Review article
The sialylation of plasma lipoproteins
John S. Millar *
Department of Medicine,Uni6ersity of Pennsyl6ania,621BRB II/III,421Curie Boule6ard,Philadelphia,PA19104-6160,USA
Received 22 February 2000; received in revised form 1 September 2000; accepted 13 October 2000
Abstract
Sialic acids are a family of amino sugars that are commonly found as terminal oligosaccharide residues on glycoproteins and glycolipids. Plasma lipoproteins are sialylated on their apolipoprotein and glycolipid constituents. The function of sialic acid on apolipoproteins is not completely understood but has been associated with secretion, lipid-binding, and plasma clearance for some apolipoproteins. The sialic acid content of individual apolipoproteins can vary in response to physiological conditions while the sialic acid content of individual sialylated glycolipids (gangliosides) is constant. Thus, the sialic acid content of plasma lipoproteins can differ considerably as a result of (1) variations in the sialylation of their apolipoprotein constituents, (2) variations in their content of sialylated apolipoproteins and gangliosides, and (3) modifications of the sialic acid on lipoprotein constituents while circulating in plasma. The significance of sialic acid on lipoproteins is not fully understood although associations have been made between sialic acid and charge (very low density lipoprotein), lipoprotein solubility, receptor binding and uptake, and interactions with vascular matrix (low density lipoprotein and Lp(a)) and with cholesterol efflux (high density lipoprotein). Further studies identifying sites of sialylation on apolipoproteins and characterizing the structures of sialylated oligosaccharides will aid in determining the enzymes responsible for their sialylation. Manipulations of the sialylation of apolipoproteins and of the quantity of apolipoproteins and gangliosides on lipoproteins will be useful methods in determining the role of lipoprotein sialic acid in the development of atherosclerosis. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Sialic acid; Gangliosides; Oligosaccharides; Very low density lipoprotein; Low density lipoprotein; High density lipoprotein; Lp(a) www.elsevier.com/locate/atherosclerosis
1. Introduction
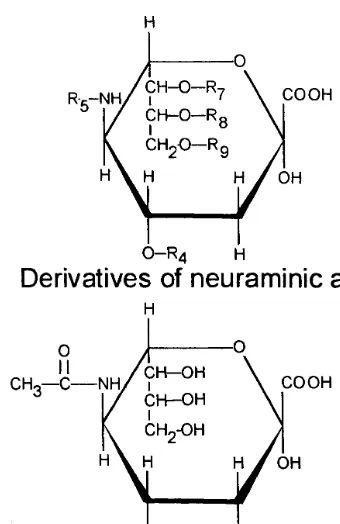
Sialic acids are a family of N- and O-substituted derivatives of neuraminic acid, an amino sugar (Fig. 1) [1]. Over 40 different naturally occurring derivatives of sialic acid have been identified [2]. These are generally found as terminal sugar residues on oligosaccharides of both glycoproteins and glycolipids [2]. The functions of sialic acids in biological systems include conformational stabilization, protease resistance, charge, enhancement of water binding capacity, cellular recognition, protein targeting, and developmental regulation [3]. The most common sialic acid found on human plasma glyco-proteins and glycolipids is 5-N-acetylneuraminic acid (Fig. 1), frequently found in an a-2,3-linkage with
galactose [1,2]. O-acetylated derivatives of neuraminic acid are normally found in plasma of some mammalian species (including mouse, rat, guinea pig, cow, and
horse). In humans O-acetylated derivatives are not normally found in plasma except during fetal develop-ment [2,4]. These derivatives are also found in abun-dance in the plasma of patients with malignant melanoma [2,4]. For the purposes of this manuscript the term ‘‘sialic acid’’ will refer to 5-N-acetylneuraminic acid.
The majority of sialic acid in plasma is protein- and lipid-bound (Fig. 2) with little occurring in the free form [2,5]. Elevations in the plasma sialic acid concen-tration have been reported for both the protein- and lipid-bound fractions [6]. Lipid-bound sialic acids have been shown to be elevated due to unregulated produc-tion of gangliosides, the sialic acid-containing family of glycolipids [6]. An increase of protein-bound sialic acids in plasma has also been observed in inflammatory disease, possibly as a result of elevations in plasma levels of acute phase proteins, many of which are sialylated [7,8].
Studies relating sialic acid to the development of coronary heart disease (CHD) tend to fall into one of
* Tel.: +1-215-8985909; fax:+1-215-5736725.
E-mail address:[email protected] (J.S. Millar).
J.S.Millar/Atherosclerosis154 (2001) 1 – 13 2
Fig. 1. (Upper) Basic molecular structure for derivatives of neu-raminic acid. Variable sites are represented by an ‘R’ with a subscript indicating the carbon number to which different groups may be attached. (Lower) 5-N-acteylneuraminic acid, the most abundant sialic acid derivative found in human plasma.
sion that individuals with low levels of lipoprotein-asso-ciated sialic acid are at increased risk of developing CHD [12 – 14]. Despite this relationship the factors that control the content of lipoprotein-associated sialic acid are not known.
The factors that influence the content of lipoprotein-associated sialic acid and its relationship to lipoprotein metabolism will be the focus of this review. The discus-sion will begin with an overview of the sialic acid-con-taining components on lipoproteins (apolipoprotein (apo), glycolipids) and their contribution to the total sialic acid content of a lipoprotein fraction, and end with the known effects that lipoprotein sialic acid has on lipoprotein metabolism. Graphical representations of some of the terms used in this manuscript are shown in Fig. 3.
2. Apolipoprotein sialic acid
Of the plasma apolipoproteins, apo A-II, 48, B-100, C-II, C-III, D, E, J, and (a) have been reported to be sialylated to some degree (Table 1) [15 – 25]. Other minor sialylated apolipoproteins including apo C-IV, H and M have been described but will not be discussed further [26 – 28].
