and PC2, respectively.
ghum leaves. Two retarded bands, PC1 and PC2, the major one (PC2) moving faster, were observed in EMSA of the protein samples from etiolated leaves (Fig. 2 lane 2). Pretreatment of the protein extracts with proteinase K, or boiling, prior to electrophoresis caused the disappearance of the retardation bands thus establishing that the shift resulted from protein-DNA interactions (Fig. 2 lanes 3 and 4).
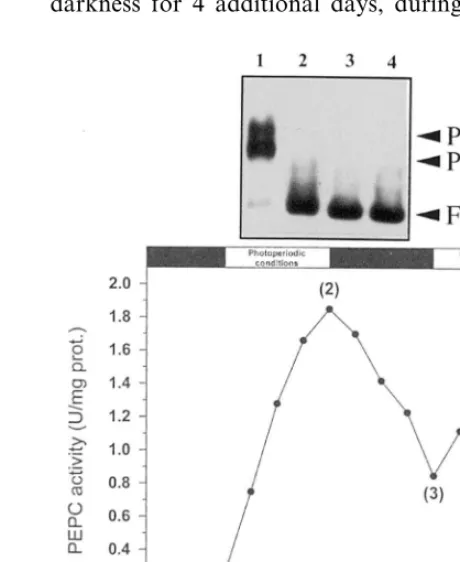
There was no detectable signal in EMSA with nuclear protein extracts from light-adapted leaves for four days under photoperiodic conditions (Fig. 3 lane 2). After the return of the greening plants to darkness for 4 additional days, during which the
Fig. 3. Effect of alternated prolonged darkness and photope-riodic light on the binding activities to the 430-bp S6C4
promoter fragment. Sorghum seeds were germinated for 11 days in darkness (thermoperiod, 27°C for 16 h and 17°C for 8 h) (stage 1), and then illuminated (4 days) under photoperi-odic conditions (16 h of white light, 700 E m−2s−1, 27°C, 8 h of darkness, 17°C) (stage 2), returned to darkness as in stage 1 (4 days) (stage 3) and finally illuminated as in stage 2 (4 days) (stage 4). Leaf and nuclear soluble proteins were extracted at the indicated time. Specific activity of PEPC was determined in leaf protein extracts (means value of three independent experiments). The binding activities to the 430-bp probe were assessed with EMSA (15 fmol of the 430 430-bp probe, 2 mg of nuclear proteins). Lanes (a – d), probe+ nu-clear proteins from stages 1 – 4, respectively. PC1, PC2 and FP, as under Fig. 2.
mM leupeptin, 50 mM HEPES – NaOH pH 8. Other assays were supplemented with 500 U of commercial, rat liver casein kinase II (CKII) or with 100 mM K252a (general protein kinase in-hibitor). After incubation for 20 min, 25°C, the reactions were blocked by 1 ml EDTA (final con-centration, 20 mM). The other components of the shift assay (poly (dI-dC) and the labeled probe in TE buffer) were added and the mix (total volume, 20ml) subjected to electrophoresis in agarose gel as described above.
3. Results
3.1. DNA-binding acti6ities for the S6C4 (430 bp)
promoter fragment in nuclear protein extracts
from etiolated Sorghum lea6es
EMSA has been devised to detect the interac-tion of nuclear proteins with the S6C4 promoter [25,26]. A S6C4 promoter fragment (430-bp, −
625 to −195 bp from the translation start site) was used in these experiments because (a) it con-tains several consensus domains homologous to light dependent boxes found in other photoregu-lated plant gene promoters ([3] and Fig. 1) and (b) it was previously shown to direct the mesophyll-specific expression of the marker gene uidA in transient expression experiments with Sorghum
-Fig. 4. Effect of Sorghum plant irradiation with red and far-red light pulses on the binding activities to the 430-bp
S6C4 promoter fragment. EMSA was performed as described
in Fig. 2 using the S6C4 promoter fragment (430-bp) as a probe. The probe was incubated with 20 mg of proteins extracted from leaves of (lane 1) etiolated plants (11 days of continuous darkness); (lane 2) green plants (11 days of pho-toperiodic conditions); (lane 3), plants treated with red light; (lane 4) plants treated with far-red light; (lane 5) plants treated with red/far-red light. C4 PEPC specific activity (U mg−1 of proteins) in crude protein extracts from Sorghum leaves is indicated below each lane. PC1, PC2 and FP are as given in Fig. 2.
