Non-equilibrium alcohol flooding model
for immiscible phase remediation: 2.
Model development and application
Stanley Reitsma* & Bernard H. Kueper
Department of Civil Engineering, Queen’s University, Kingston, Ontario, Canada K7L 3N6
(Received 16 October 1996; revised 9 June 1997; accepted 28 July 1997)
A non-equilibrium, two-phase, three-component compositional model for the simulation of alcohol flooding has been developed and tested. Inter-phase mass transfer algorithms allow for transfer of all three components at high concentrations and high mass flux rates using a two-film model. The model has been used to simulate alcohol floods where the alcohol has an affinity for either the water-rich phase, or the organic-rich phase. Calibration, using experimental effluent data from an alcohol flood which used a 2-propanol (IPA)–water–tetrachlorethene (PCE) ternary system, indicates that inter-phase mass transfer parameters can be non-unique. Sensitivity studies, completed using the non-equilibrium model for the IPA–water–PCE system, indicate that experimentally derived organic-rich phase composition data should lead to better estimates of the non-wetting phase film thickness. For alcohol flooding experiments where the primary mechanism of non-aqueous phase liquid (NAPL) removal is enhanced dissolution, near-equilibrium conditions may be achieved with NAPL recovery similar for conditions of near-equilibrium and equilibrium. However, for systems where remobilization is the primary mechanism of NAPL recovery, it is expected that although local conditions may approach equilibrium, the resulting NAPL recovery can be significantly lower than would be attained if equilibrium conditions persisted.q1998 Elsevier Science Limited. All rights reserved
Key words: alcohol flooding, non-equilibrium, multicomponent, mass transfer, DNAPL, compositional, remediation.
1 NOMENCLATURE
A1 fitting parameter for specific interfacial area [-] Ah Hand Plot fitting parameter [-]
Bh Hand Plot fitting parameter [-]
C1 constant greater than 1.0 that dictates the rate of time increment increase [-]
ct molar density [mol m¹3]
ctw,B* molar density of the wetting phase being injected at the boundary node [mol m¹3
]
~
Dib dispersion tensor for component i in phase ([m 2
s¹1] Eh Hand Plot fitting parameter [-]
Fh Hand Plot fitting parameter [-]
Iib inter-phase mass transfer of component i to or from phaseb[mol m¹3s¹1]
q1998 Elsevier Science Ltd
Printed in Great Britain. All rights reserved 0309-1708/98/$19.00 + 0.00
PII: S 0 3 0 9 - 1 7 0 8 ( 9 7 ) 0 0 0 2 5 - 0
663
*Corresponding author. Presently at University of Windsor, Department of Civil and Environmental Engineering, Windsor, ON, Canada, N9B 3P4
~
k intrinsic permeability tensor [m2]
˜
kjk harmonic mean of the permeability [m2]
ko mass transfer ratio [m-2]
krb Relative permeability to phaseb[-]
[kb*] finite flux mass transfer coefficient matrix [m s¹1] lb film thickness in phaseb[m]
m fitting parameter for the change in krwwith change in interfacial tension [-]
N denotes time step
Ni molar flux of component i [mol m¹2s¹1] Nt total molar flux [mol m¹2s¹1]
NI basis functions nc number of components nn number of nodes np number of phases Pd displacement pressure [Pa]
Pw* specified wetting phase pressure at the boundary node [Pa]
2 INTRODUCTION
Compositional simulators were initially developed in the petroleum industry to assist in the analysis and design of
enhanced oil recovery systems. One of the first
compositional models61 was used to simulate
one-dimensional condensing gas drive systems while accounting for two-phase flow with equilibrium inter-phase mass transfer of three components. Further development of
compositional models were given in Refs.11,12,15,42,59.
Ref.28 developed a fully three-dimensional three-phase
flow model (oil–water–gas) with capillary flow, gravi-tational forces, equilibrium inter-phase mass transfer, and transport of any number of oil components in both the oil and gas phases. A four-phase compositional model (UTCHEM) has been developed which can handle up to
19 components including water, oil, surfactant,
polymer, alcohol, air, and various electrolytes and tracers.9 UTCHEM’s model capabilities for modeling enhanced
oil recovery have been demonstrated by various
authors.5,13,47
Compositional models have also been developed for the simulation of non-aqueous phase liquid (NAPL) dissolution in groundwater systems. Both equilibrium, e.g. Refs.17,27,38, and non-equilibrium, e.g. Refs.10,30,39–41,52, models have been presented by various authors. Different approaches for describing the mass transfer coefficients have been used and have been the focus of much research. Simplified
NAPL blob geometry has been used in Ref.39to calculate
specific interfacial area. Certain authors have presented mass transfer coefficients based on a ‘two-domain’ approach where a part of the NAPL can dissolve rapidly
and the remaining NAPL dissolves slowly, e.g. Ref.30.
Others have derived empirical relationships for mass transfer coefficients based on experimental data, e.g. Ref.40. All the non-equilibrium models developed for the simulation of NAPL dissolution adopt a single-film linear driving force conceptual model for the calculation of rate-limited inter-phase mass transfer.
Recently, compositional models have been utilized to simulate the use of alcohol flooding to remediate sites contaminated by NAPLs. These models have utilized the assumption of local equilibrium to describe inter-phase mass transfer, e.g. Refs.7,49. In cases where significant inter-phase mass transfer of all components can occur, the single-film model may be inadequate. Through
experi-mentation, Ref.25 demonstrates that non-equilibrium
conditions may occur during an alcohol flood. Ref.25
presents expressions, based on development in Ref.26, for calculating a lumped mass transfer coefficient to describe
non-equilibrium inter-phase mass transfer. These
expressions assume that the non-aqueous phase is immobile, that insignificant amounts of alcohol and water transfer into the organic-rich phase, and that the mass transfer rate is proportional to the concentration gradient of the organic between the bulk water-rich phase and the interface between the phases.
The purpose of this paper is to present a one-dimensional control volume finite element (CVFE) numerical model designed to simulate the recovery of non-aqueous phase liquids from porous media using alcohol flooding. The developed simulator accounts for migration of both the wetting and non-wetting phase, and accounts fully for non-equilibrium mass transfer in either direction between the phases for a three-component system using a two-film model.
Qb,B volume flux representing the amount of phase (leaving the boundary cell from the boundary [m3s¹1] qib source or sink of component i in phaseb[mol m3s] ri fitting parameter to characterize the rate of change of Srw
with change in interfacial tension [-]
Srw0 irreducible wetting phase saturation for the two-phase system containing no alcohol [-]
Sb fraction of void space occupied by phaseb[-].