Many sialylated apolipoproteins, with the exceptions of apo B-100, and, possibly, B-48, D, J, and (a), are found in plasma as a mixture of glycosylated and non-glycosylated forms [17,21,29 – 31]. The significance of the heterogeneity in the glycosylation of proteins, in general, is not known and has been discussed by Dwek et al. [32]. The existence of non-glycosylated forms of some apolipoproteins in plasma would imply that gly-cosylation and sialylation are not requirements for their secretion. This has been shown to be true for those apolipoproteins where this hypothesis has been tested [30,33]. Other potential functions of sialic acid on indi-the two categories. One type of study has focused on
the relationship between plasma sialic acid and CHD. The majority of these studies have found that plasma sialic acid levels are increased in individuals with CHD, possibly due to elevations of plasma acute phase proteins released in response to inflammation [9 – 11]. The other type of study relating sialic acid to CHD has focused on the relationship between lipoprotein-associ-ated sialic acid and the development of CHD. This latter type of study has generally arrived at the
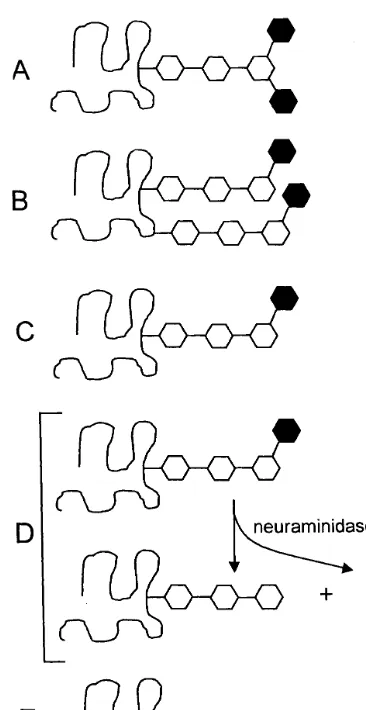
conclu-Fig. 2. Examples of lipid-bound (A) and protein-bound (B) sialic acids. Individual sugars and linkages are detailed. Neu5Aca23Gal represents
ana-linkage between carbon 2 of 5-N-acetylneuraminic acid (sialic acid) and carbon 3 of galactose. Panel A is ganglioside GM3and panel B is
Fig. 3. Examples to illustrate some terms used throughout the manuscript. All figures show a protein (curved line) that can be glycosylated with a neutral oligosaccharide core structure (open hexagons) and sialic acid (filled hexagons). (A, B) Di-sialylated proteins containing (A) a single di-sialylated oligosacharide or (B) two mono-sialylated oligosaccharides, (C) mono-sialylated protein, (D, lower left) desialylated protein, and (E) non-sialylated protein.
A-I/A-II HDL particles is reported to be non-glycosy-lated [33].
2.2. Apolipoprotein B
Apo B-100 is a multi-sialylated apolipoprotein con-taining several mono- and di-sialylated complex bi-an-tennary oligosaccharides in addition to non-sialylated, high-mannose structures [19,20]. Bartlett and Stanley [34] have shown that all low density lipoprotein (LDL) contain mono-sialylated complex bi-antennary oligosac-charides. Since these structures are not found on gan-gliosides the conclusion can be drawn that all circulating apo B-100 polypeptides contain at least one sialic acid residue [35]. Further characterization of the heterogeneity in the sialylation of apo B-100 might best be accomplished by studying the sialylation of individ-ual peptide fragments following partial proteolysis of apo B-100. This would allow individual apo B-100 peptide sequences to be associated with different glyco-sylation and sialylation patterns.
In rabbits, the number of complex bi-antennary oligosaccharides on apo B-100 containing two terminal sialic acids is variable, being lower in those animals with elevated cholesterol levels [36,37]. The proposed structure of this di-sialylated oligosaccharide (Fig. 2), which is also found on human apo B-100, is identical to the two complex bi-antennary oligosaccharides found on human transferrin [38]. It is worth noting that the number of di-sialylated oligosaccharide chains on trans-ferrin is related to plasma cholesterol levels although it is not known if there is any relationship between the glycosylation of transferrin and apo B-100 [39]. Sasak et al. [18] also studied the carbohydrate content of apo B-48 and identified mono- and di-sialylated oligosac-charides that are of similar composition to those found on apo B-100.
2.3. Apolipoprotein C-II
Apo C-II is found in di-, mono- and non-sialylated forms in plasma [21]. The sialylated form is non-glycosylated [21]. The mono-sialylated and di-sialylated forms of apo C-II constitute a small percentage (B
15%) of the total apo C-II in plasma [21]. While the sialylated forms of apo C-II have been described as precursors (i.e. propeptide) to the parent polypeptide, it is possible that mature apo C-II includes these sialy-lated forms in addition to non-glycosysialy-lated forms. The significance of the sialylation of apo C-II is unknown [21,40].
2.4. Apolipoprotein C-III
Apo C-III exists as three isoforms in plasma, apo C-III0, C-III1 and C-III2, the subscripts indicating the
vidual apolipoproteins are discussed below. The study of apolipoprotein sialylation is complicated due to spe-cies-related and tissue-related differences in glycosyla-tion and sialylaglycosyla-tion [32]. This review will focus on the sialylation of apolipoproteins isolated from human plasma unless otherwise indicated.
