(
Medicago sativa
L.) AS CLOROPHIL SOURCES
Perkecambahan In Vitro dan Mikropropagasi Alfalfa (Medicago sativa L.)
Sebagai Sumber Klorofil
Fitrahtunnisa*, Sajimin** and Selvia Dewi Anomsari***
*Assessment Institute for Agricultural Technology (AIAT) West Nusa Tenggara Jln . Paninjauan Narmada – 83371
**Indonesian Center for Animal Research and Development (ICARD) ***Assessment Institute for Agricultural Technology (AIAT) Central Java
E-mail : fit_biotek@yahoo.co.id
ABSTRAK
Alfalfa (Medicago sativa L.) dikenal sebagai tanaman penghasil klorofil yang berkhasiat menyembuhkan berbagai penyakit. Biji Alfalfa yang diproduksi di Indonesia tidak memiliki embrio dan tidak bisa berkecambah, sehingga biji masih harus diimpor. Salah satu cara yang dapat digunakan untuk menghasilkan benih alfalfa dalam jumlah besar dan relatif cepat adalah dengan menggunakan teknik mikropropagasi. Penelitian dilakukan untuk mengem-bangkan teknik germinasi (perkecambahan) dan mikropropagasi alfalfa secara in-vitro. Perkecambahan dilakukan dengan menggunakan dua teknik yang berbeda. Pertama, benih steril direndam dalam 50 mg/l GA3, dan kedua tanpa direndam GA3. Benih dikecambahkan pada media MS yang mengandung 0 atau 0,5 mg/l BA dikombinasikan dengan 0,1, 0,5 atau 1 mg/l GA3. Inisiasi dan mutiplikasi tunas dilakukan pada media MS yang mengandung 0, 0,5 atau 1 mg/l BA dikombinasikan dengan 0, 0,1 dan 0,5 mg/l Th menggunakan eksplan epikotil. Variabel yang diukur adalah tinggi tanaman, jumlah daun dan jumlah tunas. Sedangkan inisiasi kalus dilakukan pada media MS yang mengandung 0, 1 atau 3 mg/l 2.4D dan dikombinasikan dengan 0, 3 atau 5 mg/l Pic menggunakan eksplan hipoko-til. Variabel yang diukur adalah panjang, lebar dan tebal kalus. Hasil penelitian menunjukkan bahwa rata-rata perkecambahan biji yang direndam dengan GA3pada media yang berbeda berkisar antara 60-87,5%, sedangkan tanpa direndam GA3 berkisar antara 4-75%. Rata-rata perkecambahan benih paling tinggi diperoleh dari biji yang direndam dengan GA3 pada media MS tanpa BA dan GA3 (kontrol). Media terbaik untuk induksi dan multiplikasi pucuk eksplan epikotil adalah MS + 1 mg/l BA. Sedangkan media terbaik untuk induksi kalus adalah MS + 3 mg/l Pic.
Kata kunci: Medicago sativa L., perkecambahan, mikropropagasi.
ABSTRACT
Alfalfa (Medicago sativa L) known as chlorophyll producing plants are efficacious cure for various diseases. Alfalfa seeds produced in Indonesia do not have embryos and could not germinate. So seeds are still to be imported. One
way that can be used to produce alfalfa seeds in large quantities and relatively fast is the micropropagation tech
-niques. An experiment was carried out to develop technique for in-vitro germination and micropropagation of al
The seed germination rate with GA3 soaked on MS medium without BA and GA3 (control) was the highest. The
best medium for induction and shoot multiplication of epycotyl
is MS+1 mg/l BA. While the best medium for callus induction was MS+3 mg/l Pic.
Key words: Medicago sativa L., germination, micropropagation.
INTRODUCTION
Alfalfa(Medicago sativa L.) is legume
that is efficacious to treat cancer, diabetes, lu -pus, and hepatitis. Alfalfa known as a producer of chlorophyll is also used as a dietary
supple-ment. Chlorophyll is an organic molecule in
plants. Its structure such as hemoglobin at hu-mans, can increase the body resistance.
Alfal-fa also contains carotenoids, acidic acids, fla -vonoids, phyto estrogen, and saponins. Other
benefits of alfalfa are as feed of cattle and oil producer (Horne, 2010). Alfalfa has high pro
-tein, energy, vitamins, minerals, and effective fiber. Its composition of amino acids is bal
-anced (Gonzalez et al., 2001).
