Composition and bioactive factor
content of cowpea (
Vigna unguiculata
L.
Walp) raw meal and protein concentrate
Leticia Olivera-Castillo,
1∗Fabiola Pereira-Pacheco,
2Erik Polanco-Lugo,
2Miguel Olvera-Novoa,
1Jos ´e Rivas-Burgos
3and George Grant
41Centro de Investigaci ´on y de Estudios Avanzados del IPN, Unidad Merida, Antigua Carretera a Progreso Km 6, 97310 Merida, Yucatan,
Mexico
2Facultad de Ingenieria Qu´ımica, Universidad Aut ´onoma de Yucatan, Av. Ju ´arez No 421, Cd. Industrial, 97288 Merida, Yucatan, Mexico 3Instituto Tecnologico de Merida, Antigua Carretera Progreso Km 5, 97118 Merida, Yucatan, Mexico
4The Rowett Research Institute, Bucksburn, Aberdeen AB2 9SB, UK
Abstract: Analysis was done of the composition and bioactive factor content of whole meal, processed meal and protein concentrate from a cowpea cultivar (Vigna unguiculataL. Walp var. IT86D-719) grown in Yucatan, Mexico and of changes in these parameters after soaking and dehulling. Both meals had a high protein content (247.53 and 257 g kg−1dry matter (DM) respectively). The protein concentrate was rich in protein (786 g kg−1DM) and lipids
(58.47 g kg−1DM) and had an amino acid profile similar to that of the processed meal. The amino acid profiles
of the meals almost covered human dietary requirements based on FAO/WHO/UNU-suggested profiles but were deficient in sulphur amino acids. Trypsin inhibitor activity was high in both meals compared with levels found in previous studies. Trypsin inhibitor activity in the concentrate was not eliminated but was significantly reduced. Lectin activity, tannin levels, phytate levels andα-amylase inhibitor activity were relatively low in the meals, and cyanogenic glucosides were not detected. Residual amounts ofα-amylase inhibitors, tannins and phytate were observed in the concentrate, and lectin activity was not detected. Results indicate thatV. unguiculataL. Walp var. IT86D-719 meals and protein concentrate are good potential foodstuffs in the Yucatan region.
2006 Society of Chemical Industry
Keywords: Vigna unguiculata L. Walp; proximate analysis; amino acid composition; antinutritional factors; Yucatan, Mexico
INTRODUCTION
Legume grains are a primary source of dietary protein in many regions of the world.1 However, yields vary greatly according to plant species, environmental conditions and horticultural practices. The actual nutritional value of these crops is also compromised by an apparently low digestibility of their constituent proteins and by adverse effects of seed components on body metabolism, particularly bioactive/antinutritional factors such as lectins, trypsin inhibitors, tannins, etc.1,2
Most legume seeds therefore require extensive processing before they can be used safely and effectively. Various methods have been developed to improve the nutritional properties of legume seed products, including sprouting,3 soaking and cooking,4extrusion5and isolation of the major storage (globulin-like) proteins, i.e. protein concentration. Protein concentrate quality may vary, however, depending on the exact preparation conditions,
and thus new legume protein concentrates require chemical characterisation of their composition and bioactive/antinutritional factor content. Despite this variation, protein concentrates generally have good nutritional quality, partially because globulin-like proteins have low antinutritional factor levels.6
Cowpeas (Vigna unguiculata) grow well in a diverse range of conditions and environments and contain only moderate levels of bioactive/antinutritional factors.7 The National Institute of Forestry, Agricultural and Livestock Research (Instituto Nacional de Investigaciones Forestales, Agr´ıcolas y Pecuarias (INIFAP)) located in Uxmal, Yucatan, Mexico recently identifiedV. unguiculataL. Walp var. IT86D-719, a variety created at the International Institute for Tropical Agriculture (IITA) in Ibadan, Nigeria, as having good agronomic potential for use as a crop plant in the Yucatan Peninsula region. Indeed, it was found to have a high protein and starch content and a yield of up to 3.5 T ha−1, significantly higher
∗Correspondence to: Leticia Olivera-Castillo, Centro de Investigaci ´on y de Estudios Avanzados del IPN, Unidad Merida, Antigua Carretera a Progreso Km 6, 97310 Merida, Yucatan, Mexico
E-mail: [email protected]
Contract/grant sponsor: British Council/Mexico HEL Program; contract/grant number: MXC/991/83 Contract/grant sponsor: Scottish Executive Environment and Rural Affairs Department
than that achieved with other legumes grown in the region. Despite its promise, usage of cowpea by the regional population remains low; unfamiliarity with this legume, its horticulture and possible uses means that there is only a limited local market for it.
As part of a developmental programme, V.
unguiculata L. Walp var. IT86D-719 seeds were
provided to small-scale farmers in rural Yucatan and grown in accordance with local horticultural practices. The composition, amino acid profile and bioactive/antinutritional factor content of whole meal, processed meal and protein concentrate prepared from this legume are reported in this study. Additional information is provided on changes in the proximal and antinutritional composition resulting from the protein concentration process.