2.1. Apolipoprotein A-II
J.S.Millar/Atherosclerosis154 (2001) 1 – 13 4
number of sialic acid residues per polypeptide [41]. The non-sialylated form (apo C-III0) is not glycosylated
[29]. Hypertriglyceridemic subjects have an increased proportion of apo C-III as the C-III2 isoform in very
low density lipoprotein (VLDL) [42,43]. This may be due to apo C-III2 having a higher affinity for VLDL
than apo C-III0 or C-III1, [44]. Apo C-III2 is also a
poorer inhibitor of VLDL binding to the purported lipolysis stimulated receptor than apo C-III0 or C-III1,
[44]. Neuraminidase treatment of apo C-III to remove sialic acid has no effect on the ability of apo C-III to inhibit lipoprotein lipase [45].
2.5. Apolipoprotein D
Apo D, found primarily on HDL, is a highly glycosy-lated apolipoprotein (18% by weight) [23]. The different isoforms of apo D have been shown, in part, to be due to differences in the sialic acid content of apo D [31,46]. Schindler et al. [47] has shown that there are a wide range of carbohydrate structures with different num-bers of sialic acid residues that can occupy each of the two glycosylation sites. The significance of sialic acid on apo D is unknown.
2.6. Apolipoprotein E
Apo E occurs as di-, mono-, and non-sialylated forms in plasma, the non-sialylated form being non-gly-cosylated [30]. The metabolism of apo E in plasma is related to its degree of sialylation with the di-sialylated isoform cleared from plasma more rapidly than the non-sialylated isoform [48]. Marmillot et al. [49] re-ported that sialylated apo E had a higher affinity for HDL than desialylated apo E in vitro while in vivo studies have not shown any differences in the lipo-protein distribution of disialylated and non-sialylated apo E [48]. Long-term ethanol intake leads to decreased apo E sialylation in rats similar to what has been observed with apo J in rats [50,51].
2.7. Apolipoprotein J
Apo J is a sialylated apolipoprotein found on HDL [25]. Apo J sialylation in rats is alcohol sensitive, there being decreased in sialylation of the apolipoprotein following long-term ethanol intake [51]. The signifi-cance of sialic acid on apo J is unknown.
2.8. Apolipoprotein (a)
Apo (a) is the most highly sialylated apolipoprotein [15,16]. The sialic acid content of apo (a) is influenced by two factors, the number of kringle repeats in the apolipoprotein (i.e. the length of the apolipoprotein) and the degree of sialylation of each kringle [52]. The sialylation of apo (a) has been shown to inhibit its secretion from HepG2 cells [53]. There is no require-ment of sialic acid on apo (a) for the formation of Lp(a) from apo (a) and LDL [53]. While there are no reports of the effect of sialylation on the clearance rate of free apo (a) in plasma, desialylation of plasminogen, which is in part homogeneous to apo (a), has been reported to increase its clearance rate in plasma [54]. Sialic acid is required for the interaction between apo (a) and complement activation fragment iC3b although the significance of this interaction is unknown since there is no effect of apo (a) sialylation on compliment activation or degradation [55].
3. Glycolipid sialic acid
Lipid associated sialic acids are found on gan-gliosides, a family of glycolipids somewhat similar in structure to phospholipids that have a variable sialic acid-containing oligosaccharide structure attached to an acylated ceramide core (Fig. 2) [56]. Although syn-thesized to a large degree by neural tissue, plasma gangliosides are primarily liver-derived [56,57]. More than ten different gangliosides have been identified in
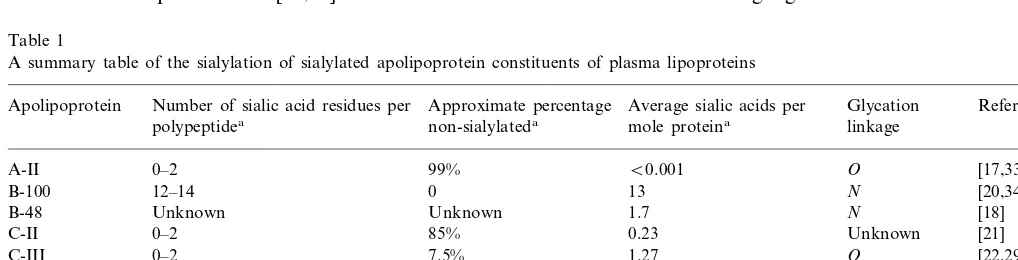
Table 1
A summary table of the sialylation of sialylated apolipoprotein constituents of plasma lipoproteins
Number of sialic acid residues per Approximate percentage
Apolipoprotein Average sialic acids per Glycation Reference
non-sialylateda
polypeptidea mole proteina linkage
0–2 99% B0.001
A-II O [17,33]
12–14 0
B-100 13 N [20,34]
1.7 [18]
Unknown Unknown
B-48 N
0–2 85%
C-II 0.23 Unknown [21]
C-III 0–2 7.5% 1.27 O [22,29]
D 0–6 Unknown 5.4 N [23,31,47]
0–2 80%
E 1.29 O [30]
J Unknown Unknown Unknown N [25]
(a)b 5.7 0 5.7 N [15,16,52]
aValues for proteins isolated from human plasma.
plasma, the majority of which are associated with lipo-proteins (Table 2) [58,59]. Gangliosides are likely to be present on newly secreted lipoproteins although there may also be transfer of gangliosides to lipoproteins in plasma.
The synthesis of plasma gangliosides requires the addition of sialic acid to glycolipid core structure. This reaction is under control of hepatic sialyltransferases, a family of enzymes that sialylate glycoproteins and gan-gliosides [60,61]. The expression of individual sialyl-transferases is unregulated in certain disease states resulting in an overproduction of individual gliosides that increases the concentration of gan-gliosides in plasma [6].
