Ratios of
cis
- and
trans
-Sabinene Hydrate in
Origanum majorana
L. and
Origanum
microphyllum
(Bentham) Vogel
Johannes Novak
!
,
*
, Christina Bitsch
!
, Jan Langbehn
"
,
Friedrich Pank
"
, Melpo Skoula
#
, Yiota Gotsiou
#
,
Chlodwig M. Franz
!
!Institute of Applied Botany, University of Veterinary Medicine, Veterina(rplatz 1, A-1210 Wien, Austria
"Institute for Breeding of Vegetables, Medicinal and Aromatic Plants, Federal Centre for Breeding Research on Cultivated Plants, Neuer Weg 22/23, D-06484 Quedlinburg, Germany
#Mediterranean Agronomic Institute of Chania, Department of Natural Products, P.O. Box 85, GR-73 100 Chania, Crete, Greece
Received 18 April 1999; accepted 14 June 1999
Abstract
(#)-cis-Sabinene hydrate and (#)-trans-sabinene hydrate are the main monoterpenes
found in marjoram (Origanum majorana), but can also be found in otherOriganumspecies as
well, as in e.g.Melaleuca alternifolia. The synthesis of sabinene hydrate in marjoram (Origanum
majorana) is performed by sabinene hydrate synthase. It is claimed, that both, (#)-cis- and (#)-trans-sabinene hydrate are produced by the same enzyme in an exact ratio of 10 : 1. To
verify this in vitro result in vivo, we analysed single plants of 20 di!erent genotypes ofOriganum
majoranaand of three di!erent populationsof Origanum microphyllumand calculated the ratios
of (#)-cis- to (#)-trans-sabinene hydrates. In Origanum majoranaa constant ratio of 20 : 1
could be found, whereas in Origanum microphyllum the ratio did not prove to be
con-stant. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Origanum majoranaL.;Origanum microphyllum(Bentham) Vogel; Lamiaceae; (#)-cis-sabinene hydrate; (#)-trans-sabinene hydrate; (#)-cis-sabinene hydrate acetate
*Corresponding author. Tel: 0043-1-250-77-3104; fax: 0043-1-250-77 3190. E-mail address:[email protected] (J. Novak)
1. Introduction
Marjoram (or&Sweet Marjoram') (Origanum majoranaL. syn.Majorana hortensis Moench) is a herbaceous, perennial plant native to Cyprus and the Eastern Mediter-ranean. In the food industry, marjoram is mainly used as a spice in sausages, but also its use in baked goods, processed vegetables, condiments, soups, snack foods and gravies is reported (Burdock, 1995). The main compounds of the essential oil of marjoram are the epimeric monoterpene alcohols trans-sabinene hydrate (2), cis-sabinene hydrate (1) and cis-sabinene hydrate acetate (3) (Scheme 1).Cis-sabinene
Scheme 1. Biosynthetic`routea to the formation oftrans-sabinene hydrate and cis-sabinene hydrate ((Hallahan and Croteau, 1988), modi"ed and conversion ofcis-sabinene hydrate tocis-sabinene hydrate acetate added by the author). (1)cis-sabinene hydrate, (2)trans-sabinene hydrate, (3)cis-sabinene hydrate acetate, (4) sabinene.
hydrate is an intensively spicy,&marjoramy'aroma compound, whereastrans-sabinene hydrate has no typical&marjoramy'properties (Franz, 1990; Lossner, 1968; Oberdieck, 1981). Higher amounts of these monoterpenes within the genus Origanum were reported for Origanum heracleoticum (Akgul and Bayrak, 1987), O. majorana var. tenuifolium(Arnold et al., 1993), Origanum ramonese(Danin et al., 1997), Origanum husnucan-baserii(Baser et al., 1998) andOriganum microphyllum(Gotsiou, 1999). The often-cited higher contents of terpinen-4-ol,a-terpinene andc-terpinene in the
essen-tial oil (examples cited in Lawrence, 1994,1997) are due to rearrangements during the distillation process (Fischer et al., 1988,1987).