3.2. Binding acti6ities depend on the light quality
Etiolated plants (8-day-old) were irradiated by pulses of red or far-red light for 3 consecutive days. Control plants were either maintained in continous darkness (etiolated plants), or illumi-nated with white light (normal photoperiodic con-dition; green plants). C4 PEPC activity was
measured in protein extracts from leaves of treated and control plants. Compared to etiolated plants, white light- and red-treated plants showed a 7.2 and 5.8-fold enrichment in leaf C4 PEPC activity,
respectively (Fig. 4 lanes 1 – 3). In far-red treated plants, this C4 PEPC activity increased to a lower
extent (3.2-fold), and far-red given after red blocked the red light effect; the red/far-red pho-toreversibility of this response was in good agree-ment with data of previous reports [6,7]. With respect to EMSA, the retarded complexes (PC1 and 2) were observed with protein extracts from etiolated leaves, but not from green leaves (Fig. 4 lanes 1 and 2), as expected. Red and far-red treated plants behaved essentially as the green and etiolated ones, respectively (Fig. 4 lanes 3 and 4). In contrast, far-red given after red pulses, partially but clearly restored the retardation signals (Fig. 4 lane 5). Overall, the results support the view that both the gain in C4 PEPC activity and the loss in
DNA-binding activities to the S6C4 promoter are
mediated by phytochrome during greening of the
Sorghum leaf.
3.3. The major nuclear binding acti6ity interacts
with an AT-rich domain within the 430-bp
fragment of the S6C4 promoter
To further identify the protein binding se-quence(s) on the promoter DNA, four subfrag-ments covering most of the 430-bp probe of the
S6C4 promoter were prepared (Fig. 1) and used in
EMSA. Retarded bands with nuclear protein ex-tracts from etiolated leaves were seen with the 430-bp probe (Fig. 5 lane 2; positive control) and the ATL, ATC and R29/R30 subfragments (Fig. 5 lanes 4 and 6). Furthermore, ATC or ATL showed only one retarded complex which, based on signal intensity, should correspond to the faster migrat-ing band in the control (PC2). The very faint retarded R29/R30 band could be due to PC2, or due to a very weak binding of PC1 to this probe. Therefore, one protein-binding domain was
en-Fig. 5. Identification of binding domains in S6C4 promoter fragments. EMSA was performed with subfragments of the 430-bp (15 fmol) and nuclear protein extracts (2 mg) from etiolatedSorghumleaves. Lane 1, 430-bp free probe; lane 2, 430-bp probe+nuclear proteins; lanes 3 – 10, subfragments of the 430-bp probe (ATL, ATC, R36, R29/R30) + / − nuclear proteins. PC1, PC2 and FP, as under Fig. 2.
activity of PEPC showed a 2.3-fold decrease, the DNA binding activities were not restored (Fig. 3 lane 3). As expected, there was no detectable signal after another round of light treatment (Fig. 3 lane 4). Therefore, the DNA-binding proteins lost their binding capacity during greening of the C4 leaf. Whether they were irreversibly inactived
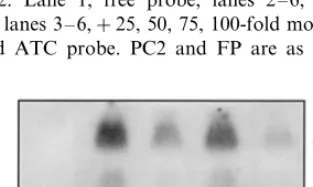
tirely located in the shorter ATC element of the
S6C4 promoter. In a competition experiment, a 25-fold molar excess of the unlabeled ATC probe caused the retarded band to decrease severely (Fig. 6 lane 3) and become almost non-detectable when higher concentrations of the competitor were used (Fig. 6 lane 4 – 6). In contrast, poly (dI-dC) had no effect on the intensity of the signal (data not shown). Thus, this DNA-protein complex, as de-tected in the EMSA, resulted from a specific interaction.