Gangliosides are able to influence lipoprotein metabolism, though the mechanisms involved are not known. Filipovic et al. [62] showed that incubation of LDL with ganglioside inhibits its binding and uptake by smooth muscle cells. Subsequent removal of the sialic acid from the ganglioside-enriched LDL with neuraminidase abolished the inhibitory effect on the uptake of LDL by smooth muscle cells. Neither free sialic acid nor other negatively charged amphipathic molecules incubated with LDL had any effect on their uptake by smooth muscle cells demonstrating a gan-glioside-specific effect. Millar et al. [63] showed that incubation of LDL with ganglioside resulted in a de-creased interaction between LDL and arterial proteo-glycans while incubation with asialoganglioside resulted in a slightly increased interaction. Thomas and Poznan-sky [64] have demonstrated that the addition of gan-gliosides to lipid vesicles resulted in increased cholesterol transfer suggesting that lipoprotein-associ-ated gangliosides can influence the activity of cholesteryl ester transfer protein (CETP).
4. Sialylation of lipoproteins
The sialic acid content of plasma lipoproteins varies considerably between different lipoprotein fractions and within lipoprotein fractions [14,63,65]. As total sialic acid content of a lipoprotein fraction is determined by the contribution of sialic acid from each of its stituents, the factors that influence the sialic acid con-tent of lipoproteins must affect one or more of the following:
1. the sialylation of apolipoproteins prior to their se-cretion into plasma,
2. the quantity of sialic acid-containing apolipo-proteins and gangliosides on lipoapolipo-proteins,
3. the sialic acid content of lipoprotein-associated apolipoproteins and gangliosides following their se-cretion in plasma either by addition, removal or modification of sialic acid.
Each of these possible mechanisms for influencing lipoprotein-associated sialic acid will be expanded upon in the discussion that follows.
4.1. The sialylation of apolipoproteins prior to their secretion into plasma
The sialylation of apolipoproteins occurs in the post-Golgi compartment where sialic acid is added to core oligosaccharide structures. Sialylated apolipoproteins can be divided into those in which all circulating polypeptides are sialylated (apo (a), B-48, B-100, D, and J) and those in which sialylated and nonsialylated polypeptides are found in plasma (apo A-II, C-III, C-III, and E) (Table 1). The non-sialylated apolipo-proteins in this latter group that have been studied are non-glycosylated [21,29,30]. Sialylated apolipoproteins have a minimum of one amino acid in a covalent linkage to a sialylated oligosaccharide. There may be subpopulations of an apolipoprotein isolate that are sialylated at only one of several potential glycosylation sites while other subpopulations are sialylated at two or more sites [2]. Furthermore, each sialylated oligosac-charide may potentially contain one or more sialic acid residues [2,47]. The existence of multiple potential gly-cosylation sites on an apolipoprotein combined with the variability in the sialylation of the oligosaccharides that can occupy these sites results in the possibility of con-siderable heterogeneity in the sialylation of many apolipoproteins.
In general, the particular type of sialylated oligosac-charides and its site of attachment to a protein is not random but instead is influenced by physiological con-ditions under which the protein is synthesized [32]. The degree of sialylation of apolipoproteins is likely related to the expression of individual sialyltransferases. Sialyl-transferase activity in various rat tissues correlates with mRNA levels of this enzyme [4]. The differing levels of expression of sialyltransferases and other glycosyltrans-ferases among different tissues and cell lines and be-tween species results in a great degree of variability in the core oligosaccharide and further variability in the sialylation of this core structure [4]. This variability is evident when comparing the sialylation of apo E in different tissues and cell lines. Apo E has been reported to contain up to six sialic acids per polypeptide [66]. The sialic acid content of apo E produced by HepG2 cells, monocytes, and astrocytes is much higher than that found in plasma, indicating a lower degree of sialylation of the apo E synthesized by normal liver [30,66 – 68]. In addition, the tissue-specific sialylation of proteins can be affected by factors such as acute alco-hol consumption, inflammation, aging, and hyperc-holesterolemia [2,37,50,51].
J
.
S
.
Millar
/
Atherosclerosis
154
(2001)
1
–
13
6
Table 2
Plasma and lipoprotein ganglioside absolute and relative concentrationsa
GD1a(%)
Ganglioside concentration GM3(%) GD3(%) GM2+MG3(%) MG4(%) GT1b(%) GD1b(%) GM1 (%) Average S.A./mol Reference
Fraction
(nmol/ml) gangalioside
4.3–7.0 4.1–6.1 3.8–6.6 2.1–4.4 0.2–1.5 ND 1.3 [104]
Plasma 4.0–8.9 55.4–66.6 17.2–21.3
ND B23b ND 3 1.3
3 [105]
Plasma 7.0–8.6 71 ND B23b
10.5+3.2 46–48 24–26 10–11 1 4–5 2–3 ND 1.4 [58]
Plasma 6–9
3 3 2 ND 1.2
12 [58]
18 11
VLDL 0.7 36
11
6.7 45 13 9 2 3 3 ND 1.2 [58]
LDL
2 16 3 3 4 ND 1.1 [58]
HDL 2.6 34 20
aND, not detected; S.A., sialic acid. bReported GD
of sialylation is, by definition, constant and for this reason will not be discussed further.
4.2. Influence o6er the quantity of sialic acid-containing
constituents on lipoproteins
The factors that control the affinity of sialic acid-con-taining constituents for lipoproteins are largely un-known. Apo B-100 is a structural component of VLDL, LDL and Lp(a), there being one apo B-100 polypeptide per lipoprotein particle [15,69,70]. In addition, Lp(a) also has a single apo (a) polypeptide per particle [15]. Sialylated exchangeable apolipoproteins associate pri-marily with lipoproteins of specific size and lipid com-position. Apo C-II, C-III and E are lipoprotein-bound in plasma, and associate primarily with VLDL and HDL [71]. Apo D and J although largely found in HDL are associated with specific HDL subfractions [25,72]. The di-sialylated form of apo C-III has a greater affinity for VLDL than the mono-sialylated or non-sialylated forms and should result in relatively high VLDL sialic acid content in hypertriglyceridemia [14,44].