Table 1
Accessions ofOriganum majoranaand their provenance
Accession Name Provenance
01 &Marcelka' Slovakia
02 &Erfo' N.L.Chrestensen GmbH, Erfurt, Germany
03 &Boilier' N.L.Chrestensen GmbH, Erfurt, Germany
04 &LOT 1' SchaKfer, GoKttingen, Germany
05 &Pfann' Pfann, Germany
06 &Nutting Sweet' Nutting, UK
07 &FranzoKsischer Stauden (MAJ 2/83)' H. Mette and Co., Quedlinburg, Germany, pro-vided by the Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany
08 &Maggiorana gentile (MAJ 11/92)' Northern Italy, provided by the Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany
09 &50846', Germany Federal Centre for Research on Agriculture, Braunschweig, Germany
10 &50847'Egypt Federal Centre for Research on Agriculture, Braunschweig, Germany
11 &Franz. Sommer' Geissler GmbH, Wals, Austria
12 &Origanum majorana' Jelitto, Schwarmstedt, Germany
13 &Franz. Maj.' Austrosaat, Vienna, Austria
14 &Sweet Marjoram' Richters & Sons Ltd., Goodwood, Ontario, Cana-da
15 &C' France
16 &G' France
17 &Marcelka'! Slovakia
18 &SAC 1' Nat. Collection England
19 &SAC 8' Nat. Collection England
20 &Schweiz' Seeds trade, provided by MAWEA GmbH,
Aschersleben, Germany
!Di!erent provenance from Accession no. 1.
an intermediate in this process and therefore not correlated to the biosynthesis of sabinene hydrate.
The aim of this study therefore, was to prove the distinct ratio in vivo and compare the results ofOriganum majoranawith results of another species of the genusOriganum containingtrans- andcis-sabinene hydrate (in this caseOriganum microphyllum).
2. Material and methods
2.1. Samples of Origanum majorana
2.2. Samples of Origanum microphyllum
155 single plants ofOriganum microphyllumdescending from four populations from Crete, Greece (Selakano (&S6'), n"35; Oropedio Lassithiou (&O14'), n"48; and
Tripiti (&T2'),n"62) were grown in the greenhouse at the Institute of Applied Botany
from seeds collected from their natural habitats. Herbarium specimens from the natural habitats of these populations were deposited at the Herbarium of the Mediter-ranean Agronomic Institute of Chania (No 5241 (Selakano), 5242 (Oropodio Lar-sithiou) and 5240 (Tripiti)). Samples were taken approximately three weeks before blooming and dried at 353C.
2.3. Extraction
0.2 g of the dried herb of each plant was extracted with 1.5 ml of dichloromethane for 15 min in an ultrasonic bath. To avoid di!erences in the composition based on di!erent leaf positions and therefore on the age of the essential oil as has been observed in marjoram especially for cis-sabinene hydrate acetate (Hallahan and Croteau, 1988), only the #ower heads and the youngest leaves were used for the extracts.
2.4. Analyses
GC/MS-analyses were performed on an HP 6890 coupled with an HP 5972 MSD and "tted with an HP 30 m]0.25 mm capillary column coated with HP-5MS (0.25lm "lm thickness). The analytical conditions were: carrier gas He, injector temperature 2503C, split ratio 50 : 1, temperature programme 60 to 2403C with 33C min~1. Components were identi"ed by comparing retention indices (Kovats index) and the spectra to data in the literature (Adams, 1995; McLa!erty, 1989).
2.5. Statistics
Three ratios were calculated: ratio 1 (sabinene/(cis-sabinene hydrate#cis-sabinene
hydrate acetate)), ratio 2 (trans-sabinene hydrate/cis-sabinene hydrate) and ratio 3 (trans-sabinene hydrate/(cis-sabinene hydrate#cis-sabinene hydrate acetate)). The
di!erences among accessions and populations were calculated using ONEWAY-ANOVA with subsequent multiple-range test (modi"ed LSD (Bonferroni)) using SPSS for Windows 6.0.