EXPERIMENTAL Seed preparation
Vigna unguiculata L. Walp (IT86D-719) seeds
from INIFAP were cultivated in the rural area of Santa Elena, Yucatan, harvested when mature and dry, cleaned and stored at 4◦C until processing. Representative samples of the seeds were ground in a hammer mill fitted with a 1 mm2 mesh. Whole V.
unguiculataseeds (3 kg) were soaked (1:4 w/v) for 16 h
at 25◦C in sodium bisulphite (2 g L−1) solution. The seeds were then cracked in a manual mill and the hulls separated off by water flotation (dehulling). The cotyledons were cleaned and then dried in a lyophiliser, though one sample of fresh cotyledons was dried in a convection oven at 40◦C to determine if dry heat reduced trypsin inhibitor levels.
Protein concentrate preparation
Protein extraction was done using a modified version of the isoelectric method.8Briefly, the cotyledons were passed through an industrial mill (Koch mod. C352Z, Kansas City, MO, USA) to produce a homogenous dough, which was then ground (1:3 w/v) in water in a colloidal mill (Micron Mod. WB-1, M´exico, DF, Mexico). The pH of the resulting slurry was adjusted to 9 using 0.05 mol L−1 NaOH. After approximately 20 min the suspension was filtered through 0.150 and 0.106 mm2 meshes to remove fibrous material. The remaining slurry was stored without agitation at 4◦C for 12 h to allow the starch to settle. The supernatant was decanted and its pH adjusted to 4.33 with 0.5 mol L−1HCl. It was then heated at 80◦C for 10 min and stored for 12 h at 4◦C. The precipitated globulin-enriched fraction (i.e. protein concentrate) was recovered by centrifugation (1500×g, 12 min) and subsequent drying in a convection oven at 40◦C for 24 h.
Proximate composition
Moisture, ash, crude fibre and crude fat contents in the whole meal, processed meal and protein concentrate were determined according to standard
methods.9 Nitrogen content was estimated by gas chromatography using a ThermoQuest NCS analyser (ThermoQuest Flash EA 1112, Rodano, Italy). Crude protein content was calculated as N × 6.25. All analyses were run in triplicate.
Amino acid analysis
Amino acid analysis of the defatted meals and defatted protein concentrate was done using a four-step Pico-Tag method.10 In the Pico-Tag station, hydrolysis was done using 3 mol L−1 HCl under vacuum at 104◦C for 24 h, fol-lowed by redrying with ethanol/water/triethylamine (2:2:1 v/v/v) solution and derivatisation with ethanol/triethylamine/water/phenylisothiocyanate (7:1:1:1 v/v/v/v) reagent. Once derivitised, aliquots were subjected to reverse phase liquid chromatography in a Waters high-performance liquid chromatography (HPLC) system (Waters Corporation, Milford, MA, USA). Tryptophan was determined by a colorimetric method.11All analyses were run in triplicate.
Analysis of antinutritional factors in meals and protein concentrate
Trypsin inhibitor activity,α-amylase inhibitor content, lectin activity, tannic acid levels, phytate content and cyanogenic glucoside levels were determined in the whole meal (WM), processed meal (PM) and protein concentrate (PC) as follows.
Trypsin inhibitor activity was assayed by the method of Liu and Markarkis12using benzoyl-DL-arginine-p -nitroanilide (BAPNA) as substrate and porcine trypsin (Type II-S, Sigma Chemical St. Louis, Mo, USA). One trypsin unit (TU) was defined as 0.01 at A410 under the assay conditions, and trypsin inhibitor activity was expressed as trypsin units inhibited (TUI) kg−1 dry matter (DM) and kg−1 protein. Trypsin inhibitor activity in the WM, PM and PC was also determined from diluted samples of the fractions with 40 – 60% inhibition of enzyme activity and expressed as g enzyme inhibited kg−1DM.
α-Amylase inhibitor content was determined by the procedure of Piergiovanni13 and expressed as g amylase inhibited kg−1DM and kg−1protein.
Lectin activity (haemagglutination) was assayed by the serial dilution method of Armour et al.14 using human, bovine and hamster red blood cells previously treated with trypsin. One haemagglutinating activity unit (HU) was defined as that contained in the amount of sample in the final dilution which caused 50% agglutination of the blood cells. Lectin activity was also determined using pure Phaseolus vulgaris lectin (Sigma Chemical) as a standard and expressed as g lectin equivalent kg−1DM.
Tannic acid levels were determined using a spectrophotometric method15 and expressed as g tannic acid kg−1DM and kg−1protein.
The extract was then mixed with ethylene diamine tetraacetic acid (EDTA)/NaOH solution and placed in an ion exchange column. The phytate was eluted with 0.7 mol L−1 NaCl solution and wet digested with HNO3/H2SO4 mixture to release phosphorus, which was measured calorimetrically at 640 nm and expressed as g phytic acid kg−1DM and kg−1protein. Cyanogenic glucoside levels were determined using the method of Lucas and Sotelo,16 based on the specific Guinard reaction, and expressed as g HCN kg−1DM.