A decrease in the concentration of B:C-III and B:E lipoproteins in plasma was reported for subjects under-going treatment with the fibric acid derivative fenofi-brate [73]. These changes should result in decreases in the sialic acid content of the apo B-containing lipo-protein fractions due to a lower content of sialylated apolipoproteins (apo C-III and E). Millar et al. [63] showed that the sialic acid content of VLDL, IDL and LDL decreased following ciprofibrate treatment al-though apo C-III and E levels were not measured in this study. Other treatments or interventions that result in changes in the content of sialylated apolipoproteins within a lipoprotein fraction would be expected to be accompanied by corresponding changes in the sialic acid content of that lipoprotein fraction.
Gangliosides are largely associated with lipoproteins in plasma with a small proportion bound to albumin [58,59]. There are no apparent differences in the pro-portion of individual gangliosides found on VLDL and LDL but there are differences between LDL and HDL [58]. This latter observation would suggest that ex-change of gangliosides between lipoproteins, if it oc-curs, is carrier-mediated. In vitro data suggest that ganglioside exchange between lipoproteins is both car-rier-mediated and spontaneous [74]. Therefore other factors, possibly lipid composition, may affect any spontaneous transfer of gangliosides between lipo-proteins. Overproduction of ganglioside GD3 has been
described in malignant melanoma resulting elevated ganglioside levels in plasma, though it is not known how this is reflected in plasma lipoproteins [6].
4.3. Addition, remo6al, or modification of
apolipoprotein and ganglioside sialic acid following secretion into plasma
Native lipoproteins and apolipoproteins are com-monly referred to as being desialylated, implying that they were secreted as sialylated entities and that sialic acid was removed, either enzymatically or chemically, in plasma (Fig. 3D) [34,75,76]. This concept gained favor in the lipoprotein field when it was noted that certain apolipoproteins secreted from cultured cells had a higher sialic acid content than the same apolipo-proteins isolated from plasma [66]. The assumption was made that the difference in sialic acid content between apolipoproteins secreted in vitro and those found in vivo was due to loss of sialic acid in plasma. It is now known that the reason for this discrepancy, as de-scribed in Section 4.1, is that there is differential expres-sion of sialyltransferases in liver and cultured cells. This differential expression can result in variability in the sialylation of the same apolipoprotein secreted from these two sources [3].
There is little evidence to support the action of desialylation in normal plasma. Neuraminidase, a lyso-somal enzyme that cleaves sialic acid from oligosaccha-ride chains, is detectable in plasma but has a pH optimum between 4 and 5 [77]. Hanson et al. [78] have detected increased plasma neuraminidase levels follow-ing acute myocardial infarction supportfollow-ing the concept that the enzyme has entered plasma following tissue damage. Several studies have been unable to demon-strate desialylation of sialylated plasma proteins in vivo, the exception being experimental peritonitis where there are high plasma levels of bacterial-derived neu-raminidase [48,79,80].
explana-J.S.Millar/Atherosclerosis154 (2001) 1 – 13 8
tion is that these are normal structures that are secreted on apo B-100 in addition to di-sialylated complex-type oligosacccharides.
Several studies report a decrease in the sialic acid content of LDL following LDL oxidation [85 – 87]. There is no accompanying increase in free sialic acid suggesting that sialic acid is oxidized to a product that is not detectable using conventional assays for sialic acid [86,87]. Van Lenten and Ashwell [88] showed that oxidized sialic acid has a molar extinction coefficient that is 45% lower than unmodified sialic acid when measured using the Warren method [89]. Thus, oxidized sialic acid is underestimated using this method of mea-surement. The resorcinol method of Svennerholm is less sensitive to this modification, oxidized sialic acid having a molar extinction coefficient that is 10% greater than unmodified sialic acid [90]. It is not clear if product of sialic acid oxidation remains bound to the oligosaccha-ride chain of its parent glycolipid or apolipoprotein [86,87]. Tertov et al. [86] showed that oxidatively modified sialic acid is not found in the free form (i.e. remains apolipoprotein- or glycolipid-bound) while re-sults from Tanaka et al. [87] were inconclusive.
Tertov et al. [86] have raised the possibility of trans-fer of sialic acid from lipoproteins to other plasma constituents. AlthoughTrypanosoma cruzi has been re-ported to express a trans-sialidase that transfers protein-bound donor sialic acid to an acceptor glyco-protein, there is no evidence of this occurring in hu-mans [2]. While sialyltransferases are demonstrable in plasma these are, as with neuraminidase, likely intracel-lular enzymes that serve no function in plasma but have entered plasma in response to tissue injury [91]. Fur-thermore, human sialyltransferases do not transfer protein-bound sialic acid to an acceptor but instead transfer nucleotide-activated sialic acid (CMP-neu-raminic acid), which is not found in plasma, to galacto-sylated acceptors [61].
5. VLDL sialic acid
Apo B-100, C-II, C-III, E and gangliosides (Table 3) are the main contributors to the sialic acid content of VLDL. Sialic acid makes a major addition of charge to VLDL, its electrophoretic mobility decreasing following neuraminidase treatment [92]. VLDL from hyper-triglyceridemic subjects was found to be a relatively poor substrate for bovine milk lipoprotein lipase whereas incubation of hypertriglyceridemic VLDL with neuraminidase normalized its lipolysis by this enzyme. Stoline et al. [43] also noted an increased proportion of apo C-III2in hypertriglyceridemic VLDL although this
had no effect on lipolysis of VLDL [44]. Lindbohm et al. [93] reported a decreased content of sialic acid on VLDL from subjects with combined hyperlipidemia
Table 3
An example of the molar contribution of each sialic acid-containing constituent to the total sialic acid content of the major apo B-100-containing lipoprotein fractions based on the average normal fasting apolipoprotein [52,71,106] and ganglioside [58] content
VLDL
Total sialic acid (mol/mol) 19.6 138.0–309.0
aRange of sialic acid contents for apo (a) containing from 12 to 41 kringle IV repeats [107].
when compared to those with primary hypercholes-terolemia. This may reflect enrichment of VLDL with protein constituents that are relatively poor in sialic acid or possibly a depletion of sialylated proteins from VLDL since sialylation was expressed on a per protein basis.