3. Results and discussion
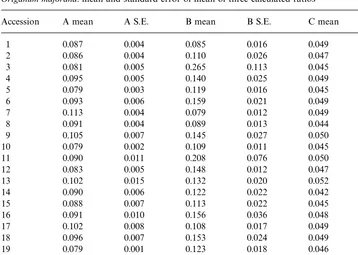
3.1. Origanum majorana(Table 2)
Table 2
Origanum majorana: mean and standard error of mean of three calculated ratios
Accession A mean A S.E. B mean B S.E. C mean C S.E.
1 0.087 0.004 0.085 0.016 0.049 0.001
2 0.086 0.004 0.110 0.026 0.047 0.002
3 0.081 0.005 0.265 0.113 0.045 0.001
4 0.095 0.005 0.140 0.025 0.049 0.002
5 0.079 0.003 0.119 0.016 0.045 0.001
6 0.093 0.006 0.159 0.021 0.049 0.001
7 0.113 0.004 0.079 0.012 0.049 0.002
8 0.091 0.004 0.089 0.013 0.044 0.006
9 0.105 0.007 0.145 0.027 0.050 0.003
10 0.079 0.002 0.109 0.011 0.045 0.001
11 0.090 0.011 0.208 0.076 0.050 0.002
12 0.083 0.005 0.148 0.012 0.047 0.002
13 0.102 0.015 0.132 0.020 0.052 0.006
14 0.090 0.006 0.122 0.022 0.042 0.005
15 0.088 0.007 0.113 0.022 0.045 0.001
16 0.091 0.010 0.156 0.036 0.048 0.002
17 0.102 0.008 0.108 0.017 0.049 0.002
18 0.096 0.007 0.153 0.024 0.049 0.002
19 0.079 0.001 0.123 0.018 0.046 0.001
20 0.084 0.004 0.114 0.021 0.042 0.005
Total 0.091 0.002 0.135 0.009 0.047 0.001
A*ratio of sabinene to (cis-sabinene hydrate#cis-sabinene hydrate acatete). B*ratio oftrans-sabinene hydrate tocis-sabinene hydrate.
C*ratio oftrans-sabinene hydrate to (cis-sabinene hydrate#cis-sabinene hydrate acetate).
exact (S.D."0.12) and similar to the &random' ratio of sabinene to cis-sabinene
hydrate (S.D."0.16). Ifcis-sabinene hydrate acetate is included and added to
cis-sabinene hydrate, the variability of the ratio of&trans- tocis-'observed was extremely small (S.D."0.01) compared to the ratio of sabinene to (cis-sabinene hydrate#
cis-sabinene hydrate acetate) and the other ratios. Furthermore, the mean values of this ratio, listed by accession, were extremely similar in contrast to the other two ratios. But the ratio is not found at 1 : 10 as observed by Hallahan and Croteau (1988) in vitro, but at 1 : 20, with the exception of one sample from accession no. 13 at 1 : 10 and two samples from the accessions 8 and 20 containingtrans-sabinene hydrate only in traces. These three distinct deviations from the supposed ratio are not in concordance with the hypothesis of only one enzyme involved in forming both isomers and strongly indicate a di!erent genetical background of these three samples. Another possibility could be the assumption of three slightly di!erent enzymes responsible fortrans- and cis-sabinene hydrate (&chemotypes'), two of them occurring extremely rarely.
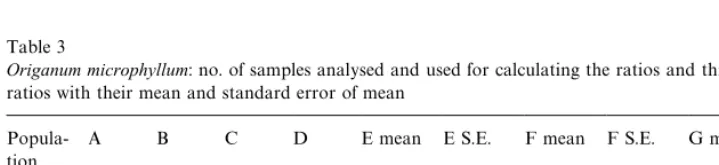
Table 3
Origanum microphyllum: no. of samples analysed and used for calculating the ratios and three calculated ratios with their mean and standard error of mean
Popula-tion
A B C D E mean E S.E. F mean F S.E. G mean G S.E.