3.4. The major nuclear binding acti6ity (PC2) is
modulated by phosphorylation
So far, reversible phosphorylation is the best documented posttranslational protein modification shown to modulate the binding activity of trans-acting factors and transcriptional activity of pho-toregulated genes [16,20,27 – 32]. To test this hypothesis, nuclear protein extracts from etiolated leaves were preincubated in the presence of ATP and Mg2+, to stimulate the activity of putative, endogenous protein kinases, and NaF, to simulta-neously reduce the activity of protein-phos-phatases, before EMSA was performed. Under these experimental conditions, the DNA binding activity (PC2) to the ATL probe was considerably reduced (Fig. 7 lanes 2 and 3). It showed a further decrease if the pretreatment was performed in the presence of commercial, rat liver casein kinase II (Fig. 7 lane 5); however, it was essentially main-tained when the preincubation medium conmain-tained the general protein serine/threonine kinase in-hibitor K252a (Fig. 7 lane 4). Similar results were observed when Mg-GTP was used during preincu-bation of the nuclear protein extracts (not shown). These findings demonstrated that a protein kinase activity, possibly a CKII-type casein kinase [33], phosphorylating the protein factor is present in nuclear protein extracts from etiolated Sorghum
leaves.
4. Discussion
In this report, we provide evidence that nuclear protein extracts from etiolated Sorghum leaves contain two DNA binding activities (PC1 and PC2) for a promoter fragment of the C4 PEPC
gene (S6C4). The major one (PC2) interacts with an AT-rich region (−473 to −447 bp) within the promoter fragment. AT-rich DNA sequences and corresponding trans-acting protein factors have been reported to regulate gene expression in plants [34]. For example, this cis-element plays a role in the transcriptional activation of the pea small subunit of ribulose bisphosphate carboxylase (rbcS-3A) gene promoter [35]; in contrast, it acts as a negative regulatory element in the Nicotiana
plumbaginifolia chlorophyll a/b binding protein
(Cab-E) gene promoter [36]. Two AT-rich do-mains are also present in the promoter of the salt
Fig. 6. Competition experiment with EMSA. The labeled ATC probe (15 fmol) was mixed with increasing amounts of the unlabeled probe and EMSA were performed with 2mg of nuclear protein extracts from etiolated leaves, as described in Section 2. Lane 1, free probe; lanes 2 – 6, probe+nuclear proteins; lanes 3 – 6,+25, 50, 75, 100-fold molar excess of the unlabeled ATC probe. PC2 and FP are as given in Fig. 2.
Fig. 7. Modulation of PC2 binding activity for the ATC subfragment of the S6C4 promoter. EMSA was performed
stress-inducible PEPC gene from the CAM plant
Mesembryanthemum crystallinum [37]. Among the
three protein factors, PCAT 1-3, with binding activity in these DNA domains, only PCAT1 is a putative salt stress-dependent regulatory activa-tor.
Both PC1 and PC2 almost completely vanished during the greening process of the etiolated Sor
-ghum leaf and cannot be restored in dark-adapted green leaves. Phytochrome and is well correlated with a phytochrome-dependent increase in tran-scriptional capacity of S6C4 [6,7]. In vitro assays have suggested that the DNA binding activity of PC2 to the AT-rich sub-fragment of the S6C4
promoter was abolished by a phosphorylation process. Along the same lines, it has been re-ported that inhibition of DNA binding by phos-phorylation is a common mode of regulation of gene expression [20]. This is the case of the nu-clear proteins AT-1 (binding an AT-rich cis -ele-ment of the Cab gene promoter from pea) and ATBP-1 (binding an AT-richcis-element from the glutamine synthetase gene (GS2) promoter from tobacco) which show a significant loss of binding activity in EMSA after preincubation with a protein kinase activator, like Mg-GTP [16,34].