6. LDL sialic acid
Sialic acid seems to have multiple functions on LDL. As with VLDL, sialic acid contributes to the charge of LDL [94,95]. It has been reported that partial desialyla-tion of LDL results in its precipitadesialyla-tion from plasma or physiological saline [96]. Orekhov et al. [12] have de-scribed a sialic acid-poor LDL fraction in patients with CHD. Presumably this fraction contains either a rela-tively low amount of sialic acid-containing constituents or contains constituents with a low degree of sialylation (or a combination of these). It has been noted that sialic acid-poor LDL isolated from plasma has in-creased propensity to aggregate [97]. This would sug-gest that sialic acid enhances the solubility of LDL in an aqueous environment. Sialic acid may also be in-volved in cellular recognition of LDL as it has been shown to be an antigenic determinant [98].
removed by the reticuloendothelial system. Lindbohm et al. [93] studied the clearance of native LDL contain-ing varycontain-ing amounts of sialic acid and found that there was no effect of sialic acid content on LDL clearance. Desialylation of LDL had no effect on its binding and uptake by fibroblasts or hepatocytes [82,99]. How-ever desialylated LDL and sialic acid-poor LDL both show an enhanced uptake by aortic smooth muscle cells [12,62]. Enrichment of LDL with ganglioside to in-crease its sialic acid content dein-creased LDL uptake by aortic smooth muscle cells [62]. The reasons for the differences in uptake by the different cell types may be due to the differential expression of lipoprotein recep-tors. Grewal et al. [85] have hypothesized the clearance of desialylated LDL via a lectin receptor. Yoshida et al. [100] recently described a lipoprotein receptor, LOX-1, that recognizes oxidized LDL. This receptor is ho-mologous to the natural killer cell antigen (NKR-P1), which has lectin-like qualities [101]. Since sialic acid-poor LDL have many of the qualities of oxidized LDL the possibility that the enhanced uptake of desialylated and sialic acid-poor LDL is due to oxidation rather than decreased sialylation or desialylation must be con-sidered [102]. It may be worthwhile to investigate if differences in binding and uptake are found with LDL that has been desialylated and subsequently resialy-lated. This would enable oxidative effects to be distin-guished from sialic acid-specific effects.
Camejo et al. [94] first reported that desialylation of LDL increased its interaction with chondroitin-6-sul-fate-rich proteoglycans isolated from arterial wall ma-trix. Enrichment of LDL with gangliosides decreased the interaction of LDL with chondroitin-6-sulfate-rich proteoglycans [63]. However there is no difference in the interaction of LDL with relatively low and high sialic acid content isolated from separate individuals [63]. This suggests that individual sialic acid-containing components on LDL rather than total LDL sialic acid can affect the interaction of LDL with chondroitin-6-sulfate-rich proteoglycans.
7. Lp(a) sialic acid
Among lipoproteins, Lp(a) is the most highly sialy-lated (Table 3). Lp(a) sialic acid is derived primarily from apo (a), with gangliosides and apo B-100 also making contributions. Apo B-100 derived from Lp(a) has the same carbohydrate content, including sialic acid, as apo B-100 from LDL [103]. Presumably the ganglioside content of Lp(a) is also similar to that of LDL. The major difference in the sialic acid content between Lp(a) and LDL is the contribution of sialic acid from apo (a) in Lp(a). The sialic acid content of Lp(a) should vary depending on the kringle length of the apo (a) polypeptide [52]. The association of apo (a)
with LDL to form Lp(a) is not dependent on the sialylation of apo (a) [53].
Tertov and Orekhov [97] demonstrated that Lp(a) from healthy subjects did not cause lipid accumulation in aortic smooth muscle cells. However, Lp(a) from subjects with CHD, which was relatively poor in sialic acid, did result in cholesterol accumulation in cultured aortic smooth muscle cells. Desialylation of Lp(a) also resulted in lipid accumulation in this cell type. These authors also divided LDL from a single donor into sialic acid-poor and sialic acid-rich fractions. The sialic acid-poor fraction resulted in lipid accumulation and was prone to aggregation while the sialic acid-rich fraction had neither of these effects [97]. Due to similar-ities between LDL and Lp(a) the possibility of these effects being due to oxidation should be considered.
8. HDL sialic acid
The major contributors of sialic acid on HDL are apo C-III, E, J and gangliosides. Due to the large variation of HDL sizes and protein contents within each size range it is difficult to calculate an average HDL sialic acid content. Lindbohm et al. [93] reported that subjects with combined hyperlipidemia had higher sialic acid per HDL protein than subjects with primary hypercholesterolemia although the significance of this is unclear. The only known function of sialic acid on HDL metabolism is related to apo E [48]. Phospholipid vesicles enriched with sialylated apo E were better at promoting cholesteryl ester uptake from HDL to HepG2 cells than neuraminidase treated (desialylated) HDL or vesicles enriched with desialylated apo E. Vesicles containing apo E that had been desialylated and then resialylated also resulted in enhanced cholesteryl ester uptake by HepG2 cells demonstrating a sialic acid-specific effect.
