The relationship between plasma glucose and insulin responses to
oral glucose, LDL oxidation, and soluble intercellular adhesion
molecule-1 in healthy volunteers
N.G. Chen, S. Azhar, F. Abbasi, M. Carantoni, G.M. Reaven *
Department of Medicine,Stanford Uni6ersity School of Medicine,Stanford,CA, USA
Received 3 March 1999; received in revised form 16 September 1999; accepted 3 November 1999
Abstract
This study was initiated to describe the relationships between plasma glucose and insulin responses to oral glucose and the concentrations of partially oxidized low density lipoprotein (poxLDL) and soluble intercellular adhesion molecule-1 (sICAM-1) in 23 healthy, non-diabetic volunteers. Results demonstrated that plasma glucose (r=0.65, PB0.002) and insulin (r=0.58,
PB0.007) responses to a 75-g oral glucose challenge were highly correlated to poxLDL concentrations. Plasma glucose (r=0.63,
PB0.002) and insulin (r=0.68, PB0.001) concentrations also significantly correlated with sICAM-1 concentrations. Further-more, concentrations of poxLDL and sICAM-1 were significantly related (r=0.55, PB0.001). These relationships remained statistically significant when adjusted for differences in age, gender, body mass index, and lipoprotein concentrations. These results provide further evidence that circulating LDL particles are more highly oxidized in insulin resistant states, and demonstrate the presence of an in vivo relationship between insulin resistance, LDL oxidized state, and sICAM-1 concentrations. These results help explain why soluble forms of adhesion molecules are increased in clinical conditions characterized by insulin resistance, and support the possibility that LDL oxidizability is increased in insulin resistant subjects, and that the increase in sICAM-1 results from stimulation of cellular adhesion molecules by more highly oxidized LDL. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Insulin resistance; Glucose response; Insulin response; Low density lipoprotein oxidizability; sICAM-1
www.elsevier.com/locate/atherosclerosis
1. Introduction
We have recently described a significant relationship between resistance to insulin-mediated glucose disposal and partially oxidized low density lipoprotein (LDL) particles in healthy volunteers [1]. A similar relationship was also seen in this population between partially oxi-dized LDL particles and the common consequences of insulin resistance-increased plasma glucose and insulin responses to an oral glucose challenge, and the combi-nation of higher plasma triglyceride (TG) and lower high density lipoprotein (HDL) cholesterol concentra-tions. Several lines of evidence have been summarized [2,3] suggesting that insulin resistance, and/or its
conse-quences, play a major role in the pathogenesis of coro-nary heart disease (CHD). Further support for this notion can be derived from our recent finding that insulin resistance, as well as its surrogate marker, hy-perinsulinemia, predicted the development of CHD in a prospective study of healthy volunteers [4]. Given the presumed central role of oxidized LDL in the initiation and propagation of the process of atherogenesis [5], the possibility exists that insulin resistance and/or hyperin-sulinemia increases risk of CHD by virtue of its ability to enhance LDL oxidation. In this context, in vitro evidence that oxidized LDL increases the expression of various adhesion molecules on cultured endothelium [6 – 9] provides a cellular mechanism that might link insulin resistance to enhanced atherogenesis. In support of this notion is the evidence that soluble forms of these adhesion molecules have been reported to occur in a variety of clinical conditions [10 – 16], associated with * Corresponding author. Present address: Shaman
Pharmaceuti-cals, Inc., 213 East Grand Avenue, South San Francisco, CA 94080-4812, USA. Tel.: +1-650-952-7070; fax: +1-650-873-8377.
E-mail address:[email protected] (G.M. Reaven).
both increased risk of CHD and insulin resistance [2,3]. Based upon these considerations, the current study was initiated to explore the relationship between plasma glucose and insulin responses to oral glucose (surrogate measures of insulin resistance), partially oxidized LDL, and the concentration of soluble intercellular adhesion molecule-1 (sICAM-1) in 23 healthy volunteers.
2. Methods
The study population consisted of 23 healthy volun-teers, eight men and 15 women, who responded to a newspaper advertisement indicating our interest in studying factors that modulate insulin action and CHD risk factors in healthy volunteers. The individuals se-lected for study were defined as healthy on the basis of medical history, physical examination, and normal re-sults from routine laboratory tests and electrocardiog-raphy and because they were found to be non-diabetic after a 75-g oral glucose load [17]. The study protocol was approved by the Stanford University Institutional Review Board, and written, informed consent was ob-tained from all subjects.