Statistical analysis
With the exception of lectin activity, results were subjected to one-way analysis of variance (ANOVA) at P<0.05. Differences between means were evaluated by the Newman – Keuls test; normality was tested using the Wilk – Shapiro test. When necessary, values were normalised using natural logarithms. Lectin activity results were compared using the Student t test (P<0.05). Means for all data were calculated from three replicates (n=3). All analyses were done with the Statistica v5.5 program (StatSoft, Tulsa, OK, USA).
RESULTS AND DISCUSSION
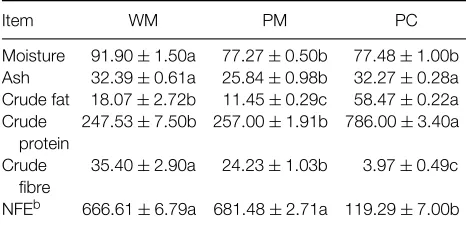
The proximate compositions of the V. unguiculata L. Walp var. IT86D-719 WM, PM and PC were generally similar to reported levels for cowpeas (Table 1). Protein content in the WM was similar to that found in other cowpea (V. unguiculata) varieties (180 – 310 g kg−1 DM)7,17,18 but slightly higher than levels previously reported for cowpeas cultivated in Yucatan (225 – 241 g kg−1 DM)19. The dehulling process increased protein content in the PM, though its protein value was not statistically different from that of the WM. This agrees with Aremu,20 although his results were not statistically evaluated. The increase in protein content observed in the present study was attributed to changes in proximate composition during dehulling. Crude fiber content dropped by 11.8 g kg−1
Table 1.Proximate composition (g kg−1dry matter)aof cowpea
(Vigna unguiculataL. Walp var. IT86D-719) whole meal (WM), processed meal (PM) and protein concentrate (PC)
Item WM PM PC
aValues are mean ± standard deviation of three determinations.
Different letters in the same row indicate significantly different (P<0.05) means.
bNitrogen-free extract, estimated by difference.
DM, raising the net proportion of protein, despite the seed coat containing 112.5±2.4 g protein kg−1 DM or 53.7 g protein kg−1total whole grain protein.
The PC had significantly (P <0.05) higher protein (786 g kg−1) and fat (58.47 g kg−1) contents and lower fibre and carbohydrate levels than the meals. Protein concentrates of similar quality have been obtained from other legumes using the same isoelectric precipitation technique.6,21,22 Protein isolates (or concentrates) from legumes normally have a high lipid content, due to a binding mechanism between protein and lipid that may result from emulsification of the lipid by the protein.23
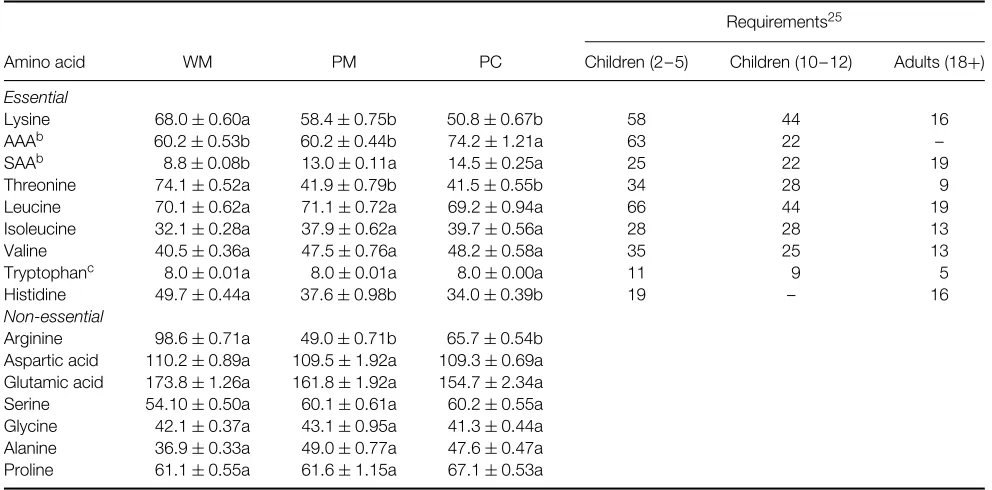
The WM, PM and PC amino acid profiles (Table 2) show that the first limiting amino acids in all three fractions were the sulphur amino acids (methionine/cysteine). This was expected, since it confirms the results of a number of other legume protein quality studies.6,17,18,20,24 Tryptophan is the second limiting amino acid in legume seeds. Its levels in the WM, PM and PC do not meet the requirements for children of 2 – 5 years but do meet those for children of 10 – 12 years and adults of 18+
years.25In comparison with other studies, the present tryptophan levels (8.0 g kg−1) are similar to the average for cowpeas reported by Kelly (cited in Ref. 26) but lower than that reported by Maia et al.18 for six Brazilian cowpea varieties (8.93 g kg−1). The levels found here may have resulted from the processing method used or simply from variations in genetics, environmental factors and/or seed maturation.26
Worth noting is that lysine content dropped significantly (P<0.05) during the soaking and dehulling process, from 68.0±0.60 g kg−1 (WM) to 58.4±0.75 g kg−1 (PM). However, there was little change between lysine values in the PM and PC (P>0.05). Lysine values in all three fractions meet the requirements for children of 2 – 12 years and adults of 18+years.25
Levels of the remaining essential amino acids in the three fractions meet the requirements for all children and adults. Although threonine and histidine did decrease significantly (P<0.05) in the PM and PC, they remained within normal ranges for these amino acids in legumes. This differs from the findings of Fern´andez-Quintela et al.6 and Aremu,20 who reported higher values for these amino acids in cowpea protein concentrate. Leucine and isoleucine values were similar (P >0.05) in all three fractions, with leucine/isoleucine ratios ranging from 1.74 to 2.18. The lowest ratio was in the PC, making it appropriate for complementing the cereal diets that predominate among the rural people of Yucatan. This ratio was similar to that (1.85) found by Chan and Phillips24 in the globulin-enriched fraction ofV. unguiculatacv. California Blackeye No 5.