S6 35 11 11 24 1.085 0.176 13.211 3.624 11.857 3.638
O14 58 0 0 58 0.757 0.046 1.665 0.535 1.554 0.515
T2 62 7 7 55 0.676 0.042 5.153 1.151 4.986 1.14
Total 155 18 18 137 0.782 0.042 5.088 0.879 4.737 0.862 A*number of total analyses.
B*number of samples withcis-sabinene hydrate not present. C*number of samples withcis-sabinene hydrate acetate not present.
D*number of samples used for calculating the ratios E, F and G (only possible where (cis-sabinene hydrate#cis-sabinene hydrate acetate'0).
E*ratio of sabinene to (cis-sabinene hydrate#cis-sabinene hydrate acetate). F*rato oftrans-sabinene hydrate tocis-sabinene hydrate.
G*ratio oftrans-sabinene hydrate to (cis-sabinene hydrate#cis-sabinene hydrate acetate).
selection process by man over the centuries. During this period, marjoram was cultivated in Europe, with wild populations of marjoram only due to gardens escapes (Adler et al., 1994), conserving consciously or unconsciously valuable or random traits in a very homogeneous way.
Regarding the di!erences in the ratios oftrans:cisof 1 : 10 in the enzyme prepara-tions (Hallahan and Croteau, 1988) and 1 : 20 in the plant extracts of this work, it is interesting to note, that also a pentane extraction of young leaves (Hallahan and Croteau, 1988) yielded a ratio of trans-sabinene hydrate to (cis-sabinene hy-drate#cis-sabinene hydrate acetate) of approximately 1 : 20, whereas the enzyme
preparations from these plants yielded a ratio of 1 : 10. Although the evidence of one enzyme is very strong (con"rmed by cochromatography, di!erential thermal inactiva-tion, proteolysis and inhibition studies (Hallahan and Croteau, 1988), only the puri"cation of the enzyme(s) to homogeneity or, on the other hand, inheritance studies (as e.g. done for the bisaboloids in chamomile (Horn et al., 1988)) would deliver the de"nite evidence for one or two enzymes being responsible in the formation of trans-sabinene hydrate andcis-sabinene hydrate.
3.2. Origanum microphyllum (Table 3)
InO. microphyllum, a strong dependency of the ratio on the sampled populations was observed. In general, O. microphyllumcontained more trans-sabinene hydrate thancis-sabinene hydrate, with the exception of population&O14', where this has been observed for approximately 25% of the population only. The other part of population
acetate were only observed in trace amounts with trans-sabinene hydrate being present in percentages clearly above 1%, as has been also observed by Baser et al. (1998) forOriganum husnucan-baserii. Although the populations ofO. microphyllum showed high variability regarding the calculated ratios, population&S6'was signi" -cantly di!erent from the other two populations. In all the populations, no strict ratio could be observed as was found inO. majorana, leading again to the assumption of independent inheritance and therefore indicating the presence of more than one enzyme in the biosynthesis oftrans-sabinene hydrate andcis-sabinene hydrate, respec-tively. Due to the fact that most research work in otherOriganumspecies has been done starting from the essential oil and the known enormous rearrangements during the distillation process (Fischer et al., 1987,1988), they are not directly comparable with these results. Southwell and Sti!(1990) observed di!erent ratios ofcis: trans-sabinene hydrates of ethanolic extracts of#ush growth of two di!erent Melaleuca species. InM. alternifoliathey found a ratio ofcis:transat 7.1 : 1 and inM. linariifolia a predominanttrans isomer (cis:trans at 0.65 : 1). They argued that the di!erences between the species may be caused by the use of di!erent precursors, since Hallahan and Croteau (1989) observed in marjoram a cis:trans-ratio of 10 : 1 when using (3R)-linalyl pyrophosphate and 0.8 : 1 when using (3S)-linalyl pyrophosphate as substrate. In contrast toOriganum majorana,Melaleuca alternifoliaandM. linariifolia (Southwell and Sti!, 1990), O. microphyllumwas extremely variable in the ratio of trans:cis-sabinene hydrate, not only between, but also within the populations exam-ined.