All studies were performed at the General Clinical Research Center of Stanford University Medical Cen-ter. Venous blood was drawn after an overnight fast for measurement of plasma cholesterol, triglyceride, and HDL cholesterol concentrations [18]. Plasma glucose [19] and insulin [20] concentrations were determined before and 30, 60, 90, 120, and 180 min after oral administration of 75 g glucose. The total integrated area of the plasma concentrations during this 180-min period was used to quantify plasma glucose and insulin responses.
An additional aliquot of fasting venous blood (20 ml) was also drawn from each individual into EDTA tubes and immediately centrifuged, plasma flushed with nitro-gen, and stored frozen at −70°C until used to measure degree of LDL oxidizability and concentration of sICAM-1 as described below. Partially oxidized (pox) LDL particles were quantified as described previously [1,21]. Briefly, LDL particles were isolated by sequential ultracentrifugation using solid KBr for density adjust-ment, dialyzed against PBS without EDTA, and the protein content determined by a modification of the method of Lowry et al. [22]. The susceptibility of LDL to oxidation was estimated as initially described by Picard et al. [22], and the validity of this approach has been discussed in detail in our recent publication [1]. Isolated LDLs are diluted in PBS to obtain a final concentration of 200 mg/ml, and oxidation initiated by adding a concentrated solution of copper sulfate to obtain a final concentration of 5 mmol/l. For each sample, the amount of conjugated dienes (CDs) formed during LDL oxidation was monitored every 5 min for 4
h by the change in absorbance at 234 nm in the presence or absence of 0.1 mmol/l of D,L alanine. Alanine inhibits LDL fatty acid oxidation, an effect dependent on the amount of peroxides present in LDL particles. Thus, the difference between the CD forma-tion half-time (time corresponding to half of the ab-sorbance) for the curve with and without alanine reflects LDL oxidative status, i.e. the greater the differ-ence, the greater the antioxidant effect of alanine on LDL, and the lower the oxidative state of the LDL. As indicated by Picard et al. [21], the increase in CD formation half-time induced by alanine can be ex-pressed as the alanine oxidation coefficient (AOC), which can be calculated as the percentage increase in CD formation due to alanine. As AOC is inversely correlated with the degree of LDL oxidation, Picard et al. expressed their results as the cAOC, which is equal to 150-AOC based on their experience that AOC never exceeded 150%. The cAOC varies in direct proportion to the degree of LDL oxidation. We determined cAOC, but for clarity of presentation, will refer to this value as the amount of partially oxidized LDL, or poxLDL. The term poxLDL is used to emphasize the fact that we have no information as to the biological effect of poxLDL, and to avoid any implication that what we are measuring has the biological effects of what has been designated as minimally oxidized LDL [22 – 24]. On the other hand, in our previous publication [1], we demonstrated that the measurements of poxLDL are highly reproducible, stable over time in storage, and that the values of poxLDL were significantly correlated with the lag phase after the addition of copper before any change in absorbance, the maximum CD forma-tion, and total lipid peroxide formation at 100 min. In addition, plasma concentrations of soluble ICAM-1 were determined using enzyme-linked immunoassay (ELISA) Kit (Immunotech, Marseille, France).
Results are expressed as mean9S.E.M. Statistical significance of the relationships between variables was determined by calculating correlation coefficients and by multiple regression analysis.
3. Results
The clinical and metabolic characteristics of the ex-perimental population are listed in Table 1. It can be seen that the values of all of these varied widely within the study group.
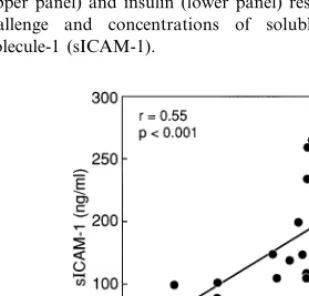
statisti-cally significant relationships remained between poxLDL and both the glucose (r=0.67,PB0.005) and insulin (r=0.59, PB0.03) responses to oral glucose. Fig. 2 illustrates the relationships between plasma glucose and insulin responses to oral glucose and con-centrations of sICAM-1. The simple correlation coeffi-cients, which were highly statistically significant between both glucose (r=0.63,PB0.002) and insulin (r=0.68,PB0.001) responses and sICAM-1, remained so when adjusted for differences in age, gender, BMI,
Fig. 2. Relationship between the total integrated plasma glucose (upper panel) and insulin (lower panel) responses to an oral glucose challenge and concentrations of soluble intercellular adhesion molecule-1 (sICAM-1).