DSwt target wetting phase saturation change in a time step [-]
DSwmax maximum change in wetting phase saturation over previous time step [-]
T temperature [K]
t time [s]
Vi molar volume of component i at its normal boiling point [m3mol¹1]
vb flux of phaseb[m s¹1] WI a very large number (1020) [-]
x*I mole fraction at the interface chosen to calculate the equilibrium composition at the interface [-]
xib* specified mole fraction of component i in phasebat a boundary node [-]
xib mole fraction of component i in phaseb[-] xibb mole fraction of component i in bulk phaseb[-] xibI mole fraction of component i in phasebat the phase
interface [-]
DxT target change in mole fraction from one time step to the next [-]
Dxbmax maximum change in composition in the entire domain from one time step to the next [-]
Greek symbols
aL longitudinal dispersivity [m]
gIJ represents the area available to flow divided by the flow path length between nodes I and J multiplied by the medium’s permeability [m3]
gIJ9 represents the area available to flow divided by the flow
path length between nodes I and J multiplied by a dispersion term [m3s¹1]
hc set of nodes connected to node I
l pore size distribution index [-]
mb viscosity of phaseb[Pa s]
rb mass density of phaseb[kg m¹3]
j0 interfacial tension for the two-phase system containing no alcohol [N m¹1]
f porosity of the medium [-]
Ji association factor for component i
wb fluid potential in phase (expressed as pressure) [Pa] Subscripts
B indicates a boundary node
i component: 1-water, 2-organic, and 3-alcohol
3 NUMERICAL MODEL DEVELOPMENT
3.1 Governing equation discretization
Assuming that the solid phase is inert and immobile, the partial differential equations governing isothermal multi-phase flow with multicomponent transport in porous media are given by:1,3
]
]t(ctbfSbxib)þ=:(ctbxibvb)¹=:[fSb ~
Dib=(ctbxib)]¹qib
¹Iib¼0 b¼1:::np, i¼1:::nc ð1Þ
where npis the number of phases,bis the phase of interest,
nc is the number of components, i is the component of
interest, ctbis the molar density of phaseb, Sbis the
frac-tion of void space occupied by phaseb,fis the porosity of the medium, vb is the flux of phase b, xib is the mole
fraction of component i in phase b, D~ib is the dispersion tensor for component i in phaseb, qibis a source or sink of
component i in phaseb, and Iib represents the inter-phase
mass transfer of component i to or from phaseb.
Using the CVFE method presented in Refs.18,20 and a
lumped mass time derivative, the final discretized equations representing eqn (1) are given by
fSbctbxib
where krb is the relative permeability to phaseb,mbis the
viscosity of phase b, nn is the number of nodes, gIJ
represents the product of a permeability term and the cross-sectional area available for flux divided by the distance between nodes I and J, gIJ9 is the product of a
dispersion term and the cross-sectional area available to flux divided by the distance between nodes I and J, and
wbis the fluid potential in phaseb. The termDt represents
the time increment, N þ 1 denotes values at the present
time step, and N denotes values at the previous time step. Subscript I represents the node of interest, I, while subscript J represents all nodes connected to node I. The term hc
represents the set of nodes connected to I such that J[hc. The volume associated with node I, VI, is given by
VI¼ Z
VNIdV (3)
where NIare Lagrange polynomial C0basis functions, e.g.
Ref.24. The term gIJis given by
where the harmonic mean permeability,k˜jk, is given by
˜
kjk¼
2kIjkkJjk
kIjkþkJjk
(5)
The advective flux terms are taken as the upstream weighted value to give
ctbxib
where PbIis the pressure in phasebat node I. Subscript IJ
þ1/2 represents the midpoint between nodes I and J. The
mass density,rb, and the molar density, ctb, are taken as the
central weighted value between nodes I and J such that
r
where Dibjk is the dispersion term for component i in phase b, and where the term(Sb)NIJþþ11
The advective flux, source/sink, and inter-phase mass trans-fer terms are all dealt with implicitly. The non-advective flux term is dealt with by combining both implicit and explicit terms as indicated by eqn (9).
conditions of local non-equilibrium, a fine grid spacing is required to capture many of the physical processes occurring during the flood. In other words, numerical dispersion as a result of upstream weighting of the advection terms is likely to be of less significance than hydrodynamic dispersion, therefore making it unnecessary to use a more complex spatial weighting scheme for one-dimensional
simulations. Due to computer memory and speed
limitations, higher order weighting schemes and/or flux limiting may be required for two- or three-dimensional simulations.
3.2 Numerical solution scheme
Due to the highly non-linear nature of the governing equations, full Newton–Raphson iteration is used to solve the discretized equations. The resulting sparse Jacobian matrix is solved using a direct tri-diagonal block solver.
The presence of either a single phase or two phases at a node will dictate its nodal state. For nodes containing only wetting phase (state 1), the primary variables at that node are the wetting phase pressure, Pw, the mole fraction of
organic in the wetting phase, x2w, and the mole fraction of
alcohol in the wetting phase, x3w. For nodes containing two
phases (state 2), the primary variables are Pw, x2w, x3w, the
wetting phase saturation, Sw, the mole fraction of water in
the non-wetting phase, x1nw, and the mole fraction of alcohol
in the non-wetting phase, x3nw.
For one-phase nodes that are adjacent to nodes containing two phases, a check is made at the beginning of an iteration to determine if the wetting phase at the single phase node is miscible with the non-wetting phase at the two-phase node. If these phases are immiscible, the single-phase node is given a state of 2 and the wetting phase saturation is set equal to 1.0. If, at the end of an iteration, the wetting phase saturation remains equal to 1.0 at that node, i.e. no non-wetting phase has entered that cell from an adjacent two-phase cell, the state at that node is reset to 1. The mass balance equations for the three components in the non-wetting phase are not independent if inter-phase mass transfer, at the node being invaded by the non-wetting phase, is equal to zero. Invoking the assumption that inter-phase mass transfer is zero at the node being invaded by the non-wetting phase allows for a simplifi-cation of the mass balance equations at that node. The three mass balance equations for the components in the non-wetting phase are summed together. The primary variables at the node being invaded by non-wetting phase then become Pw, x2w, x3w, and Sw. If no
inter-phase mass transfer occurs at the node being invaded, the composition of the non-wetting phase at that node will be equivalent to the non-wetting phase composition at the upstream node.
The mass conservation equations for each component in each phase (eqn (2)) can be rewritten as a series of equations in the form F(x)¼0 to given nc3np3nn independent
equations (note exception above)
Rbi,I; fSbctbxib
The independent equations are ordered into a vector of functions (F) as follows:
(F)T¼(R1,1w ,Rw2,1,Rw3,1,Rnw1,1,Rnw2,1,Rnw3,1,:::,Rw
The unknown variables corresponding to this set of equations are ordered into a vector (x) as follows
(x)T¼(Pw,1,xw2,1,xw3,1,xnw1,1,Sw,1,xnw3,1,:::,Pw,n
n,
xw2,nn,xw3,nn,xnw1,nn,Sw,nn,xnw3,nn) ð13Þ
The ordering of the primary variables in (x) is done in such a way as to maximize diagonal dominance in the Jacobian matrix. If the nodal state is equal to 2, all Jacobian terms must be calculated by finding the partial derivatives of Rbi,I with respect to each primary variable. However, if the nodal state is equal to 1, only the partial derivatives of Rwi,I with respect Pw, xw2, and xw3 are required, since
Rnwi,I¼0 at these nodes. When the nodal state is equal to
1, the main diagonal values in the Jacobian matrix associated with the mass conservation equations of each component in the non-wetting phase are set equal to 1.0. This enables simple utilization of the tri-diagonal block
solver since, regardless of nodal state, a 6 3 6 block
structure is maintained throughout the Jacobian matrix with non-zero values on the main diagonal.
Numerical differentiation is used to calculate the Jacobian terms. Due to the nature of the constitutive equations used in the governing equations, the partial derivative terms are not easily determined and may require more computational time than would be required for numerical differentiation.19,56It is necessary to choose the correct incremental change in primary variable size for cal-culation of the Jacobian terms for two reasons. Firstly, too small an increment may lead to large numerical error which is especially important when certain secondary variables are calculated using iterative techniques such as the inter-phase mass transfer terms. Secondly, if a change in the primary variable causes a change in state at a particular cell, con-vergence will likely not occur.19It was found that absolute
values of DPw¼10
toDSwwas always negative so that SwþDSwa 1.0. The
sign assigned toDxibwas positive unless the mole fraction
of either the organic or the alcohol in the wetting phase, or the water or the alcohol in the non-wetting phase, approached the plait point. This would ensure that the algorithms for calculating the equations of state received appropriate input compositions.