Alfalfa propagation is by seed. In tropi-cal regions such as Indonesia, alfalfa plants do
not produce seeds. According to Mercer (1943)
and Hirnyck et al. (2004), in order to produce alfalfa seeds, pollination of flowers need the
help of insects such as the honey bee, Apis mel-lifera, as apollinator. In addition, alfalfa plants are less developed in the tropics due to pests, diseases, and weeds (Ashigh et al., 2009). To
prevent and address pest problems, diseases and weeds, cultivation of alfalfa should be very concerned about pesticides being used. This
is related to the quality and safety of alfalfa
plants that will be consumed by humans as a drug or a source of chlorophyll, or as animal feed. If these issues are not addressed, it will
reduce the quality of the protein of alfalfa to 9%.
Propagation through in vitro culture can
be done in three ways, namely the formation of adventitious shoots, lateral shoots
prolifera-tion and somatic embryogenesis. Proliferaprolifera-tion
costs in order to obtain fast multiplication of shoots.
Each shoots produced can be used as a source for further multiplication, so obtain a lot of
shoots in a relatively short time (Kosmiatin et al., 2005). According to Mariska and Sukm -adjaja (2003) propagation by in vitro culture
technique is much faster than the conventional
way. Additionally, this technology also ensures uniformity, disease-free and cheaper freight costs.
The success of in vitro propagation of plants through shoot multiplication, organo-genesis, and somatic embryogenesis is strongly
influenced by genotype and explant, basic me -dia types, as well as the type and concentration
of used growth regulator hormones (Hutami et
al., 2002; Liz and Levith, 1997). The purpose of
this study is to obtain a method of in vitro ger-mination of alfalfa seed, and the formulation of appropriate media for shoot induction and multiplication.
MATERIALS AND METHOD
The study was conducted at the Tissue
Culture Laboratory of Research Group of Biol
-ogy Cell and Tissue, Indonesian Agency for Ag
-ricultural Research and Development, Ministry of Agriculture from February to October 2009. Alfalfa seeds were sterilized using 70% alco
-hol for 5 minutes, 0.02 ppm HgCl2 for 2 min,
30% clorox for 10 minutes and 20% clorox for 5 min, andsteriledistilled water and antiseptic
solution as a rinse.
In vitro germination
Alfalfa seed were cultured in two
tech-niques. First, the seeds were soaked in GA3 50mg/l; second, the seeds were not soaked in GA3. Then, seeds were germinated on MS medi
-um containing 0 or 0.5 mg/l BA and combined with 0.1, 0.5 or 1 mg/l GA3. Observations were
Shoot induction and multiplication
The plant material used as explants in this study was epycotyl and hypocotyl. Epycot-yl and hypocotEpycot-yl of normal seedling were cut
along 1 cm and cultured on shoot regenera -tion medium. Epycotyl of normal seedling was
cultured on MS medium containing 0 , 0.5 or 1 mg/l BA combined with 0, 0.1 or 0.5 mg/l Th. Variables measured were plant height, leaves
and shoots numbers. While the initiation of callus was done on MS medium combined with
0, 1 or 3 mg/l 2.4D and 0, 3, or 5 mg/l Pic. The
variables measured were length, width, and thick of callus.
MS medium (Murashige and Skoog,
1962) used equipped with 3% sucrose (w/v), and made solid by adding 0.2% (Phytagel Gel
-rite). pH of media was made 5.8 by adding 1 N NaOH or 1 N HCl before autoclaved at temper
-ature of 121oC for 15 minutes. Cultures were incubated at 25 ± 2°C under fluorescent light of 1.000-2.000 lux for 16 hours.
RESULT AND DISCUSSION
In vitro germination
Seed germination by in vitro can be per-formed on the appropriate medium for germi-nation.
This technique is often used for plants or seeds that is difficult to germinate at starting with a
high risk of miscarriage of embryo.