Table 1
Clinical and metabolic characteristics (n=23)
Variable Value Range
5592 34–75 Age (years)
– 8/15
Gender (M/F)
20.4–33.0 27.390.8
BMI (kg/m2)
11.7–31.5 19.891.0
Glucose response (mmol/l per 3 h)
11579143 389–2549 Insulin response (pmol/l per 3 h)
135915
Triglyceride (mg/dl) 49–300
Total cholesterol (mg/dl) 194911 95–300 4493 28–86 HDL-cholesterol (mg/dl)
LDL-cholesterol (mg/dl) 123910 38–242 86–133
poxLDL 11593
sICAM-1 (ng/ml) 190910 120–280
Fig. 1. Relationship between the total integrated plasma glucose (upper panel) and insulin responses (lower panel) to an oral glucose challenge and concentration of partially oxidized low density lipo-protein (poxLDL).
Fig. 3. Relationship between concentrations of partially oxidized low density lipoprotein (poxLDL) and soluble intercellular adhesion molecule-1 (sICAM-1).
and lipoprotein concentrations, with values of r=0.71 and r=0.67 for the glucose and insulin responses, respectively.
In an effort to further define the relationships be-tween the experimental variables, multiple regression analysis was performed. The first model tested had poxLDL as the dependent variable, and is seen in Table 2. Since the plasma glucose and insulin responses to oral glucose are highly correlated, only the plasma insulin response was entered. These results demonstrate that the insulin response and poxLDL were signifi-cantly related, independent of differences in age, gen-der, BMI, and lipoprotein concentrations. An essentially similar result was seen when the insulin response was replaced with the glucose response. Fur-thermore, the r2 of the two models were essentially identical, i.e. 0.63 and 0.61, respectively.
The results of multiple regression analysis of the relationship between sICAM-1 and the metabolic vari-ables in Table 2 are shown in Table 3. These data show that insulin response was significantly related to sICAM-1 concentrations (PB0.001). Replacing the in-sulin response with the glucose response gave essentially the same result, with an r2 value of 0.70.
Since concentration of poxLDL was highly corre-lated with both the glucose and insulin responses, it was also substituted for the insulin response in the model shown in Table 3. These results are given in Table 4, and demonstrate that poxLDL was also significantly related to sICAM-1. Furthermore, the r2
of the model was quite similar to the value when either the insulin or the glucose response was the independent variable in the model.
Given the high degree of correlation between glucose response, insulin response and poxLDL, it was not surprising that when all three, or any two of the three, were simultaneously entered into the model shown in Table 3, none of them were significantly related to sICAM-1.
4. Discussion
The present study was initiated to further explore the relationship between insulin resistance and the oxidized state of circulating LDL, attempting to see if these variables were also related to plasma concentrations of sICAM-1. More specifically, we wished to confirm our previous finding that the more insulin resistant an individual, the more highly oxidized their circulating LDL particles [1], and to test the hypothesis that these changes would be associated with higher circulating levels of sICAM-1. Although the data presented provide support for both of these points of view, it must be understood at the outset that the fact that two variables are associated does not mean that there is a causal relationship between them. However, given this caveat, the results in Fig. 1 clearly demonstrate that the integrated plasma and glucose responses to oral glucose were highly correlated with our measurement of poxLDL. As such, these results are consistent with our previous finding that the plasma concentrations of poxLDL are increased in proportion to the degree of insulin resistance.
The conclusion that insulin resistance and the oxi-dized state of LDL are related is only justified if the measurements we made provide reasonable estimates of the variables in question. In the case of insulin resis-tance, there is evidence that this variable is highly correlated with measurements of the plasma glucose and insulin responses to oral glucose [25 – 27]. The evidence that our measure of poxLDL provides a meaningful estimate of the oxidized state of circulating Table 2
Multiple regression analyses of the relationship between poxLDL and metabolic variablesa
Variable Coefficient S.E. P
0.619
Age (years) 0.113 0.222
−4.236
Gender (M/F) 5.296 0.438
1.571
BMI (kg/m2) 0.776 0.074
−0.029
Triglyceride (mg/dl) 0.038 0.463 0.310 0.207
HDL-cholesterol (mg/dl) 0.158
0.074
LDL-cholesterol (mg/dl) 0.058 0.226 0.004
Multiple regression analyses of the relationship between sICAM-1 and metabolic variablesa
Coefficient S.E.