At the completion of each Newton iteration, the state of each cell is determined. To switch from a state of 1 to a state of 2, either the composition of the single phase at that node must fall below the binodal curve and a second phase is precipitated or, as mentioned above, the non-wetting phase invades that node from neighboring nodes. If the switch is made due to precipitation of a second phase, the two new phases are assumed to be in equilibrium with an overall composition equivalent to that of the original single phase.
To switch from a state of 2 to a state of 1 at a particular node, either the composition of the two bulk phases at that node are miscible, or the wetting phase saturation is greater than 1 at the end of an iteration. If the switch is made due to miscibility of the two phases, the composition of the new single phase is equal to the overall composition of the two original phases. If the switch is made due to SwN
þ1
s1.0, the composition remains equal to the composition of wetting phase found at the end of the iteration, and the wetting phase saturation is set equal to 1.0.
Adaptive time stepping in a formulation similar to Ref.56 is used for calculation of the time increment at the present time step,DtNþ1, given by
whereDtNis the previous time step increment,DSw T
is the target change in wetting phase saturation, DSwmax is the
maximum change in wetting phase saturation in the entire
domain from time step N¹ 1 to time step N, DxTis the
target change in composition, Dxbmax is the maximum
change in composition in the entire domain from time
step N ¹ 1 to time step N expressed as a mole fraction,
and C1is a constant greater than 1.0 that dictates the rate of
time increment increase. Input target changes in saturation and composition represent the desired maximum changes of these variables in the entire domain from one time step to the next.
3.3 Boundary conditions
Incorporation of boundary conditions is completed using a
method similar to Ref.16. The boundary conditions are
imposed by adding source/sink terms to the boundary nodes. When the wetting phase pressure at the boundary node is constant, the boundary conditions are imposed by adding the source/sink terms to the water component mass balance equation in the wetting phase, as follows
q1w,B¼W1(Ppw¹Pw,B)x1w,Bctw,B (15)
where WI is a very large number, e.g. 1020, Pw* is the
specified wetting phase pressure at the boundary node, and subscript B indicates a boundary node. This ensures a very large number in the Jacobian matrix for change in pressure at the boundary node. When the ‘‘correction’’
matrix is solved, the change in Pw,B will be minimal.
Note that all the pressures, saturations, mole fractions, and molar densities in the boundary node source/sink terms are implicit and updated with each Newton iteration. For a constant composition at a boundary node, B, the source/sink terms for that node are given by
qib,B¼WI(xpib¹xib,B)ctb (16)
where xi*bis the specified mole fraction of component i in
phaseb. Composition in the wetting phase, the non-wetting phase, or in both phases may be specified at the boundary node using eqn (16). For the wetting phase, the mole frac-tion of organic and alcohol would be specified and for the non-wetting phase, the mole fraction of water and alcohol would be specified if the compositions in the respective phases are fixed.
When free exit boundary conditions are specified, the source/sink terms for the wetting phase mass conservation equations for the boundary nodes are given by
qiw,B¼ ¹Qw,Bctw,Bxiw,B (17)
where Qw,B is a volume flux representing the amount of
wetting phase leaving the domain at the boundary node, B. For inflow Cauchy-type boundary conditions, the source/ sink terms for the wetting phase mass conservation equations for the boundary nodes are given by
qiw,B¼ ¹Qw,Bcp
tw,Bxpiw,B (18)
where ctw,B* is the molar density of the wetting phase being
injected at the boundary node, B, and xiw,B* is the mole
fraction of component i in the injected wetting phase. The source/sink terms for the non-wetting phase equations for the boundary nodes, when a free exit boundary condition is specified, are given by
qinw,B¼
¹Qnw,Bctnw,Bxinw,B Qnw,Bs0
0 Qnw,Ba0
(
(19)
where Qnw,B is the volumetric flux of non-wetting phase
from the nodes connected to the boundary node.
3.4 Solution of inter-phase mass transfer equations
The equations used for calculation of the inter-phase mass transfer are developed in Ref.44. A two-film model for mass transfer calculations is used. The compositions at the interface between the two phases are assumed to be at equi-librium and diffusion is assumed to be the only mechanism
of mass transfer. Ref.55 recommends using Newton–
two-phase, three-component alcohol–water–organic system, the inter-phase mass transfer equations can be writ-ten as a series of equations in the form F(x)¼0 to give two equations for the molar fluxes in the wetting phase55
(Rw);ctw[k
where (Rw) is the residual vector for the wetting phase
inter-phase mass transfer equations, [kw
•
] is the finite flux mass transfer coefficient matrix, (xbw¹x
I
w) is the vector containing the differences in mole fractions of each com-ponent in the bulk wetting phase and at the interface in the wetting phase, Nt is the total molar flux, and (N) is the
vector of molar fluxes. Two equations for the molar fluxes in the non-wetting phase can be written as
(Rnw);ctnw[k
where the corresponding variables are defined as above. The equation set is completed using equations of state. The independent equations are ordered into a vector of functions (F) as follows (modified after Ref.55)
(F)T¼(Rw1,R
The unknown variables corresponding to this set of equations are ordered into a vector (x) as follows (modified after Ref.55)
(x)T¼(N1,N2,N3,x I
p) (23)
where x*I is one mole fraction at the interface. Note that for
a Type 1 ternary system, the equilibrium composition of the two phases are distinctly defined by the mole fraction of one component in one phase from which all other mole fractions can be determined. The mole fraction at the inter-face, x*I, has been chosen to be the mole fraction of water in
the wetting phase at the interface, x1wI , in this work.
The Newton–Raphson structure to eqn (20) and eqn (21) is given by
The Jacobian terms in eqn (24) are calculated numerically. Several terms in eqn (24) may be approximated as sug-gested by Ref.55to give
xb1w¹1 x
During development of the mass transfer algorithms, both numerically determined and estimated Jacobian terms have been used for solution to eqn (20) and eqn (21). The com-putational savings afforded by using the estimated Jacobian matrix were found to be offset by the additional iterations required for convergence and decreased algorithm robust-ness for the three-component systems tested here. While both methods may be used in the non-equilibrium model, all subsequent simulations shown here use the Jacobian matrix given by eqn (24).
4 MODEL TESTING
Due to the complexity of physical phenomena occurring during an alcohol flood and the non-linear equations required for numerical simulation of this system, only partial model verification can be accomplished. Partially verification was carried out using exact analytical solutions for two-phase flow33and for mass transport.21In both cases, agreement between the numerical model and the analytical solution was excellent. Both Cauchy-type inlet conditions and free exit boundary conditions were shown to be properly solved.
4.1 Mass transfer algorithm testing using Lewis Cell simulations
A series of simulations was conducted to test the developed inter-phase mass transfer algorithm using a configuration similar to the experimental apparatus employed in Ref.31, thus referred to as the Lewis Cell. The ternary systems used in the simulations were the 2-propanol (IPA)–water– tetrachlorethene (PCE) system shown in Fig. 1 and the 1-propanol–water–trichloroethene (TCE) system shown in
Fig. 1. Ternary phase behavior for the 2-propanol (IPA)–water–
tetrachlorethene (PCE) system with Hand Plot fit to experimental data from Ref.4. Several experimental tie line data have been
Fig. 2. The simulations, representing a closed system containing a water-rich phase and an organic-rich phase initially having non-equilibrium conditions, were completed using various different initial bulk phase compositions. The bulk phases were assumed to be well mixed. A constant film
thickness of 10¹4m for each phase was used in all
simulations.