Table 2.Amino acid content (g kg−1protein)aof cowpea (Vigna unguiculataL. Walp var. IT86D-719) whole meal (WM), processed meal (PM) and
protein concentrate (PC) in comparison with daily requirements for children (2–5 and 10–12 years) and adults (18+years)
Requirements25
Amino acid WM PM PC Children (2– 5) Children (10– 12) Adults (18+)
Essential
Lysine 68.0±0.60a 58.4±0.75b 50.8±0.67b 58 44 16 AAAb 60.2±0.53b 60.2±0.44b 74.2±1.21a 63 22 –
SAAb 8.8±0.08b 13.0±0.11a 14.5±0.25a 25 22 19 Threonine 74.1±0.52a 41.9±0.79b 41.5±0.55b 34 28 9 Leucine 70.1±0.62a 71.1±0.72a 69.2±0.94a 66 44 19 Isoleucine 32.1±0.28a 37.9±0.62a 39.7±0.56a 28 28 13 Valine 40.5±0.36a 47.5±0.76a 48.2±0.58a 35 25 13 Tryptophanc 8.0±0.01a 8.0±0.01a 8.0±0.00a 11 9 5
Histidine 49.7±0.44a 37.6±0.98b 34.0±0.39b 19 – 16 Non-essential
Arginine 98.6±0.71a 49.0±0.71b 65.7±0.54b Aspartic acid 110.2±0.89a 109.5±1.92a 109.3±0.69a Glutamic acid 173.8±1.26a 161.8±1.92a 154.7±2.34a Serine 54.10±0.50a 60.1±0.61a 60.2±0.55a Glycine 42.1±0.37a 43.1±0.95a 41.3±0.44a Alanine 36.9±0.33a 49.0±0.77a 47.6±0.47a Proline 61.1±0.55a 61.6±1.15a 67.1±0.53a
aValues (except for tryptophan) are mean±standard deviation of five determinations. Different letters in the same row indicate significantly different
(P<0.05) means.
bAAA, total aromatic amino acids; SAA, total sulphur amino acids. cValues are mean±standard deviation of three determinations.
Table 3.Trypsin inhibitor activityain cowpea (Vigna unguiculataL. Walp var. IT86D-719) whole meal (WM), processed meal (PM) and protein
concentrate (PC)
Trypsin inhibitor activityb WM PM (lyophilised) PM (dry heat treated) PC
TUI kg−1dry matter (DM) (34.52±0.06)×106a (34.60±0.06)×106a (33.27±0.06)×106b (27.86±0.06)×106c
TUI kg−1protein (141.52±0.24)×106a (134.50±0.23)×106ab (134.00±0.12)×106b (35.45±0.73)×106c
g enzyme inhibited kg−1DM 49.08±0.43a 49.52±0.15a 48.75±0.59a 44.04±0.84b
aValues are mean±standard deviation of three determinations. Different letters in the same row indicate significantly different (P<0.05) means. bTUI, trypsin units inhibited. One TU is defined as 0.01 atA
410under the assay conditions (pH 8.1, 37◦C, 4 mL assay volume, porcine trypsin).
a complement to cereal-rich diets, thus providing a complete amino acid profile. If any one of the three fractions was used as the sole protein source in human or animal diets, it would need to be enriched with this legume’s limiting amino acids.
Of the non-essential amino acids, aspartic acid and glutamic acid were abundant in all three fractions. This is common in cowpea meals and protein concentrates17,18,24,27 and may adversely affect the solubility of the protein concentrate.27 Overall, the PC’s amino acid profile was similar to that of the PM. The exception was the aromatic amino acids (tyrosine + phenylalanine), which rose in the PC because tyrosine increased (28.8±0.40 g kg−1).
Trypsin inhibitor activity (Table 3) was statisti-cally similar (P>0.05) between the WM and the lyophilised PM in all three quantifications (i.e. TUI kg−1 DM, TUI kg−1 protein, g enzyme inhibited kg−1 DM), though there were very minor differences between the lyophilised and dry heat-treated PMs. Trypsin inhibitor activity in the WM was comparable to that reported by other researchers,12,28,29 though
Maia et al.18 found lower trypsin inhibitor activity
values (expressed as g kg−1) using Brazilian cowpea cultivars. When compared with soya bean cultivars, the present cowpea trypsin inhibitor levels were approxi-mately five times lower than those of some soya bean meals.12Nevertheless, Armouret al.14reported results (between 39.5 and 49.7 TUI kg−1 DM) similar to those for cowpeas in nine of the 26 soya bean cultivars they studied. The differences between different soya bean cultivars as well as between them and cowpeas may be due to environmental differences in the areas of cultivation.29
this was a combined result for four cultivars, with no analysis of variation by cultivar.