The loss of PC2 binding activity to the ATC fragment fromS6C4 promoter was observed after
pretreatment of the nuclear protein extracts from etiolated leaves with protein kinase activators, Mg-ATP and Mg-GTP. Furthermore, this effect was strongly enhanced when CKII was supple-mented in the incubation mixture and markedly inhibited in the presence of the general protein serine/threonine kinase inhibitor, K252a. Collec-tively, the data pointed to a CKII-type casein kinase of nuclear protein extracts from etiolated
Sorghum leaves. This multifunctional, nuclear
protein kinase is involved in central cellular func-tions including the regulation of gene expression. It has been formerly characterized in nuclear protein extracts from tobacco [38], pea [33,39],
Arabidopsis thaliana [40] and broccoli [32].
Broc-coli CKII was found to co-purify with phospho-rylate HMG proteins in in vitro assays [41]. Some subgroups of these chromatin binding proteins interact with AT-rich regions of the promoters [34,42].
Altogether, these data support the view that the
trans-acting factor PC2 is present in
non-phos-phorylated form in etiolated Sorghum leaves and binds the ATC domain of the S6C4 promoter. Based on these data, PC2 might well be a ho-molog of AT-1 already shown in pea to bind rbcs
promoters, also presumably Cab promoters, and suggested to be required for the regulation of gene expression [16]. It would come as no surprise if the machinery underlying light-regulated gene expression have some regulatory devices in com-mon. During the greening process of etiolated leaves, PC2 would undergo a phosphorylation-de-pendent unbinding from its cognate DNA do-main. Whether and how the activity of the putative converter enzymes (protein kinase/ protein phosphatase) is regulated by light (via phytochrome) in mesophyll cell nuclei to cause the corresponding target protein factor to become phosphorylated remains to be investigated. Since (1) phytochrome apparently mediates both the loss in binding activity of PC2 (and also of PC1) and an increase in the transcriptional efficiency of
S6C4 [6,7], and (2) PC2 is not restored in
dark-adapted green leaves, one hypothesis is that this protein factor at least contributes to the negative control of S6C4 as far as the skotomorphogenetic
program of the etiolated C4 leaf is pursued.
Acknowledgements
This work was partly supported by Polish-French Biotechnology Centre (CNRS, KBN) and KBN grant 385/P04/95/08. Jose Luis Prieto is supported by a fellowship from the Spanish ‘Min-isterio de Educacion y Cultura’.
References
[1] R. Chollet, J. Vidal, M.H. O’Leary, Phosphoenolpyru-vate carboxylase: a ubiquitous, highly regulated enzyme in plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 4 (1996) 273 – 298.
[2] J. Vidal, R. Chollet, Regulatory phosphorylation of C4 PEP carboxylase, Trends Plant Sci. 2 (1997) 230 – 237.
[3] L. Lepiniec, E. Keryer, K. Philippe, P. Gadal, C. Cre´tin, The phosphoenolpyruvate carboxylase gene family of
Sorghum: structure, function and molecular evolution, Plant Mol. Biol. 21 (1993) 487 – 502.
[5] L. Lepiniec, E. Keryer, D. Tagu, P. Gadal, C. Cre´tin, Complete nucleotide sequence of a Sorghumgene cod-ing for the phosphoenolpyruvate carboxylase involved in C4 photosynthesis, Plant Mol. Biol. 19 (1992) 339 – 342.
[6] M. Thomas, C. Cre´tin, E. Keryer, J. Vidal, P. Gadal, Photocontrol of Sorghum leaf phosphoenolpyruvate carboxylase: characterisation of messenger RNA and of the photoreceptor, Plant Physiol. 85 (1987) 243 – 246.