The first series of seeds germination were soaked with GA3, while the second series seeds was without soaked on GA3. Seeds then
were germinated on MS medium added with 0
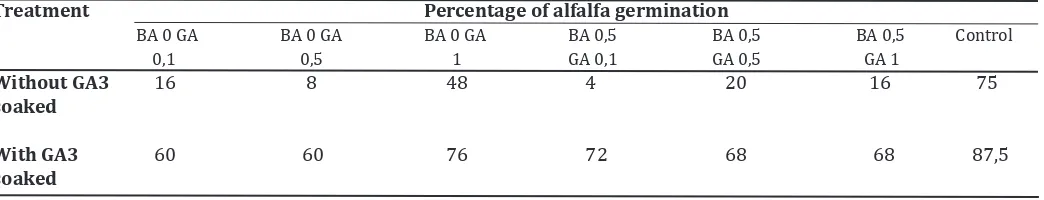
or 0.5 mg/l BA and combined with 0.1 , 0.5 or 1 mg/l GA3. The results showed that the addition of BA and GA3 on the medium in which both of seeds soaked or not soaked GA3 did not result better germination than controls (Table 1). Ko -smiatin et al. (2005) showed that the addition of GA3 in the medium only slightly increased
the percentage of germination of agarwood. The addition of cytokinin with strong activity (BA) could also improve germination, although
it was only 25%. Media without vitamins could
improve germination and reduce the formation
of abnormal sprouts (Kosmiatin et al., 2005).
The media was also successful for germinating vanilla beans (Mariska et al., 1998).
Table 1. Average of alfalfa seeds germination on MS with and without GA3 soaked Treatment Percentage of alfalfa germination
BA 0 GA BA 0 GA BA 0 GA BA 0,5 BA 0,5 BA 0,5 Control 0,1 0,5 1 GA 0,1 GA 0,5 GA 1
Without GA3 16 8 48 4 20 16 75 soaked
With GA3 60 60 76 72 68 68 87,5 soaked
Shoot induction and multiplication
Multiplication of alfalfa using seeds in
Indonesia produces flower without seeds. In
vitro propagation can use various explants. Explants used in this study was epycotyl and
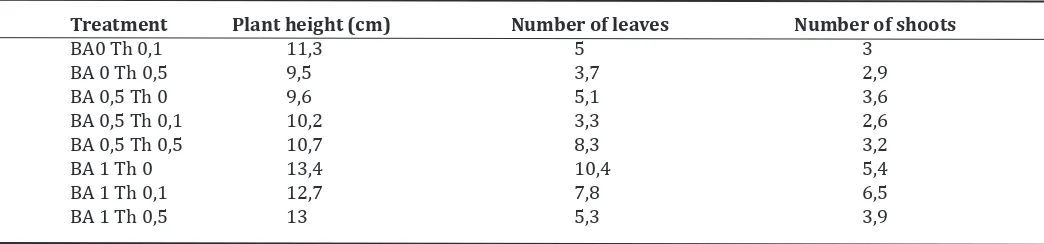
The addition of BA and Th did not give a
significant difference on plant height and num -ber of shoots produced. However, the num-ber
of leaves produced by the addition of BA 1 mg/l
resulted in plants with more leaves in the
in-duction and shoot multiplication. Kosmiatin et al. (2005) stated that the addition of BA 1 mg/l
resulted the highest number of shoots with
the fastest time of shoot induction. Research
in Malaysia showed that MS medium plus BA gave the best shoot multiplication (Normah
et al., 1995). However Goh et al. (1990), men -tioned that the best shoot multiplication was
obtained from medium WPM + BA 5 mg/l. The
results of the study in Solok showed that
spray-ing the source of explants (shoot) with BA 0.5 ppm + 1 ppm GA3 before cultured, gave the highest number of growing explants (42%),
whereas explants from cotyledons provided more leaves than shoot buds (Triatminingsih
et al., 2001).
Shoot induction using hypocotyl did not give better results than using epycotyl ex-plants. Shoots induction using hypocotyl
pro-duced callus at first so that the shoots induc -tion process was longer. Shoot induc-tion using hypocotyl explants were listed in Table 3.
Table 2. Plant height, leaves and shoot number of induction and multiplication of shoots using epycotyl explants.
Treatment Plant height (cm) Number of leaves Number of shoots
BA0 Th 0,1 11,3 5 3
BA 0 Th 0,5 9,5 3,7 2,9
BA 0,5 Th 0 9,6 5,1 3,6
BA 0,5 Th 0,1 10,2 3,3 2,6
BA 0,5 Th 0,5 10,7 8,3 3,2
BA 1 Th 0 13,4 10,4 5,4
BA 1 Th 0,1 12,7 7,8 6,5
BA 1 Th 0,5 13 5,3 3,9
Table 3. Induction of shoots by using hypocotyl explant.