The NRTL46thermodynamic properties model was used
for calculation of the diffusion coefficients for the IPA–
water–PCE system. The UNIQUAC2 thermodynamic
properties model was used for the 1-propanol–water–TCE system because it was found to be more robust than the NRTL model for this system. A set of thermodynamic para-meters for the two ternary systems can be found in Tables 4–8, below. A specific interfacial area of 10 m¹1was used in the simulations. This translates to a cube-shaped con-tainer with sides measuring 0.1 m, where the interfacial area between the two phases is equal to the cross-sectional area of the container. A simulation time of 105seconds was chosen to allow phases to approach equilibrium.
The results of three different simulations are shown in Fig. 3. These simulations were performed to illustrate pro-cesses occurring during inter-phase mass transfer for the IPA–water–PCE system. The figures present the initial phase compositions, the overall system composition, and the phase composition pathways followed during the simu-lations. The simulation represented in Fig. 3(a) was per-formed because it closely represents the introduction of an alcohol-rich wetting phase to a porous medium containing PCE. Note that the composition path of the organic-rich phase starts at pure PCE and follows along the binodal curve to its final equilibrium value. The water-rich phase composition path curves sharply rather than following a straight line to the final equilibrium composition. This illustrates the strong molecular interaction between the different components during diffusion.54
The simulation represented in Fig. 3(b) is initiated with a high alcohol concentration in the organic-rich phase. The composition path trends compare favorably to those
observed experimentally in Ref.29 for a similar system.
Again, curvature in the composition traces indicates molecular interaction during diffusion.
Fig. 3(c) is used to illustrate the composition path for initial phase compositions that both lie close to the binodal curve. It might be expected that the composition paths would follow a straight line from the initial composition to the final equilibrium composition. If this were to occur, either a water-rich phase would precipitate from the organic-rich phase, or the organic-rich phase would become super-saturated in response to the composition of the phase falling below the binodal curve. However, due to the change in diffusion coefficient with composition, the composition paths follow along the binodal curve such that neither phase becomes super-saturated.
Fig. 2. Ternary phase behavior for the 1-propanol–water–
trichlorethene system with Hand Plot fit to experimental data from Ref.35.
Fig. 3. Phase composition histories from Lewis Cell simulations
for: (a) high alcohol concentration in the water-rich phase, (b) high alcohol concentration in the PCE-rich phase, and (c) initial
The results of two additional Lewis Cell simulations are shown in Fig. 4. These were performed to illustrate pro-cesses occurring during inter-phase mass transfer for the IPA–water–TCE system. Fig. 4(a) illustrates a system where the organic-rich phase consists entirely of TCE and the water-rich phase contains large amounts of alcohol. The composition history illustrated in Fig. 4a closely resembles that which would occur during injection of an alcohol– water solution in a porous medium containing separate phase TCE. The alcohol transfers from the water-rich phase into the organic-rich phase, thus swelling the organic-rich phase. Note that the composition path of the organic-rich phase closely follows the binodal curve.
Fig. 4(b) illustrates a system where the organic-phase contains a large amount of alcohol and the water-rich phase is pure water. Alcohol transfers from the organic-rich phase to the water-organic-rich phase. This emulates mass trans-fer conditions at the tail end of an injected alcohol slug. Again, as for the IPA–water–PCE system, the composition paths follow close to the binodal curve if the original com-positions lie near the binodal curve. Note that only small differences in the overall composition cause large changes in the mass transfer direction.
4.2 Model calibration
Mass transfer coefficients are not available in the literature
for systems involving alcohol, water, and organic in porous media where significant amounts of alcohol partition into the organic-rich phase. Mass transfer coefficients may also vary dramatically from one system to another. Because of the large uncertainty associated with extrapolating the mass transfer coefficients determined for one system to another and the lack of published values, it is deemed necessary to determine these coefficients through model calibration.
The experimental data used for model calibration in this
study is taken from Ref.8. A laboratory experiment was
performed in a one-dimensional vertical glass column. The porous medium used in the experiment was made up of equal amounts by weight of 0.1 mm, 0.3 mm, and 0.5 mm diameter glass beads. The column had an inside diameter of 2.5 cm and the porous medium length ranged from 60 to 65 cm. For the experiment of interest, the porous medium length was assumed to be 62.5 cm. The porous medium was initially saturated with water. PCE was introduced into the top of the column at a gradient of 1.0 until approximately 1.5 pore volumes of PCE had been injected. Water was injected into the top of the column following the injection of PCE at a rate of 100 ml hr¹1and was continued until no more separate phase PCE exited the bottom of the column. Once residual PCE saturation was achieved, one pore volume of an IPA–water mixture, made up of 60% by volume IPA, was injected into the bottom of the column at a gradient of 0.3. The IPA–water mixture was followed by 3 pore volumes of water, also at a gradient of 0.3. Ref.8 states that a spike in the PCE effluent concentration at approximately 2.25 pore volumes could have been due to sample collection problems. As a result, these data points are not included in the calibration exercise.
Three parameters defining the mass transfer coefficients were determined by matching PCE effluent concentrations from the numerical simulations to those observed experi-mentally. The three fitted parameters include constants representing the film thickness in the wetting and non-wetting phase, lw and lnw, and the specific interfacial area
fitting parameter, A1. A non-linear least squares fitting
routine58was used to determine the best-fit parameters. The three-parameter calibration simulation was com-pleted using the modeling parameters, soil properties, fluid properties, Hand Plot fit, and NRTL parameters shown in Table 1, Table 2, Table 3 and Table 4. The longitudinal dispersivity,aL, has been set to 0.0 since numerical
disper-sion, caused by upstream weighting of the advection terms, results in dispersion of the concentration fronts. A uniform Fig. 4. Phase composition histories from Lewis Cell simulations
with initial phase compositions near the binodal curve for: (a) high alcohol concentration in the water-rich phase, and (b) high alcohol
concentration in the TCE-rich phase.
Table 1. Model parameters used in calibration simulations for the IPA–water–PCE system.
Nodal spacing 6.25310¹3
Maximum time step 100 s
Newton–Raphson convergence criterion
DPw 4.0 Pa
DSw 1.0310¹5
grid spacing equal to 6.25310¹3m resulted in numerical dispersion similar toaLequal to 0.01 m. This was
deter-mined by comparison of a single-phase one-component numerical simulation to a one-dimensional analytical solution for mass transport.34
Results from the three-parameter fit for two fitting runs, each using different initial estimates, are shown in Table 5. The high degree of correlation between the film thickness in the wetting phase and the specific interfacial area constant suggests that the chosen fitting parameters should be com-bined in some manner for this set of experimental data. It is also clear from Runs 1A and 1B that the confidence in the best-fit values is low since different initial guesses result in different best-fit values. This is likely due to the high degree of correlation between wetting phase film thickness and specific interfacial area. Different combinations of A1and
film thickness result in similar fits to the experimental data. To further illustrate the non-uniqueness of these two parameters, the fits from Run 1A and 1B are compared in Fig. 5. Although the values of lwand lnware very different
for the two simulations, the final effluent curve results are similar.
Because the best-fit values for the three fitting parameters are uncertain, four fitting runs were completed using A1as
the only fitting parameter while fixing lwand lnw. The film
thicknesses were assumed to be equal. Model input para-meters are shown in Table 1 Table 2 Tables 3 and 4. Runs 2
through 4 were completed to examine how A1varied as a
function lw and lnw. Run 5, using identical input as Run 2
except for the initial guess of A1, was completed to ensure
that the fitting run converged to similar best-fit values of A1.