Variable P
−0.494 0.807 0.551 Age (years)
Triglyceride (mg/dl) 0.266
0.286 HDL-cholesterol (mg/dl) 0.833 0.749
0.250
LDL-cholesterol (mg/dl) 0.211 0.258 0.016
0.052 B0.001
Insulin response
ar2=0.67.
Table 4
Multiple regression analyses of the relationship between sICAM and other metabolic variables replacing insulin response with poxLDLa
Coefficient S.E. P
Variable
−0.873
Age (years) 0.872 0.335
18.467 Triglyceride (mg/dl)
−0.228
HDL-cholesterol (mg/dl) 0.733 0.761 LDL-cholesterol (mg/dl) −0.025 0.206 0.905
poxLDL 2.043 0.795 0.023
LDL particles is not as well-established, and was dis-cussed in detail in our previous paper [1]. Briefly, the theoretical basis of the measure is reasonable, and both we [1] and Picard et al. [20] have shown it to be reproducible. Furthermore, this is now the third study in which the poxLDL measurements have been shown to be highly related to relevant biological events. There-fore, it seems reasonable to suggest that measurements of poxLDL provide an estimate of the oxidized state of circulating LDL.
The relationships shown in Fig. 2 are less ambiguous, and demonstrate highly significant relationships be-tween the plasma glucose and insulin responses to oral glucose and plasma sICAM-1 concentrations. Further-more, the relationship shown in Fig. 2 appeared to be independent of any of the variables known to be associ-ated with insulin resistance. Results of previous studies have shown that adhesion molecules were elevated in patients with type 2 diabetes, hypertension, and hyper-triglyceridemia [10 – 16]. Since insulin resistance is com-mon in these three conditions [2,3] it could be argued that the defect in insulin action is sufficient to lead to an increase in the plasma concentration of soluble adhesion molecules, and these changes can be seen in the absence of hyperglycemia, hypertension, or hyper-triglyceridemia. On the other hand, these additional metabolic abnormalities may also directly stimulate ex-pression of cellular adhesion molecules and/or mononu-clear cell binding as has been shown with hyperglycemia in in vitro studies [28].
The fact that the surrogate measures of insulin resis-tance used in this study correlated with both poxLDL and sICAM-1 raises the possibility that poxLDL and sICAM-1 are also highly related. The evidence in Fig. 3 demonstrates that this is the case, and the relationship remained when adjusted for differences in age, gender, BMI, and lipoprotein concentrations. The fact that poxLDL and sICAM-1 are highly correlated is consis-tent with previous in vitro data showing that oxidized LDL stimulates expression of various cellular adhesion molecules. On the other hand, it is important to again emphasize that the presence of statistically significant correlations does imply causality.
In conclusion, the results presented provide further evidence that circulating LDL particles are more highly oxidized in insulin resistant individuals. It was hypothe-sized that this change in LDL state would stimulate the expression of ICAM-1, consistent with results of in vitro studies showing that oxidized LDL stimulates expression of various cellular adhesion molecules [6 – 9], thereby increasing the plasma concentration of sICAM-1. Demonstration of highly significant relationships be-tween surrogate measures of insulin resistance, poxLDL, and sICAM-1 does not establish the causal relationships that we have postulated, but the consis-tency of the experimental data certainly provides ample support for it.
Acknowledgements
This work was supported by Research Grants (RR-00070 and HL-08506) and Training Grant (HL-07708) from the National Institutes of Health.
References
[1] Carantoni M, Abbasi F, Warmerdam F, Klebanov M, Wang P-W, Chen Y-DI, et al. Relationship between insulin resistance and partially oxidized LDL particles in healthy, non-diabetic volunteers. Arterioscler Thromb Vasc Biol 1998;18:762 – 7. [2] Reaven GM. Role of insulin resistance in human disease.
Dia-betes 1988;37:1595 – 607.
[3] Chen Y-DI, Reaven GM. Insulin resistance and atherosclerosis. Diabetes Rev 1992;5:332 – 42.
[4] Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998;83:2773 – 6.
[5] Chisolm GM, Penn MS. Oxidized lipoproteins and atherosclero-sis. In: Fuster V, Ross R, Topol EJ, editors. Atherosclerosis and coronary artery disease. Philadelphia, PA: Lippincott-Raven, 1996:129 – 49.
[6] Jeng J-R, Chang C-H, Shieh S-M, Chiu H-C. Oxidized low-den-sity lipoprotein enhances monocyte-endothelial cell binding against shear-stress-induced detachment. Biochem Biophys Acta 1993;1178:221 – 7.