[7] M. Thomas, C. Cre´tin, J. Vidal, E. Keryer, P. Gadal, E. Mo¨singer, Light regulation of phosphoenolpyruvate car-boxylase mRNA in leaves of C4 plants: evidence for phytochrome control on transcription during greening and for rhytmicity, Plant Sci. 69 (1990) 65 – 78. [8] A.R. Sha¨ffner, J. Sheen, Maize C4 photosynthesis
in-volves differential regulation of phosphoenolpyruvate carboxylase genes, Plant J. 2 (1992) 221 – 232.
[9] S. Yanagisawa, J. Sheen, Involvement of maize Dof Zinc fingers proteins in tissue-specific and light-regu-lated gene expression, Plant Cell 10 (1998) 75 – 89. [10] A. Morishima, Identification of preferred binding sites
of a light-inducible DNA-binding factors (MNF1) within 5%-upstream sequence of C4-type
phospho-enolpyruvate carboxylase gene in maize, Plant Mol. Biol. 38 (1998) 633 – 646.
[11] Y. Kano-Murakami, I. Suzuki, T. Sugiyama, M. Mat-suoka, Sequence-specific interactions of maize factors with a GC-rich repeat in the phosphoenolpyruvate carboxylase gene, Mol. Gen. Genet. 225 (1991) 203 – 208.
[12] S. Yanagisawa, K. Izui, Multiple interactions between tissue-specific nuclear proteins and the promoter of the phosphoenolpyruvate carboxylase gene for C4 photo-synthesis in Zea mays, Mol. Gen. Genet. 224 (1990) 325 – 332.
[13] S. Yanagisawa, K. Izui, Maize nuclear factors interact-ing with the C4 photosynthetic phosphoenolpyruvate carboxylase gene promoter, in: N. Murata (Ed.), Re-search in Photosynthesis, vol. 3, Kluwer Academic Pub-lishers, The Netherlands, 1992, pp. 839 – 842.
[14] S. Yanagisawa, K. Izui, MNF1 A leaf tissue-specific DNA-binding protein of maize, interacts with the cauliflower mosaic virus 35S promoter as well as the C4 photosynthetic phosphoenolpyruvate carboxylase gene promoter, Plant Mol. Biol. 19 (1992) 545 – 553.
[15] S. Yanagisawa, K. Izui, Molecular cloning of two DNA-binding proteins of maize that are structurally different but interact with the same sequence motif, J. Biol. Chem. 268 (1993) 16028 – 16036.
[16] N. Datta, A.R. Cashmore, Binding of a pea nuclear protein to promoters of certain photoregulated genes is modulated by phosphorylation, Plant Cell 1 (1989) 1069 – 1077.
[17] G. Neuhaus, C. Bowler, R. Kern, N.H. Chua, Calcium/ calmodulin-dependent and -independent phytochrome signal transduction pathways, Cell 73 (1993) 937 – 952.
[18] C. Bowler, G. Neuhaus, H. Yamagata, N.-H. Chua, Cyclic GMP and calcium mediate phytochrome photo-transduction, Cell 77 (1994) 73 – 81.
[19] C. Bowler, H. Yamagat, G. Neuhaus, N.-H. Chua, Phytochrome signal transduction pathways are regu-lated by reciprocal control mechanisms, Gene. Develop. 8 (1994) 2188 – 2202.
[20] T. Hunter, M. Karin, The regulation of transcription by phosphorylation, Cell 70 (1992) 375 – 387.
[21] K. Steinmu¨ller, A. Batschauer, K. Apel, Tissue-specific and light-dependent changes of chromatin organization in barley (Hordeum 6ulgare), Eur. J. Biochem. 158
(1986) 519 – 525.
[22] M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[23] P.J. Green, S.A. Kay, N.-H. Chua, Sequence specific interactions of a pae nuclezar factor with light-respon-sive elements upstream of the rbcS-3A gene, EMBO J. 6 (1987) 2543 – 2549.
[24] T. Maniatis, E.F. Fritsch, J. Sambrook, Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 1989. [25] M.M. Garner, A. Revzin, The use of gel electrophoresis
to detect and study nuclear acid-protein interactions, TiBS 11 (1986) 39 – 40.