Although cold soaking may not eliminate significant amounts of trypsin inhibitor activity, the present results indicate that dry heat treatment at 40◦C also did not substantially lower trypsin inhibitor activity. Indeed, the differences between the lyophilised and dry heat-treated PMs was not significant in two of the quantifications (TUI kg−1protein, g enzyme inhibited kg−1 DM) and barely significant in the other (TUI kg−1DM) (Table 3). Apparently, g enzyme inhibited kg−1 DM most accurately reflects trypsin inhibitor activity and may be the most appropriate way of quantifying this antinutritional factor.
In all three quantifications the humid heat (80◦C) of the concentration process significantly reduced trypsin inhibitor activity in the PC compared with levels in the WM and PM. It did not, however, completely eliminate trypsin inhibitor activity as was reported by Armouret al.14for some Japanese soya bean (Glycine
max) cultivars, meaning that the trypsin inhibitor in
V. unguiculataL. Walp (ITD86-719) is partially
heat-resistant.
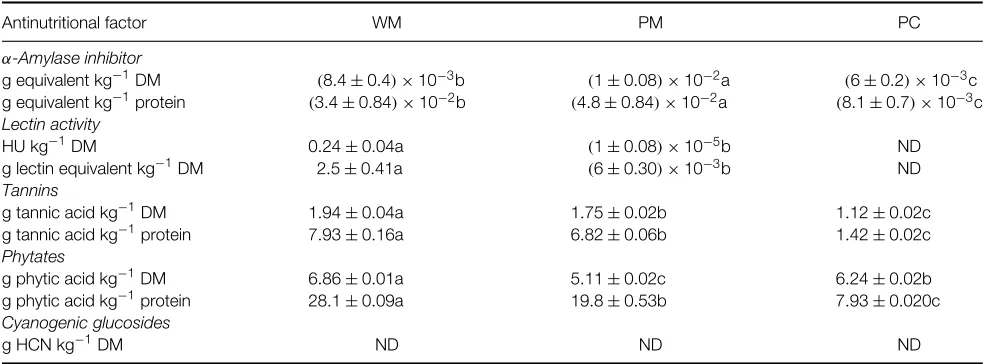
α-Amylase inhibitor was detected in very low concentrations in the WM, PM and PC (Table 4). When the values were expressed as g equivalent kg−1 DM, they were statistically different (P <0.05), with the highest inhibitor activity in the PM and the lowest in the PC. Piergiovanni13also reported higher activity in the PM when dehulling light-coloured cowpea seeds, explaining that this may result from inhibition of theα-amylase inhibitor by polyphenolic compounds in the testa. That is why inhibitor activity was higher in whole seeds and why there was less inhibitor activity in seeds with darker testae. Grant et al.31 reported a value of 0.2±0.1 g equivalent kg−1 DM for whole grain cowpeas, using the same method as here, and considered this inhibitor activity to be low compared with that of kidney bean (Phaseolus vulgaris), haricot
bean (P. vulgaris), pinto bean (P. vulgaris) and runner bean (Phaseolus coccineus).
When expressed as g equivalent kg−1 protein,
α-amylase inhibitor activity was again significantly different (P<0.05) among the three fractions, with the highest value in the PM and the lowest in the PC. This means thatV. unguiculataL. Walp var. IT86D-719 has a very low concentration of this protease inhibitor, particularly in the PC.