Acknowledgements
Special thanks to Dr. C.B. Johnson for valuable discussions. This research was
"nanced by the European Commision in the FAIR program, project no. CT96 1914.
References
Adams, R.P., 1995. Identi"cation of Essential Oil Components by Gas Chromatography/Mass Spectro-scopy. Allured Publishing Corporation, Carol Stream IL.
Adler, W., Oswald, K., Fischer, R., Fischer, M.A., Knab, O., HoKrandl, E., Franz, W.R., Grims, F., Schubert, B., Speta, F., Walter, J., Maurer, W., Starlinger, F., Englmaier, P., 1994. Exkursions#ora von OGsterreich. Eugen Ulmer, Stuttgart.
Akgul, A., Bayrak, A., 1987. Constituents of essential oils from Origanum species growing wild in Turkey. Planta Med. 53, 114.
Arnold, N., Bellomaria, B., Valentini, G., Arnold, H.J., 1993. Comparative study of the essential oils from three species of Origanum growing wild in the eastern mediterranean region. J. Essent. Oil Res. 5, 71}77. Baser, K.H.C., KuKrkcuKoglu, M., Duman, H., Aytac, Z., 1998. Composition of the essential. oil ofOriganum husnucan-baseriiH.Duman, Z.Aytac et A.Duran, a new species from Turkey. J. Essent. Oil Res. 10, 419}421.
Burdock, G.A. (Ed.), 1995. Fenaroli'Handbook of Flavor Ingredients. Vol. I*Natural Flavors. CRC Press, Boca Raton.
Fischer, N., Nitz, S., Drawert, F., 1987. Original#avour compounds and the essential oil composition of Marjoram (Majorana hortensisMoench). Flavour Frag. J. 2, 22}61.
Fischer, N., Nitz, S., Drawert, F., 1988. Original composition of marjoram#avor and its changes during processing. J. Agric. Food Chem. 36, 996}1003.
Franz, Ch., 1990. Sensorial versus analytical quality of marjoram. Herba Hung. 29, 79}86.
Gotsiou, P., 1999. Variation of the volatile compounds ofOriganum microphyllum. Master Thesis, Mediter-ranean Agricultural Institute of Chania, Greece.
Hallahan, T.W., Croteau, R., 1988. Monoterpene biosynthesis: demonstration of a geranyl pyrophos-phate:sabinene hydrate cyclase in soluble enzyme preparations from sweet marjoram (Majorana horten-sis). Arch. Biochem. Biophys. 264, 618}631.
Hallahan, T.W., Croteau, R., 1989. Monoterpene biosynthesis: mechanism and stereochemistry of the enzymatic cyclization of geranyl pyrophosphate to (#)-cis- and (#)-trans-sabinene hydrate. Arch. Biochem. Biophys. 269, 313}326.
Horn, W., Franz, Ch., Wickel, I., 1988. Genetics of bisaboloids in chamomile. Plant Breeding 101, 307}312. Lawrence, B., 1994. Progress in essential oils*Marjoram oil. Perfum. Flavorist 19, 39}40.
Lawrence, B.M., 1997. Progress in essential oils*Marjoram oil. Perfum. Flavorist 22, 49}56. Lossner, G., 1968. Der Majoran*phytochemisch betrachtet. Planta Med. 16, 54}57. McLa!erty, F.W., 1989. Wiley Registry of Mass Spectral Data. Wiley, New York.
Oberdieck, R., 1981. Ein Beitrag zur Kenntnis und Analytik von Majoran (Majorana hortensisMoench.). Deutsch. Lebensm. Rundsch. 77, 63}74.
Southwell, I.A., Sti!, I.A., 1990. Di!erentiation between Melaleuca alternifolia and M. linariifolia by monoterpenoid comparison. Phytochemistry 29, 3529}3533.