A summary of the results for Runs 2 through 5 are shown in
Table 6. The results from these runs show that A1 is
proportional to lwand lnw, and that the value of A1is not a
function of the initial guess. A combination of mass transfer terms, defined as a mass transfer ratio, ko, and given by
ko¼ A1 lw
(26)
has been found to be constant regardless of the choice of lw,
given that lw and lnw are equal to each other. It is then
possible that the mass transfer ratio can be defined to adequately represent mass transfer for a specific system. Similar expressions for mass transfer coefficients have been found where interfacial area and film thickness are combined in single-film models, e.g. Refs.22,41.
It should be noted that in systems where significant trans-fer of all components takes place, the two-film model is required to properly account for chemical gradients that exist on either side of the NAPL–water interface. Com-bining mass transfer parameters removes a number of fitting parameters, but does not change the fact that a two-film approach is used for calculating inter-phase mass transfer. While a single-film linear driving force model may provide an adequate fit to experimental data in a system where significant inter-phase mass transfer of all components takes place, it is only through using a two-film modeling approach that mass transfer phenomena of all components will be captured properly. However, a single-film model may be appropriate in systems where one or more of the components does not experience significant inter-phase mass transfer.
Runs 1A and 1B are compared to Run 2 in Fig. 5. The three-parameter formulation does not show significant improvement in fit to the experimental data. The integral square error (ISE) is defined as48
ISE¼
where y represents the model output and y represents theˆ
observed experimental results. For the single-parameter fit from Run 2, the ISE is equal to 4.5%, and for the three-parameter fit from Run 1, the ISE is equal to 4.2%. The ISE increases only slightly with removal of two of the fitting parameters, further justifying the use of a single-parameter fit. For comparison, the ISE values for hydrograph Table 2. Fluid properties used in calibration simulations for the IPA–water–PCE system.
Molecular weight Water60 1.8310¹2kg mol¹1 Density Water14 997 kg/m3
PCE36 1.66310¹1kg mol¹1 PCE36 1610 kg/m3
IPA8 6.0096310¹2kg mol¹1 IPA8 781 kg/m3
Association parameter Water23 PCEa
2.26 1.0
Molar volume at normal boiling point (m3mol¹1)a
Viscosity of pure fluid Water14 8.9310¹4
Pa·s Solubility of water in PCE37 9.67310¹4
PCE36 9.0310¹4 Pa·s IPA60 2.09310¹3
Pa·s Solubility of PCE in water36(mole fractions) 1.62310¹5 a
Indicates estimated parameters.
Table 3. Porous medium properties used in calibration simu-lations for the IPA–water–PCE system
Srw Temparaturea 258C
a
Indicates estimated parameters. b
modelling48 can be examined to give an indication of the goodness of fit.
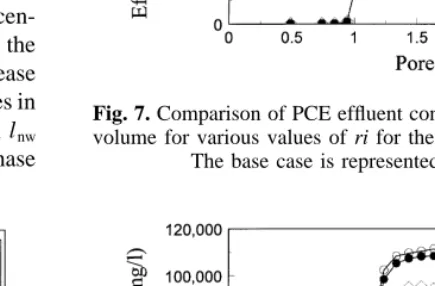
The values of m and ri, which influence the relative per-meability functions, are estimated in the model calibration. Fig. 6 illustrates the impact of changing m. It can be seen that the model output is relatively insensitive to this parameter. Fig. 7 illustrates the impact of changing ri. Although model output is more sensitive to ri than it is to m, model output is again quite insensitive to ri in the range tested.
The sensitivity of A1on model output is shown in Fig. 8.
The effluent concentrations are sensitive to the specific
interfacial area parameter, showing lower values in response to a decreased interfacial area available for mass transfer. There is a stronger response when the interfacial area is reduced by a factor of two than when it is increased by a factor of two. This indicates that the compositions of the phases near the exit of the column approached equilibrium conditions for the performed experiment. Had the column been shorter, or flow rates higher, this condition may not have occurred. Confidence in the predicted values of speci-fic interfacial area and film thickness is expected to decrease as the experimental conditions used in the calibration approach equilibrium.
4.3 Analysis of mass transfer parameters
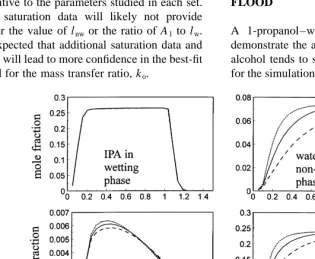
Several simulations were completed to determine what type of experimental data, in addition to effluent concentration measurements, may assist in uniquely determining the values of the specified mass transfer parameters. Using input parameters from Table 1, Table 2, Table 3, and Table 4, and the exact domain and boundary conditions adopted for simulation of the IPA–water–PCE alcohol flooding experiments,8two sets of runs were completed to study the affect of the mass transfer parameters on phase composition. One pore volume of an IPA–water solution (60% IPA by volume) was injected into the porous medium followed by several pore volumes of water.
For the first set of runs, the input value of lnwwas varied.
A base case was chosen using the best-fit and input parameters from Run 4 shown in Table 6. The film thickness was then multiplied by a factor of two and four. Results Table 4. Hand Plot and NRTL parameters used in calibration simulations for the IPA–water–PCE system
Hand Plot fitting parameters43 Ah1 0.0917 Eh1 5.659
Ah2 2.308 Eh2 0.5707
Bh1 ¹3.654 Fh1 1.857
Bh2 ¹1.590 Fh2 0.6380
NRTL parameters53Units oftin Kelvin t(1,2) 1877.8 t(1,3) 319.11
t(2,1) 708.87 t(2,3) 328.53
t(3,1) ¹280.94 t(3,2) ¹100.12
a(i,j) 0.2 for i,j¼1,2,3(iÞj)
Table 5. Results of the three-parameter fit to the IPA–water–PCE flooding experiment
Fitted parameter Initial guess Best-fit Standard deviation
Run 1A Run 1B Run 1A Run 1B Run 1A Run 1B
lw(m) 5.18310¹4 4.16310¹4 4.34310¹3 1.97310¹5 3.77310¹3 2.36310¹4 lnw(m) 2.72310¹5 4.00310¹3 2.66310¹4 3.23310¹3 9.93310¹5 7.45310¹4 A1(m¹1) 1.283103 1.43103 1.163103 1.493103 1.033103 1.783103 Correlation matrices
lw lnw A1
Run 1A Run 1B Run 1A Run 1B Run 1A Run 1B
lw 1.0 1.0
lnw ¹0.577 ¹0.134 1.0 1.0
A1 0.959 0.999 ¹0.366 ¹0.133 1.0 1.0
Fig. 5. Comparison of numerical simulation results and
experi-mental results8for PCE effluent concentration versus number of pore volumes injected. For Runs 1A and 1B the best-fit parameters include separate values for lw, lnw, and A1. For Run 2, the best-fit parameters include only A1with lwand lnwset equal to 1310
from these simulations are shown in Fig. 9 and Fig. 10. Because the composition profiles for the wetting phase are similar for each simulation, it can be concluded that the
non-wetting phase film thickness cannot be accurately
determined given the wetting phase composition over the length of the domain at a certain time in the experiment (Fig. 9), or at a specific location over the course of the experiment (Fig. 10). However, if the composition in the
non-wetting phase is determined, lnw may be determined
with greater confidence since the compositional profiles are sensitive to lnw. The confidence in best-fit values for
lnwshould increase for experimental data collected from a
system where stronger non-equilibrium conditions persist. The non-wetting phase compositions presented in Fig. 10 show significant variation with changing lnw. For example,
the mole fraction of water in the non-wetting phase is essen-tially doubled at early times when the non-wetting film
thickness is decreased by a factor of 4 from 23 10¹3m
to 5 3 10¹4m. As the non-wetting film thickness is
decreased, the non-wetting phase approaches equilibrium more rapidly. As is shown in Fig. 10, the concentrations of both water and alcohol increase more rapidly in the
non-wetting phase with decreased lnw when in contact
with an alcohol-rich wetting phase. When alcohol concen-trations in the wetting phase decrease at the end of the alcohol slug, concentrations of alcohol and water decrease more rapidly with decreased lnw. The composition profiles in
the wetting phase are not influenced by the changes in lnw
because of the relatively large volume of wetting phase
present and the relatively small volumes of alcohol and water that are being transferred to the non-wetting phase.