[7] Cominacini L, Garbin U, Pasini AF, Davoli A, Campagnola M, Contessi GB, et al. Antioxidants inhibit the expression of inter-cellular cell adhesion molecule-1 and vascular cell adhesion molecule-1 induced by oxidized LDL on human umbilical vein endothelial cells. Free Radic Biol Med 1997;22:117 – 27. [8] Cominacini L, Garbin U, Pasini AF, Paulon T, Davoli A,
Campagnola M, et al. Lacidipine inhibits the activation of the transcription factor NF-kappa B and the expression of adhesion molecules induced by pro-oxidant signals on endothelial cells. J Hypertens 1997;15:1633 – 9.
[9] Erl W, Weber PC, Weber C. Monocytic cell adhesion to en-dothelial cells stimulated by oxidized low density lipoprotein is mediated by distinct endothelial ligands. Atherosclerosis 1998;136:297 – 303.
[10] Cominacini L, Pasini AF, Garbin U, Davoli A, De Santis A, Campagnola M, et al. Elevated levels of soluble E-selectin in patients with IDDM and NIDDM: relation to metabolic control. Diabetologia 1995;38:1122 – 4.
[11] Lip GYH, Blann AD, Zarifis J, Beevers M, Lip PL, Beevers DG. Soluble adhesion molecule P-selectin and endothelial dysfunction in essential hypertension: implications for atherogenesis? A pre-liminary report. J Hypertens 1995;13:1674 – 8.
[12] Ceriello A, Falleti E, Bortolotti N, Motz E, Cavarape A, Russo A, et al. Increased circulating intercellular adhesion molecule-1 levels in type II diabetic patients: the possible role of metabolic control and oxidative stress. Metabolism 1996;45:498 – 500. [13] Desouza CA, Dengel DR, Macko RF, Cox K, Seals DR.
Elevated levels of circulating cell adhesion molecules in uncom-plicated essential hypertension. Am J Hypertens 1997;10:1335 – 41.
[14] Abe Y, El-Masri B, Kimball KT, Pownall H, Reilly CF, Os-mundsen K, et al. Soluble cell adhesion molecules in hyper-triglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol 1998;18:723 – 31.
[16] Abe Y, El-Masri B, Kimball KT, Pownall H, Reilly CF, Os-mundsen K, et al. Soluble cell adhesion molecules in hyper-triglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol 1998;18:723 – 31.
[17] National Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039 – 57.
[18] Jeppesen J, Zhou M-Y, Chen Y-DI, Reaven GM. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care 1994;17:1093 – 9. [19] Kadish AH, Litle RH, Sternberg JC. A new and rapid method
for determination of glucose by measurement of rate of oxygen consumption. Clin Chem 1968;14:117 – 31.
[20] Hales CN, Randle PJ. Immunoassay of insulin with insulin-anti-body precipitate. Biochem J 1968;88:137 – 46.
[21] Picard S, Talussot C, Serusclat A, Ambrosio N, Berthezene F. Minimally oxidized LDL as estimated by a new method. In-crease in plasma of type 2 diabetic patients with atherosclerosis or nephropathy. Diabetes Metab 1996;22:25 – 30.
[22] Markwell MAK, Hass SM, Bieber LL, Tolbert NE. A modifica-tion of the Lowry procedure to simplify protein determinamodifica-tion in membrane and lipoprotein samples. Anal Biochem 1978;87:206 – 10.
[23] Berliner JA, Territo MC, Sevanian A, Ramin S, Kim JA,
Bamshad B, et al. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest 1990;85:1260 – 6.
[24] Cushing SD, Berliner JA, Valentine AJ, Territo MC, Navab M, Parhami F, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA 1990;87:5134 – 8.
[25] Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 1990;344:254 – 7. [26] Hollenbeck CB, Chen N, Chen Y-DI, Reaven GM. Relationship
between the plasma insulin response to oral glucose and insulin-stimulated glucose utilization in normal subjects. Diabetes 1984;33:460 – 3.
[27] Reaven GM, Hollenbeck CB, Chen Y-DI. Relationship between glucose tolerance, insulin secretion, and insulin action in non-obese individuals with varying degrees of glucose tolerance. Diabetologia 1989;32:52 – 5.
[28] Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, et al. Leukocyte-endothelial interaction is aug-mented by high glucose concentrations and hyperglycemia in an NF-kB-dependent fashion. J Clin Invest 1998;101:1905 – 15.