9. Future areas of research
Thus far, research into lipoprotein sialylation has uncovered some interesting findings but is far from complete. Tools currently available to scientists study-ing carbohydrate linkages, oligosaccharide structures, and gene expression should facilitate further research into this area. Some of the more important questions brought up in this review are summarized below.
J.S.Millar/Atherosclerosis154 (2001) 1 – 13 10
are needed to provide a clearer picture of the role of sialic acid in lipoprotein metabolism. The effect of desialylation of apolipoproteins should also be exam-ined to determine the role of sialic acid on apolipo-protein secretion, clearance, lipid binding, and function. The effects of enrichment of lipoproteins with indi-vidual gangliosides should be investigated to determine the effect on lipoprotein clearance and uptake, the effects on intracellular cholesterol accumulation and the development of atherosclerosis. Similarly, the effects of desialylation on cholesterol accumulation should be studied in more detail to determine if these effects are truly due to desialylation or due to mild oxidation of lipoproteins.
This basic information regarding apolipoprotein sia-lylation would aid the development of cell and animal systems in which the sialylation of individual apolipo-protein(s) and/or gangliosides is manipulated. Manipu-lation of sialyManipu-lation could be through gene over-expression, gene knockout, or through site directed mutagenesis by which glycosylation sites on proteins are eliminated or added. These systems would be useful in studying the metabolic effects of sialylation and its effect on the development of atherosclerosis.
10. Conclusion
Lipoprotein sialylation appears to be an inherently complex process that is under control of factors that are not fully understood. Studies thus far have impli-cated a role for sialic acid in the affinity of some apolipoproteins for lipoproteins, apolipoprotein secre-tion into and their clearance from plasma. Sialic acid on plasma lipoproteins has been associated with several processes thought to influence the development of atherosclerosis. As understanding of the complexity of lipoprotein sialylation improves, the role of sialic acid in these processes should become clearer. Manipulation of lipoprotein sialylation may prove to be a useful tool to slow or prevent the development of atherosclerosis.
References
[1] Budavari S, editor. Merck Index, 11th ed. Rahway: Merck, 1989:1345.
[2] Schauer R, Kamerling JP. Chemistry and biochemistry of sialic acids. In: Montreuil J, Vliegenthart JFG, Schacter H, editors. Glycoproteins II. Amsterdam: Elsevier, 1997:243 – 402. [3] Paulson JC. Glycoproteins: what are the sugar chains for? TIBS
1989;14:272 – 6.
[4] Ravindranaths MH, Paulson JC, Irie FR. Human melanoma antigenO-acetylated ganglioside GD3is recognized by Cancer antennariuslectin. J Biol Chem 1988;263:2079 – 86.
[5] Waters PJ, Lewry E, Pennock CA. Measurement of sialic acid in serum and urine: clinical applications and limitations. Ann Clin Biochem 1992;29:625 – 37.
[6] Sillanaukee P, Ponnio M, Jaasskelainen IP. Occurrence of sialic acids in healthy humans and different disorders. Eu J Clin Invest 1999;29:413 – 25.
[7] Sarria A, Moreno LA, Mur M, Lazaro A, Lasierra MP, Roda L, Giner A, Larrad L. Relationship between immunoinflamma-tory proteins containing sialic acid and low-density lipoprotein serum concentrations. Clin Chim Acta 1996;252:21 – 31. [8] Lindberg G, Eklund G, Gullberg B, Rastam L. Serum sialic
acid concentration and cardiovascular mortality. Br Med J 1991;302:143 – 6.
[9] Watts GF, Crook MA, Haq S, Mandalia S. Serum sialic acid as an indicator of change in coronary artery disease. Metabolism 1995;44:147 – 8.
[10] Rastam L, Lindberg G, Folsom AR, Burke GL, Nilsson-Ehle P, Lundblad A. Association between serum sialic acid concen-tration and carotid atherosclerosis measured by B-mode ultra-sound. Int J Epidemiol 1996;25:953 – 8.
[11] Ruelland A, Gallou G, Legras B, Paillard F, Cloarec L. LDL sialic acid content in patients with coronary artery disease. Clin Chim Acta 1993;221:127 – 33.
[12] Orekhov AN, Tertov VV, Mukhin DN, Mikhailenko IA. Mod-ification of low density lipoprotein by desialylation causes lipid accumulation in cultured cells: discovery of desialylated with altered cellular metabolism in the blood of atherosclerotic patients. Biochem Biophys Res Commun 1989;162:206 – 11. [13] Jaakkola O, Solakivi T, Tertov VV, Orekhov AN, Miettinen
TA, Nikkari T. Characteristics of low-density lipoprotein sub-fractions from patients with coronary artery disease. Coron Artery Dis 1993;4:379 – 85.
[14] Anber V, Millar JS, McConnell M, Shepherd J, Packard CJ. Interaction of very-density, intermediate-density, and low-density lipoproteins with human arterial wall proteoglycans. Arterioscler Thromb Vasc Biol 1997;17:2507 – 14.
[15] Fless GM, ZumMallen ME, Scanu AM. Physicochemical prop-erties of apolipoprotein(a) and lipoprotein(a) derived from the dissociation of human plasma lipoprotein (a). J Biol Chem 1986;261:8712 – 8.
[16] Seman LJ, Breckenridge WC. Isolation and partial characteri-zation of apolipoprotein (a) from human lipoprotein (a). Biochem Cell Biol 1986;64:999 – 1009.
[17] Lackner KJ, Edge SB, Gregg RE, Hoeg JM, Brewer HB, Jr. Isoforms of apolipoprotein A-II in human plasma and thoracic duct lymph. Identification of proapolipoprotein A-II and sialic acid-containing isoforms. J Biol Chem 1985;260:703 – 6. [18] Sasak WV, Lown JS, Colburn KA. Human small-intestinal
apolipoprotein B-48 oligosaccharide chains. Biochem J 1991;274:159 – 65.