The second set of sensitivity runs was completed using different values of A1 and lwwhile maintaining the mass
transfer ratio given by eqn (26). lnwwas set at 1310
¹3m
for the three simulations. It can be concluded from the results shown in Fig. 11 that additional composition information for both phases gathered during experimenta-tion will likely not provide the informaexperimenta-tion necessary to uniquely define lwand A1since very little variation in the
compositional profiles occurs with changing values of these two parameters.
Table 6. Results of the one-parameter fit to the IPA–water–PCE flooding experiment.
Run # lw& lnw(m) A1 Standard
deviation (m¹1)
ko¼A13lw¹1 (m¹1) Initial guess Best-fit value
(m¹1) (m¹1)
2 1310¹3 3.153102 3.163102 1.013102 3.163105
3 2310¹3
5.553102 6.313102 2.023102 3.163105
4 5310¹4
1.63102 1.583102 5.063101 3.163105
5 1310¹3 1.03102 3.163102 1.013102 3.163105
Fig. 6. Comparison of PCE effluent concentration versus injected
volume for various values of m for the IPA–water–PCE system. The base case is represented by m¼0.01.
Fig. 7. Comparison of PCE effluent concentration versus injected
volume for various values of ri for the IPA–water–PCE system. The base case is represented by ri¼0.05.
Fig. 8. Sensitivity of PCE effluent concentration to the specific
Saturation profiles for the above two sets of sensitivity runs were insensitive to the parameters studied in each set. Thus additional saturation data will likely not provide insight into either the value of lnwor the ratio of A1to lw.
However, it is expected that additional saturation data and composition data will lead to more confidence in the best-fit value determined for the mass transfer ratio, ko.
5 SIMULATION OF A SWELLING ALCOHOL FLOOD
A 1-propanol–water–TCE system has been chosen to demonstrate the ability to simulate a ternary system where alcohol tends to swell the organic phase. Input parameters for the simulations are given in Table 7 and Table 8. Porous
Fig. 9. Comparison of spatial composition profiles with changing lnwafter 0.5 pore volumes of alcohol solution were injected. Note that the
vertical axis scales vary between figures.
Fig. 10. Comparison of compositions at 2.5 cm from injection boundary with changing lnw. Note that the vertical axis scales vary between
medium properties and modeling parameters are identical to those used in the IPA–water–PCE simulations (Table 1 and Table 3). For non-equilibrium simulations, the mass transfer parameters lw, lnw, and A1were given values equal to those
for Run 4 shown in Table 6. Ternary phase behaviour for the 1-propanol–water–TCE system is shown in Fig. 2. Simulations were also completed using the assumption of local equilibrium by employing the compositional alcohol flooding model described in Ref.45.
Effluent concentration and recovery results from both the equilibrium and the non-equilibrium simulations are shown in Fig. 12. The equilibrium model predicts 100% recovery of the TCE due to the formation of a well-defined non-wetting phase bank ahead of the alcohol flood. Once the non-wetting phase bank has been swept out of the porous medium, very little TCE is left in the medium and TCE concentrations drop off rapidly to zero with no tailing. For
the non-equilibrium simulations, the non-wetting phase bank is not as well defined and the front of the bank is essentially coincident with the front of the alcohol flood. Much of the column contains non-wetting phase throughout the duration of the simulation, unlike the equilibrium simulation where all the non-wetting phase is swept out near the front of the alcohol slug. With some non-wetting phase remaining in the porous medium, tailing effluent concentrations of the alcohol and the organic can occur.
Significant differences in NAPL recovery may result for swelling alcohol–water–organic systems where local conditions only approach equilibrium rather than reach equilibrium. For the IPA–water–PCE system, where alcohol does not significantly swell the organic-rich phase, it has been shown that effluent concentrations of PCE approach equilibrium values. Increasing the specific interfacial area from best estimates, for example, does not
Fig. 11. Comparison of spatial compositional profiles with variation of ratio of lwto A1after 0.5 pore volumes of alcohol solution were
injected. The mass transfer ratio is equal to 3.343105
for each simulation. Note that the vertical axis scales vary between figures.
Table 7. Fluid properties used in the alcohol flooding simulations for the 1-propanol–water–TCE system
Molecular weight Water60 1.8310¹2kg mol¹1 Density Water14 997 kg m¹3
TCE36 1.314310¹1
kg mol¹1 TCE36 1460 kg m¹3
1¹Propanol60 6.01310¹2
kg mol¹1 1-Propanol8 781 kg m¹3
Association parameter Water23 TCEa 1-Propanola
2.26 1.0 1.0
Molar volume at normal boiling point (m3mol¹1)a
Water TCE 1-Prop
1.88310¹5 9.00310¹5 7.69310¹5 Viscosity of pure fluid Water14 8.9310¹4
Pa·s Solubility of water in TCE37 2.02310¹3
TCE36 5.7310¹4
Pa·s 1-Propanol60 1.98310¹3
Pa·s Solubility of TCE in water36 (mole fractions)
1.2310¹4
a
significantly change the simulated results (see Fig. 8). How-ever, for the 1-propanol–water–TCE system, where alcohol swells the organic-rich phase, effluent concentrations for non-equilibrium conditions can be significantly different than for equilibrium conditions (see Fig. 12). As local con-ditions approach equilibrium, much of the non-wetting phase in the domain approaches the plait point, but may never actually become miscible with the wetting phase such that two phases remain. If equilibrium were to occur,
miscibility would be achieved and only one phase would exist at that location. Having two phases present in the porous medium allows for bypass of the non-wetting phase, and may allow for counter-current flow as a result of density contrasts.
Variation in relative permeability functions may cause variation in the simulation results because flow rates, which are dependent on the particular relative permeability function employed, will dictate contact time and therefore vary the amount of inter-phase mass transfer. High peak concentrations corresponding to the development of a non-wetting phase bank ahead of the alcohol flood may occur during a simulation, depending on the relative permeability function employed, as they would using an equilibrium model.
6 CONCLUSIONS
A non-equilibrium, two-phase, three-component composi-tional model has been developed for simulation of alcohol flooding, with specific attention given to inter-phase mass transfer. A fully implicit scheme using upstream weighting of advection terms and a Newton–Raphson iterative tech-nique enables solution of the highly non-linear governing equations. Important factors such as density, viscosity, interfacial tension, relative permeability, and inter-phase mass transfer are all considered. Both phases may be mobile, which is important for simulation of alcohol floods where alcohol tends to swell the organic phase. Versatile boundary condition algorithms are used to allow for flow of either phase out of the domain while maintaining Cauchy-type inlet and free exit conditions.