Treatment Callus size
Length (mm) Height (mm) Width (mm)
2,4D0 Pic3 12,9 4,6 6
2,4D0 Pic5 13,1 3,6 5,1
2,4D1 Pic3 13,1 4,2 5,2
2,4D1 Pic5 13,5 3,7 4,3
2,4D3 Pic0 11,7 2,7 3,6
2,4D3 Pic3 12,2 2,4 3,3
2,4D3 Pic5 11,8 2,2 3,3
Table 3 shows that length, width and
thickness of callus were not significantly dif -ferent. But after combined, size of the largest
callus was obtained from the addition Pic3 mg/l into MS medium(Figure 1). The greater concentration of 2,4D + Pic into the media, the less callus was formed. In vitro Callus induc -tion in wheat showed that the concentra-tion
and type of hormones used greatly affected
the ability of explants to form callus or tissue
(Rashid et al., 2002;Satyavathi et al., 2004).
REFERENCES
Ashigh J., Craig M. and Lauriault L. 2009. Managing weeds in alfalfa. Guide A-325.
N.M. State University. Mexico. http://
aces.nmsu.edu/pubs/a/a.325pdf. Cited 19 Mei 2014.
Goh HKL., Rao AN. and Loh CS. 1990. Direct
shoot bud formation from leaf explants of seedlings and mature mangosteen (Garcinia mangostana L.). Annals of Bot-any, 62:87-93.
Gonzalez J., Varia-Marmol J.,Rodriguez CA. and Alvir MR. 2001. Effects of stage harvest
on the protein value of fresh lecurne for ruminants. Reprod. Nutr. Dev., 41:
381-392.
He GY. and Lazzeri PA. 2001. Improvement of
somatic embryogenesis and plant
re-generation from durum wheat (Triticum
turgidum var. durum Desf.) scutelum
and inflorescence cultures. Euphytica,
119:369-376.
Hirnyck L., Downey L. and Coats SO.2004. Pest
Management Strategic Plant for Produc -tion. Western Alfalfa Seed/Clover Seed PMSP.
Horne, S. 2010. Alfalfa (Medicago sativa).
Na-tures Field 23 (6): 1. http://www. treelite.com/articles/search?search_Q uery=alfalfa+(Medicago+sativa). (Cited on Mei 19, 2014)
Hutami S., Mariska I., Purnamaningsih R.,
Her-man M., Damayanti D. and Utami TI.
2002. Regenertion of papaya (Carica
papaya) through somatic embryogen-esis. Proc.
The 2nd Indonesian Biotechnology Con
-ference. Indonesian Biotechnology Con -sorcium, Jakarta.
Kosmiatin M., Husni A. dan Mariska I. 2005. Perkecambahan dan perbanyakan ga -haru secara in vitro. Jurnal Agrobiogen,
1(2):62-67.
Litz RE.and Levith Y.1997.Effect of 1-amino cy clopropane-1-carbolic acid, amine -othoxivinilglycine, methylgluxolatbis-(gluanylhydroane) and dicyohexiamo-nium sulfat on induction of embryogenic compotence of mango nuclear explants. Plant Cell Tiss. Org. Cult., 6:171-176.
Mariska I. and Sukmadjaja D. 2003. Kultur jar
ingan abaka. Balai Penelitian Biote
-knologi dan Sumber daya Genetik Per -tanian.
Mariska I., Hobir, Mulya K., Husni A.,
Kosmiatin M and Rusyadi Y. 1998. Pe -nyelamatan embrio
hasil persilangan antara panili hasil bu-didaya dan panili liar. Laporan Hasil Pe-nelitian. Balitbio.
Mercer RD. 1943. Alfalfa Seed Production. Bulletin No.218.Montana State College
and United States. Department of Ag-riculture. Distributed in furtherance of
the Acts of Conggress. Montana. Normah MN., Noor-Azza AB dan Aliudin R.
1995. Factors affecting in-vitro prolif-eration and ex-vitro establishment of mangosteen. Plant Cell Tiss. Org. Cul.,
43(3): 291-294.
Rahmayanti E dan Sitanggang M. 2006. Takluk
kan penyakit dengan klorofil alfalfa. PT. Agromedium Pustaka. Jakarta.
Rashid H., Ghani RA and Chaudhry Z. 2002. Effect of media, growth regulator and
genotipes on callus induction and
re-generation in wheat (Triticum aestivum
L.). Biotechnology, 1(1):49-54.
Satyavathi VV., Jauhar PP. and Elias EM. 2004. Effects of growth regulator on in vitro
plant regeneration in durum wheat.
He and Lazzeri (2001) research showed
that the addition of auxin in the medium did
not significantly affect embryogenesis. But the effect on regeneration, in which the culture
was induced in media containing picloram showed a higher regeneration compared to
. Crop Sci., 44:1839-1846.