When estimated as haemagglutinationversus ham-ster blood cells, lectin activity was detected in the WM and PM but not in the PC (Table 4). No lectin activity was detected in any of the fractions when bovine or human type A+red blood cells were used. For the WM this result is comparable to that reported by Armour
et al.14 for four Japanese soya bean cultivars that
had the lowest lectin activity values (2.4±0.8 kg−1 DM) of 30 cultivars studied, with values ranging from 9.6±3.2 to 4.8±1.6 kg−1DM. They concluded that soya bean lectin activity is relatively heat-resistant and consequently that heat treatment reduces but does not eliminate it. Other tropical grains and kidney bean31,32have reported lectin activity levels lower than in the cowpeas studied here; in fact, lectin activity in kidney bean was completely eliminated after heating for 5 min at 100◦C. However, Maia et al.18reported higher lectin activity levels than in the present study in six cultivars of Brazilian cowpeas using trypsinated blood cell treatment. These results are only generally comparable with the present ones, because the authors used rabbit blood cells (trypsinated rat, bovine, human and hamster blood was used in the present study) and no heat treatment was applied, which may affect lectin affinity for carbohydrates in the blood cell membrane. As mentioned above, no appreciable lectin activity was observed in the PC, making it significantly (P<0.05) lower than in the WM. This is most likely a result of the soaking process. Fern´andez-Quintela
et al.6 reported similar results in that they observed
no detectable lectin activity in protein concentrates
Table 4.Antinutritional factorsa(except trypsin inhibitors) in cowpea (Vigna unguiculataL. Walp var. IT86D-719) whole meal (WM), processed meal
(PM) and protein concentrate (PC)
Antinutritional factor WM PM PC
α-Amylase inhibitor
g equivalent kg−1DM (8.4±0.4)×10−3b (1±0.08)×10−2a (6±0.2)×10−3c
g equivalent kg−1protein (3.4±0.84)×10−2b (4.8±0.84)×10−2a (8.1±0.7)×10−3c
Lectin activity
HU kg−1DM 0.24±0.04a (1±0.08)×10−5b ND
g lectin equivalent kg−1DM 2.5±0.41a (6±0.30)×10−3b ND
Tannins
g tannic acid kg−1DM 1.94±0.04a 1.75±0.02b 1.12±0.02c g tannic acid kg−1protein 7.93±0.16a 6.82±0.06b 1.42±0.02c
Phytates
g phytic acid kg−1DM 6.86±0.01a 5.11±0.02c 6.24±0.02b
g phytic acid kg−1protein 28.1±0.09a 19.8±0.53b 7.93±0.020c
Cyanogenic glucosides
g HCN kg−1DM ND ND ND
from peas (Pisum sativum), faba bean (Vicia faba) and soya bean, which they explained as being caused by concentrate preparation; they used the same isoelectric point method as in the present study.
Tannin was present in the WM, PM and PC frac-tions (Table 4). Tannin levels dropped progressively as the meal was processed, producing significant differ-ences among fractions whether expressed as g tannic acid kg−1 DM or kg−1protein. Ogunet al.4reported similar tannin content results for one raw cultivar (Kano 1696) of the four cultivars they studied. After processing (dehulling, cold soaking, hot soaking), no tannins were detected in any of the four cultivars. Cold soaking caused a non-significant decrease in tannins in comparison with the raw grains, and hot soaking led to a greater but still non-significant drop in tannins in comparison with the raw grains. The tannin levels in the present PM may have originated in tannins from other parts of the grain besides the testa or from residual hulls. If the latter is true, dehulling efficiency was notably lower in the present study than in that of Ogunet al.4
Despite slight variations in different studies, dehulling and the protein concentration process clearly affect tannin content in the final product. For example, Fern´andez-Quintela et al.6 observed that antinutri-tional factor content, including tannins, drops after protein concentrate preparation. Because the high-est tannin concentrations inV. unguiculataand other seeds are generally found in the testa,4,33 the lack of detectable tannins in the PC probably resulted from removal of the testa prior to protein extraction, as well as retention of low-molecular-weight polyphenols34in the water-soluble (pH 4.3) phase.
There were significant differences (P <0.05) in phytate content among the WM, PM and PC fractions on both a DM and protein basis (Table 4), though each basis exhibited a different pattern. The phytate concentration on a DM basis in the PM was significantly lower (P <0.05) than in the WM and PC. When compared in terms of protein, however, the concentration decreased progressively, with significantly lower (P <0.05) values between the WM and PM and between the PM and PC. The phytate concentration value (DM and protein basis) in the WM was comparable to those found in many other legumes.33,35,36Hidvegi and Lasztity36reported that phytic acid content in cowpea meal can vary from 2.9 to 8.6 g kg−1, while in soya protein concentrate they reported a phytic acid content of 8.2 g kg−1. Oluwatosin29 found higher dry matter basis phytic acid values in cowpeas than in the present study, with values ranging from 25.07 to 16.05 g kg−1. This discrepancy may be explained by the fact that some of their varieties, including var. IT86D-534 from the same source as var. IT86D-719, resulted from recent breeding programmes aimed at improving resistance to pests and diseases.
Processing (i.e. dehulling and soaking) notably affected phytic acid concentration in the present
study. Ogun et al.4, however, reported that it had no significant effect on phytic acid, with average values of 1.2±0.02 g kg−1with dehulling and 1.1±0.02 g kg−1 with cold soaking for four cultivars; these are quite different from the present results for the PM. This contrast may be due to the extraction method or simply to phytate solubility in var. IT86D-719. Comparison of phytate concentration variation in terms of protein content is not possible, since Ogun et al.4 did not express their data on that basis.
On a protein basis the present PC had 3.5 times less phytic acid than the WM and 2.5 times less than the PM, with its value of 7.93 g kg−1 being relatively close to the values of 9.03 g kg−1 for soya bean isolate and 10.1 g kg−1 for protein concentrate reported by Hidvegi and Lasztity.36 The difference between the meals and the PC is probably due to most of the phytates being in the cotyledon, meaning that they would have been extracted at pH 9. However, the remaining phytates may be highly water-soluble and therefore did not precipitate during protein concentrate preparation. Phytic acid was not completely eliminated in the PC, meaning that the processing method did not remove it all and/or the raw material had high concentrations. This results from the phytic acid concentration in protein concentrates or isolates being dependent on the raw materials from which they are prepared as well as the processing method. The final concentration may also be affected by the protein – phytic acid interaction, which is highly pH-dependent. A pH above a protein’s isoelectric point can diminish the protein – phytic acid interaction, while a pH below this point can raise the interaction, though binding begins to decrease below pH 2.5.