Mass transfer algorithms have been tested using Lewis Cell type simulations. The simulations indicate that during inter-phase mass transfer, precipitation within either phase should not be expected, but rather that compositions will remain above or close to the binodal curve. The Lewis Cell simulations also demonstrate the model’s ability to simulate multicomponent mass transfer under a broad range of con-centrations for ternary systems where alcohol has an affinity for either the water-rich phase or the organic-rich phase.
Model calibration using effluent data from an IPA–
water–PCE ternary system8 indicates that inter-phase
mass transfer parameters may be non-unique. Addition of Table 8. Hand Plot and NRTL parameters used in the alcohol flooding simulations for the 1-propanol–water–TCE system
Hand Plot fitting parameters43 Ah1 1.203102 Eh1 2.573105
Ah2 4.25 Eh2 6.653102
Bh1 ¹2.61310¹1 Fh1 4.10
Bh2 ¹2.05 Fh2 1.00
UNIQUAC parameters53Units oftin Kelvin t(1,2) 734.05 t(1,3) 330.63
t(2,1) 965.96 t(2,3) 330.68
t(3,1) ¹105.89 t(3,2) ¹108.26
q(1) 1.400 r(1) 0.920
q(2) 2.860 r(2) 3.3092
q(3) 2.512 r(3) 2.7799
Fig. 12. Comparison of non-equilibrium and equilibrium model
more inter-phase mass transfer terms, although possibly providing better fit to experimental data, can lead to diffi-culty in determining actual best-fit values. The problem of determining unique values for each term may be due to a high degree of correlation between terms such as the wetting phase film thickness and the specific interfacial area, or to the small influence of a term on the final model results, such as the non-wetting phase film thickness.
Additional experimental data should provide more insight into which mass transfer terms can be uniquely determined and which terms can be combined as effective parameters. From sensitivity studies, it can be concluded that additional saturation and wetting phase concentration data will likely not provide information regarding the non-wetting film thickness, or the ratio of the wetting phase film thickness to the interfacial area. Non-wetting phase composition data should, however, provide insight into the non-wetting phase film thickness.
From model calibration using alcohol flooding data for an
IPA–water–PCE ternary system, conditions that
approached equilibrium were found to occur. Increased mass transfer rates had very little impact on simulation out-come. For alcohol flooding using a 1-propanol–water–TCE system, significant differences were found when comparing results from the equilibrium and the non-equilibrium models. Although these findings are preliminary, it is sug-gested that application of an equilibrium assumption to an alcohol flood where alcohol tends to swell the organic should be used with caution.
ACKNOWLEDGEMENTS
The work contained in this paper was supported by Martin Marietta Energy Systems Inc. of Oak Ridge National Laboratories in Oak Ridge, Tennessee under subcontract No. 19Y-GUG26V. Additional funding was provided by the Solvents in Groundwater Consortium which receives support from Boeing, Ciba-Geigy, Eastman Kodak, General Electric, Motorola, PPG, and UTC. Further acknowledg-ment is given to the Natural Science and Engineering Research Council of Canada and Queen’s University in Kingston, Ontario. A special acknowledgment is extended to Dr. Ross Taylor at Clarkson University for advice on multicomponent mass transfer and for review of the mass transfer equation development.
REFERENCES
1. Abriola, L.M. & Pinder, G.F. A multiphase approach to the modeling of porous media contamination by organic com-pounds; numerical simulation. Water Resour. Res., 1985,
21(1), 19–26.
2. Adams, D.S. & Prausnitz, J.M. Statistical thermodynamics of liquid mixtures: A new expression for the excess Gibbs Energy of partly or completely miscible systems. AIChE J., 1975, 21, 116–128.
3. Baehr, A.L. & Corapcioglu, M.Y. A compositional multiphase model for groundwater contamination by petroleum products 2. numerical solution. Water Resour. Res., 1987, 23(1), 201–213.
4. Bergelin, O., Lockhart, F.J. & Brown, G.G. Liquid-liquid extraction. Trans. Am. Inst. Chem. Engrs., 1943, 39, 173– 200.
5. Bhuyan, D., Pope, G.A. & Lake, L.W. Mathematical model-ing of high-pH chemical floodmodel-ing. SPE Res. Eng., 1990, 5(2), 213–220.
6. Blunt, M. & Rubin, B. Implicit flux limiting schemes for petroleum reservoir simulation. J. Comp. Phys., 1992, 102, 194–210.
7. Brame, S. E., Development of a Numerical Simulator for the In Situ Remediation of Dense, Non-aqueous Phase Liquids Using Alcohol Flooding. M.Sc. Thesis. Clemson University, Clemson, SC, 1993.
8. Brandes, D. & Farley, K.J. Importance of phase behavior on the removal of residual DNAPLs from porous media by alcohol flooding. Water Environ. Res., 1993, 65(7), 869–878. 9. Brown, C.L., Pope, G.A., Abriola, L.M. & Sepehrnoori, K. Simulation of surfactant-enhanced aquifer remediation. Water Resour. Res., 1994, 30(11), 2959–2977.
10. Brussseau, M.L. Rate-limited mass transfer and transport of organic solutes in porous media that contain immobile immiscible organic liquid. Water Resour. Res., 1992, 28(1), 33–45.
11. Corteville, J., Van Quy, N., and Simandoux, P., A numerical and experimental study of miscible or immiscible fluid flow in porous media with interphase mass transfer. In 46th Annual Fall Meeting of the SPE of AIME, New Orleans, LA, Oct. 3-6, 1971.
12. Culham, W. E., Farouq Ali, S. M., and Stahl, C. D., Experi-mental and numerical simulation of two-phase flow with interphase mass transfer in one and two dimensions, SPEJ, September (1969) 323–37.
13. Datta Gupta, A., Pope, G.A., Sepehrnoori, K. & Thrasher, R.L. A symmetric, positive definite formulation of a three-dimensional micellar/polymer simulator. SPE Res. Eng., 1986, 1(6), 622–632.
14. Debler, W. R., Fluid Mechanics Fundamentals. Prentice Hall, New Jersey, 1990.
15. Farouq Ali, S. M., and Stahl, C. D., Computer models for simulating alcohol displacement in porous media, SPEJ, March (1965) 89-99.
16. Forsyth, P.A. Comparison of the single-phase and two-phase numerical formulation for saturated-unsaturated groundwater flow. Comput. Methods Appl. Mech Engr., 1988, 69, 243– 259.
17. Forsyth, P. A., Simulation of nonaqueous phase groundwater contamination, Research Report CS-88-02, Computer Science Department, University of Waterloo, 1988. 18. Forsyth, P. A., A finite volume approach to NAPL
ground-water contamination, Research Report CS-89-46, University of Waterloo, October, 1989.
19. Forsyth, P.A. Radioactive waste disposal heating effects in unsaturated rock. Numerical Heat Transfer, Part A, 1990, 17, 29–51.
20. Forsyth, P.A. A control volume finite element approach to NAPL groundwater contamination. SIAM J. Sci. Stat. Comput., 1991, 12(5), 1029–1057.
21. Gershon, N. & Nir, A. Effects of boundary conditions of models on tracer distribution in flow through porous media. Water Resour. Res., 1969, 5(4), 830–839.