[26] J. Carey, Gel retardation, in: R.T Sauer (Ed.), Methods in Enzymology.Protein-DNA Interactions, Academic Press, San Diego, 1991, pp. 103 – 117.
[27] L.C. Romero, B. Biswal, P-S. Song, Protein phosphory-lation in isolated nuclei from etiolated A6ena seedlings: effects of red/far-red light and cholera toxin, FEBS Lett. 282 (1991) 347 – 350.
[28] L.C. Romero, D. Sommer, C. Gotor, P-S. Song, G-proteins in etiolated A6ena seedlings. Possible
phy-tochrome regulation, FEBS Lett. 282 (1991) 341 – 346. [29] L.P. Sarokin, N.-H. Chua, Binding sites for two novel
phosphoproteins, 3AF5 and 3AF3, are required for rbcS-3A expression, Plant Cell 4 (1992) 473 – 483. [30] L.C. Romero, E. Lam, Guanine nucleotide binding
protein involvement in early steps of phytochrome-regu-lated gene expression, Proc. Natl. Acad. Sci. USA. 90 (1993) 1465 – 1469.
[31] K. Harter, S. Kircher, H. Frohnmeyer, M. Krenz, F. Nagy, E. Scha¨fer, Light-regulated modification and nu-clear translocation of cytosolic G-box binding factors in parsley, Plant Cell 6 (1994) 545 – 559.
[32] L.J. Klimczak, U. Schindler, A.R. Cashmore, DNA-binding activity of theArabidopsisG-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli, Plant Cell 4 (1992) 87 – 98.
[33] N. Datta, M.B. Schell, S.J. Roux, Spermine stimulation of a nuclear NII kinase from pea plumules and its role in the phosphorylation of a nuclear polypeptide, Plant Physiol. 84 (1987) 1397 – 1401.
[34] G. Tjaden, G.M. Coruzzi, A novel AT-rich DNA bind-ing protein that combines an HMG I-like DNA bindbind-ing domain with a putative transcription domain, Plant Cell 6 (1994) 107 – 118.
[36] C. Castresana, I. Garcia-Luque, E. Alonso, V.S. Malik, A.R. Cashmore, Both positive and negative elements mediated expression of a photoregulated CAB gene from
Nicotiana plumbaginifolia, EMBO J. 7 (1988) 1929 – 1936.
[37] J.C. Cushman, H.J. Bohnert, Salt stress A/T-rich-bind-ing factor interactions within the PEPC promoter from
Mesembryanthemum crystallinum, Plant Mol. Biol. 20 (1992) 411 – 424.
[38] H. Erdmann, M. Bocher, K.G. Wagner, Two protein kinases from nuclei of cultured tobacco cells with prop-erties similar to the cyclic nucleotide-independent en-zymes (NI and NII) from animal tissue, FEBS Lett. 137 (1982) 245 – 248.
[39] H. Li, S.J. Roux, Purification and characterization of a
casein kinase 2-type protein kinase from pea nuclei, Plant Physiol. 99 (1992) 686 – 692.
[40] L. Klimczak, M.A. Collinge, D. Farini, G. Giuliano, J.C. Walker, A.R. Cashmore, Reconstitution of Ara
-bidopsiscasein kinase II from recombinant subunits and phosphorylation of transcription factor GBF1, Plant Cell 7 (1995) 105 – 115.
[41] L.J. Klimczak, A.R. Cashmore, Microheterogeneous cy-tosolic high-mobility group proteins from Broccoli co-purify with and are phosphorylated by casein kinase II, Plant Physiol. 105 (1994) 911 – 919.
[42] E. Czarnecka, J.C. Ingersoll, W.B. Gurley, AT-rich ele-ments of soybean heat shock gene Gmhsp17.5E bind two distinct sites of nuclear proteins in vitro, Plant Mol. Biol. 19 (1992) 985 – 1000.