No cyanogenic glucosides were detected in the WM and thus they were absent in the PM and PC fractions. This contrasts with the result of Colom´e
et al.,19 who found 2.9 mg HCN kg−1 in whole V.
unguiculatameal. The difference between the present
data and those of Colom´e et al.19 is probably due to the fact that cyanogenic glucoside levels can vary considerably depending on a crop’s location and environmental conditions, as is the case with many other antinutritional factor contents.
CONCLUSIONS
combined with cereals to balance their amino acid profile.
Trypsin inhibitors, α-amylase inhibitor and lectins in many legume seeds are albumin-like proteins and thus soluble in aqueous solution. The low concentrations of some antinutrients and the absence of lectin observed in the cowpea PC (pH 4.3 insoluble) would be consistent with the constituent bioactive factors being albumin-like proteins. The persistence of some trypsin inhibitor in the PC may be a carry-over from its high concentration in the WM. Repeating the solubilisation/precipitation procedures used here with the PC may further reduce trypsin inhibitor levels. Completely eliminating all antinutrients in the PC, however, may not necessarily be the most advantageous overall approach, since at certain levels they can aid in disease prevention.In vivonutritional studies will be required to firmly establish these properties and better understand this legume’s high potential.
ACKNOWLEDGEMENTS
The authors thank C´esar Puerto-Castillo and Wilberth Ch´e-Le ´on for their laboratory assistance, Laura Escobar-Brillones for help with the statistical analysis, and Dr Luis Chel-Guerrero for facilitating the tryptophan analysis. This research was done as part of a British Council/Mexico HEL Program (MXC/991/83). Support was also provided by the Scottish Executive Environment and Rural Affairs Department and the International Foundation for Science (grant E/3171-1).
REFERENCES
1 Lalles JP and Jansman AJM, Recent progress in the under-standing of the mode of action and effects of antinutritional factors from legume seeds in non-ruminant farm animals, in
Recent Advances of Research in Antinutritional Factors in Legume Seeds and Rapeseed, ed. by Jansman AJM, Hill GD, Huis-man J and van der Poel AFB. Wageningen Pers, Wageningen, pp 219–232 (1998).
2 Ter´an S, Rasmussen HC and May CO, Frijoles, inLas Plantas
de la Milpa entre los Mayas: Etnobot ´anica de las Plantas Cultivadas por Campesinos Mayas en las Milpas del Noreste de Yucat ´an, M´exico, ed. by G ¨uemez-Pineda MA, Fundaci ´on Ben Kin AC/DANIDA, M´erida, pp. 129–152 (1998).
3 Njintang NY, Mbofung FMC and Waldron WK,In vitro
pro-tein digestibility and physicochemical properties of dry red
bean (Phaseolus vulgaris) flour: effect of processing and
incor-poration of soybean and cowpea flour. J Agric Food Chem
49:2465–2471 (2001).
4 Ogun PO, Markarkis P and Chenoweth W, Effect of processing
on certain antinutrients in cowpeas (Vigna unguiculata).J Food
Sci54:1084–1085 (1989).
5 Alonso R, Aguirre A and Marzo F, Effects of extrusion and
traditional processing methods on antinutrients andin vitro
digestibility of protein and starch in faba and kidney beans.
Food Chem53:259–265 (2000).
6 Fern´andez-Quintela A, del Barrio SA, Macarulla TM and Mart´ınez AJ, Nutritional evaluation and metabolic effects in rats of protein isolates obtained from seeds of the legumes.
J Sci Food Agric78:251–260 (1998).
7 Prinyawiwatkul W, McWatters KH, Beuchat LR and Phillips RD, Cowpea flour: a potential ingredient in food products.
Crit Rev Food Sci Nutr36:413–436 (1996).
8 P´erez V, Efecto de los par´ametros de remojo sobre el rendimiento y la composici ´on proximal de las fracciones proteicas y almidonosas obtenidas durante la molienda
h ´umeda deV. unguiculata. Undergraduate Thesis, Faculty of
Chemical Engineering, Universidad Aut ´onoma de Yucat´an, M´erida, pp. 32–43 (1996).
9 AOAC,Official Methods of Analysis(16th edn). Association of
Official Analytical Chemists, Washington, DC (1997).
10 Waters Corporation, Pico-Tag Amino Acid Analysis System
Owner’s Manual. Waters Corporation, Milford, MA (1984).
11 Spies RJ and Chambers CD, Chemical determination of
tryptophan in proteins.Anal Chem21:1249–1266 (1949).
12 Liu K and Markarkis P, An improved calorimetric method for
determining antitryptic activity in soya bean products.Cereal
Chem66:415–422 (1989).
13 Piergiovanni AR, Effects of some experimental parameters on
the activity of cowpea α-amylase inhibitors. Lebensm Wiss
Technol25:321–324 (1992).