23. Hayduk, W. & Laudie, H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AIChE J, 1974, 20, 611–615.
24. Huyakorn, P. S., and Pinder, G. F., Computational Methods in Subsurface Flow. Academic Press, New York, NY, 1983. 25. Imhoff, P.T., Gleyzer, S.N., McBride, J.F., Vancho, L.A., Okuda, I. & Miller, C.T. Cosolvent-enhanced remediation of residual dense nonaqueous phase liquids: Experimental investigation. Environ. Sci. Technol., 1995, 29, 1966–1976. 26. Imhoff, P.T., Jaffe´, P.R. & Pinder, G.F. An experimental study of complete dissolution of a nonaqueous phase liquid in saturated porous media. Water Resour. Res., 1994, 30(2), 307–320.
27. Kaluarachchi, J.J. & Parker, J.C. An efficient finite element method for modelling multiphase flow. Water Resour. Res., 1989, 25(1), 43–54.
28. Kazemi, H., Vestal, C. R., and Deane, G., An efficient multi-component numerical simulator, SPEJ, October (1978) 355-68. 29. Krishna, R., Low, C.Y., Newsham, D.M.T., Olivera-Fuentas, C.G. & Standart, G.L. Ternary mass transfer in liquid-liquid extraction. Chem. Eng. Sci., 1985, 40, 893–903.
30. Lamarche, P., Dissolution of immiscible organics in porous media. Ph.D. thesis, University of Waterloo, Waterloo, Ontario, 1991.
31. Lewis, J.B. The mechanism of mass transfer of solutes across liquid-liquid interfaces. Part I: The determination of individual transfer coefficients for binary systems. Chem. Eng. Science, 1954, 3, 248–259.
32. Liu, J., Delshad, M., Pope, G.A. & Sepehrnoori, K. Applica-tion of higher-order flux-limited methods in composiApplica-tional simulation. J. Transport Porous Media, 1994, 16, 1–29. 33. McWhorter, D.B. & Sunada, D.K. Exact integral solutions
for two-phase flow. Water Resour. Res., 1990, 26(3), 399– 413.
34. Ogata, A., and Banks, R. B., A solution of the differential equation of longitudinal dispersion in porous media, U.S. Geol. Surv. Prof. Paper 411-A, 1961.
35. Orlandini, M., Fermeglia, M., Kikic, I. & Alessi, P. Liquid-liquid equilibria for water-propanol- and water-butanol-chlorocompound systems. Chem. Eng. Journal, 1983, 26, 245–250.
36. Pankow, J. F., Johnson, R. L., Appendix: Physical and chemical properties of dense non-aqueous phase liquid (DNAPL) compounds. In Dense Chlorinated Solvents and Other DNAPLs in Groundwater, eds. J. F. Pankow and J. A. Cherry, Waterloo Press, Portland, Oregon, (1996) 507-512.
37. Parrish, F. P., Industrial solvents. In Kirk-Othmer Encyclo-pedia of Chemical Technology, Third Edition, Vol. 21, ed. M. Grayson, John Wiley & Sons, New York, 1983, pp. 377-401. 38. Pinder, G.F. & Abriola, L.M. On the simulation of non-aqueous phase compounds in the subsurface. Water Resour. Res., 1986, 22(9), 109–119.
39. Powers, S.E., Loureiro, C.E., Abriola, L.M. & Weber, W.J. Jr. Theoretical study of the significance of nonequilibrium dissolution of nonaqueous phase liquids in subsurface sys-tems. Water Resource Research, 1991, 27(4), 463–477. 40. Powers, S.E., Abriola, L.M. & Weber, W.J. Jr. An
experi-mental investigation of nonaqueous phase liquid dissolution in saturated subsurface systems: Steady state mass transfer rates. Water Resour. Res., 1992, 28(10), 2691–2705. 41. Powers, S.E., Abriola, L.M. & Weber, W.J. Jr. An
experi-mental investigation of nonaqueous phase liquid dissolution in saturated subsurface systems: Transient mass transfer rates. Water Resour. Res., 1994, 30(2), 321–332.
42. Price, H. S., and Donohue, D. A. T., Isothermal displacement
processes with interphase mass transfer. SPEJ, June (1967) 205-220.
43. Reitsma, S., Equilibrium and non-equilibrium compositional alcohol flooding models for recovery of immiscible liquids from porous media. Ph.D. Thesis, Queen’s University, ON, September, 1996.
44. Reitsma, S., and Kueper, B. H., Non-equilibrium compo-sitional alcohol flooding model for immiscible phase remediation 1. Equation development, Advances in Water Resour., this issue (1996).
45. Reitsma, S., and Kueper, B. H., Compositional modelling study of alcohol flooding for recovery of DNAPL. In Pro-ceedings of ASCE 1996 Annual Convention and Exposition, Non-Aqueous Phase Liquids (NAPLs) in Subsurface Environment: Assessment and Remediation, Washington, D. C., November 12-14 (1996).
46. Renon, H. & Prausnitz, J.M. Local compositions in thermo-dynamic excess functions for liquid mixtures. AIChE J., 1968, 14(1), 135–144.
47. Saad, N.G., Pope, G.A. & Sepehrnoori, K. Application of higher-order methods in compositional simulation. SPE Res. Eng., 1990, 5(4), 623–630.
48. Sarma, P.B.S., Delleur, J.W. & Rao, A.R. Comparison of rainfall-runoff models for urban areas. Journal of Hydrology, 1973, 18, 329–347.
49. Sleep, B.E. A method of characteristics model for equation of state compositional simulation of organic compounds in groundwater. J. of Contam. Hydrol., 1995, 17, 189–212. 50. Sleep, B.E. & Sykes, J.F. Compositional simulation of
groundwater contamination by organic compounds. 1. Model development and verification, . Water Resource Research, 1993, 29(6), 1697–1708.
51. Sleep, B.E. & Sykes, J.F. Compositional simulation of groundwater contamination by organic compounds. 1. Model applications. Water Resource Research, 1993, 29(6), 1709–1718.
52. Sleep, B.E. & Sykes, J.F. Modeling the transport of volatile organics in variably saturated media. Water Resour. Res., 1989, 25(1), 81–92.
53. Sorensen, J. M., and Arlt, W., Liquid-Liquid Equilibrium Data Collection, Ternary Systems. Chemistry Data Series, Vol. V, Part 2, Dechema, Frankfurt, Germany, 1980. 54. Standart, G.L., Cullinan, H.T., Paybarah, A. & Louizos, N.
Ternary mass transfer in liquid-liquid extraction. AIChE J, 1975, 21, 554–559.
55. Taylor, R., and Krishna, R., Multicomponent Mass Transfer. John Wiley & Sons, Inc., Toronto, 1993.
56. Therrien, R., Three-Dimensional Analysis of Variably-saturated Flow and Solute Transport in Discretely-fractured Porous Media. Ph.D. University Thesis, of Waterloo, Waterloo, Ontario, 1992.
57. Unger, A.J.A., Forsyth, P.A. & Sudicky, E.A. Variable spatial and temporal weighting schemes for use in multi-phase compositional problems. Advances in Water Resources, 1996, 19(1), 1–27.
58. van Genuchten, R., Documented Computer Programs: Part 1. U.S. Salinity Laboratory, Agric. Res. Serv., U.S. Dep. of Agric., Riverside, California, 1987.
59. Van Quy, N., Simandoux, P., and Corteville, J., A numerical study of diphasic multicomponent flow, SPEJ, April (1972) 171-184.
60. Weast, R. C., CRC Handbook of Chemistry and Physics. 66th Edition, CRC Press Inc., Boca Raton, Florida, 1985. 61. Welge, H. J., Johnson, E. F., Ewing, S. P., and Brinkman, F.