14 Armour JC, Perera RLC, Buchan WC and Grant G, Protease inhibitors and lectins in soya beans and effects of aqueous
heat-treatment.J Sci Food Agric78:225–231 (1998).
15 Rivas-Burgos JI, Evaluacai ´on nutricional de concentrados
proteicos de hojas deSesbania grandiflorayArachis hypogaea
utilizadas en dietas de tilapia (Oreochromis niloticus).Doctoral
Dissertation, Centro de Investigaci ´on y de Estudios A vanzadas del Instituto Polyt´ecnico Nacional, Unidad M´erida (1993). 16 Lucas B and Sotelo A, A simplified test for the quantification of
cyanogenic glucoside in wild and cultivated seeds.Nutr Rep
Int29:719–726 (1984).
17 Kochhar N, Walker FA and Pike JD, Effect of variety on protein content, amino acid composition and trypsin inhibitor activity
of cowpeas.Food Chem29:65–78 (1988).
18 Maia HMF, Oliveira AT, Matos TRM, Moreira AR and Vas-concelos M, Proximate composition, amino acid content and haemagglutinating and trypsin-inhibitory activities of some
Brazilian Vigna unguiculata(L) Walp cultivars. J Sci Food
Agric80:453–458 (2000).
19 Colom´e C, Bilbao T, Ledesma L and Zum´arraga R, Evaluaci ´on preliminar de algunos t ´oxicos naturales en leguminosas de
mayor consumo en el estado de Yucat´an.Tecnol Alim28:8–13
(1993).
20 Aremu YC, Proximate and amino acid composition of cowpea (Vigna unguiculataL Walp) protein concentrate prepared by
isoelectric point precipitation.Food Chem37:61–68 (1990).
21 Kohnhorst LA, Smith MD, Vebersax AM and Bennink RM, Production and characterization of a protein concentrate from
Navy beans (Phaseolus vulgaris).Food Chem41:33–42 (1991).
22 Cheung CK and Chau CF, Effect of various processing methods
on antinutrients andin vitrodigestibility of protein and starch
of two Chinese indigenous legume seeds.J Agric Food Chem
45:4773–4776 (1997).
23 Gueguen J, Legume seed extraction, processing and end product
characteristics. Qual Plant Foods Hum Nutr 32:267–303
(1983).
24 Chan C and Phillips RD, Amino acid composition and
subunit constitution of protein fractions from cowpea (Vigna
unguiculataL Walp) seeds.J Agric Food Chem42:1857–1860 (1994).
25 FAO/WHO/UNU, Energy and protein requirement report of a
joint FAO/WHO/UNU expert consultation.Tech Rep No 724,
World Health Organisation, Geneva (1985).
26 Bressani R and Elias GL, Nutritional value of legume crops
for humans and animals, in Advances in Legume Science,
Proceedings of the International Legume Conference, Vol. 1, ed. by Summerfield RJ and Bunting HA. Royal Botanical Gardens, Ministry of Agriculture, Fisheries and Food, UK, pp. 135–155 (1978).
27 Carbonaro M, Cappelloni M, Nicoli S, Lucarini M and Carnovale E, Solubility –digestibility relationship of legume
28 Singh P and Bhattacharya L, Trypsin inhibitor activity of five
improved varieties of cowpea. Ann Agric Res 12:425–426
(1991).
29 Oluwatosin BO, Genotype× environment influence on
cow-pea (Vigna unguiculata(L) Walp) antinutritional factors: 1.
Trypsin inhibitors, tannins, phytic acid and haemagglutinat-ing.J Sci Food Agric79:265–272 (1999).
30 Wang LH, Swain WE, Hesseltine WC and Heath DH, Hydrata-tion of whole soybeans affects solids losses and cooking
quality.J Food Sci44:1510–1513 (1979).
31 Grant G, Edwards EJ and Pusztai A, Alpha-amylase inhibitor
levels in seeds generally available in Europe.J Sci Food Agric
67:235–238 (1995).
32 Grant G, More LJ, Mckenzie NH, Dorward PM, Buchan WC,
Telek L,et alNutritional and haemagglutination properties
of several tropical seeds.J Agric Sci124:437–445 (1995).
33 Grant G, Dorward PM, Buchan WC, Armour JC and Pusz-tai A, Consumption of diets conPusz-taining raw soya beans
(Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: effects on body composition and organ
weights.Br J Nutr73:17–29 (1995).
34 Saini HS, Distribution of tannins, vicine and convicine activity in
legume seeds, inRecent Advances of Research in Antinutritional
Factors in Legume Seeds, ed. by van der Poel AFB, Huisman J and Saini HS. Wageningen Pers, Wageningen, pp. 95–100 (1993).
35 Bartholomai GB, Tosi E and Gonz´alez R, Polifenoles y taninos
condensados, in Caracterizaci´on de Compuestos Nutritivos y
Calidad Proteica, ed. by. Programa Iberoamericano de Ciencia y Tecnolog´ıa para el Desarrollo (CYTED), EUDEBA, Buenos Aires, Argentina, pp. 57–58 (2000).
36 Hidvegi M and Lasztity R, Phytic acid content of cereals and
legumes and interaction with proteins.Period Polytech – Chem