[19] Swaminathan N, Aladjem F. The monosaccharide composition and sequence of the carbohydrate moiety of human serum low density lipoprotein. Biochemistry 1976;15:1516 – 22.
[20] Taniguchi T, Ishikawa Y, Tsunemitsu M, Fukuzaki H. The structure of the asparagine-linked sugar chains of human apo B-100. Arch Biochem Biophys 1989;273:197 – 205.
[21] Fojo SS, Taam L, Fairwell T, Ronan R, Bishop C, Meng MS, Hoeg JM, Sprecher DL, Brewer HB, Jr. Human preproapolipo-protein C-II. Analysis of major plasma isoforms. J Biol Chem 1986;261:9591 – 4.
[22] Vaith P, Assman G, Uhlenbruck G. Characterization of the oligosaccharide side chains of apolipoprotein C-III from human plasma. Biochim Biophys Acta 1978;541:234 – 40.
[23] McConathy WJ, Alaupovic P. Studies on the isolation and partial characterization of apolipoprotein D and lipoprotein D of human plasma. Biochemistry 1976;15:515 – 20.
[25] Burkey BF, deSilva HV, Harmony JA. Intracellular processing of apolipoprotein J precursor to the mature heterodimer. J Lipid Res 1991;32:1039 – 48.
[26] Zhang LH, Kotite L, Havel RJ. Identification, characterization, cloning, and expression of apolipoprotein C-IV, a novel sialo-glycoprotein of rabbit plasma lipoproteins. J Biol Chem 1996;271:1776 – 83.
[27] Gambino R, Ruiu G, Pagano G, Cassader M. Qualitative analysis of the carbohydrate composition of apolipoprotein H. J Protein Chem 1997;16:205 – 12.
[28] Xu N, Dahlback B. A novel human apolipoprotein. J Biol Chem 1999;274:31286 – 90.
[29] Ito Y, Breslow JL, Chait BT. Apolipoprotein C-III0 lacks carbohydrate residues: use of mass spectrometry to study apolipoprotein structure. J Lipid Res 1989;30:1781 – 7. [30] Wernette-Hammond ME, Lauer SJ, Corsini A, Walker D,
Taylor JM, Rall SC, Jr. Glycosylation of human apolipoprotein E. J Biol Chem 1989;264:9094 – 191.
[31] Sprecher DL, Taam L, Brewer HB, Jr. Two-dimensional elec-trophoresis of human plasma apolipoproteins. Clin Chem 1984;30:2084 – 92.
[32] Dwek RA, Edge CJ, Harvey IDJ, Wormald MR, Parekh RB. Analysis of glycoprotein-associated oligosaccharides. Ann Rev Biochem 1993;62:65 – 100.
[33] Remaley AT, Wong AW, Schumacher UK, Meng MS, Brewer HB, Jr., Hoeg JM.O-linked glycosylation modifies the associa-tion of apolipoprotein A-II to high density lipoproteins. J Biol Chem 1993;268:6785 – 90.
[34] Bartlett AL, Stanley KK. All low density lipoprotein particles are partially desialylated in plasma. Atherosclerosis 1998;138:237 – 45.
[35] Watanabe K, Mizuta M. Fluorometric detection of glycosphin-golipids on thin-layer chromatographic plates. J Lipid Res 1995;36:1848 – 55.
[36] Fujioka Y, Taniguchi T, Ishikawa Y, Shiomi M, Yokoyama M. Relation ofN-glycosylation of apolipoprotein B-100 to cellular metabolism of low density lipoprotein. Atherosclerosis 1994;108:91 – 102.
[37] Tsunemitsu M, Ishikawa Y, Taniguchi T, Fukuzaki H. Hetero-geneity of N-linked sugar chains of apolipoprotein B-100 in Watanabe heritable hyperlipidemic and fasting rabbits. Arte-riosclerosis 1990;10:386 – 93.
[38] Wada Y, Nishikawa A, Okamoto N, Inui K, Tsukamoto H, Okada S, Taniguchi N. Structure of serum transferrin in carbo-hydrate-deficient glycoprotein syndrome. Biochem Biophys Res Comm 1992;189:832 – 6.
[39] Nikkari ST, Koivu TA, Anttila P, Raunio I, Sillanaukee P. Carbohydrate-deficient transferrin and gamma-glutamyltrans-ferase are inversely associated with lipid markers of cardiovas-cular risk. Eur J Clin Invest 1998;28:793 – 7.
[40] Havel RJ, Kotie L, Kane JP. Isoelectric heterogeneity of the cofactor protein for lipoprotein lipase in human blood plasma. Biochem Med 1979;21:121 – 38.
[41] Brewer HB, Jr., Shulman R, Herbert P, Ronan R, Wehrly K. The complete amino acid sequence of alanine apolipoprotein, (apo C-III), an apolipoprotein from human plasma very low density lipoproteins. J Biol Chem 1974;249:4975 – 84.
[42] Holdsworth G, Stocks J, Dodson P, Galton DJ. An abnormal triglyceride-rich lipoprotein containing excess sialylated apolipoprotein C-III. J Clin Invest 1982;69:932 – 9.
[43] Stoline AM, Saku K, Hynd BA, Kashyap ML. Effect of desialylation of very low-density lipoproteins on their catabolism by lipoprotein lipase. Metabolism 1985;34:30 – 5. [44] Mann CJ, Troussard AA, Yen FT, Hannouche N, Najib J,
Fruchart J-C, Lotteau V, Andre P, Bihain BE. Inhibitory effects of specific apolipoprotein C-III isoforms on the binding of triglyceride-rich lipoproteins to the lipolysis-stimulated re-ceptor. J Biol Chem 1997;272:31348 – 54.
[45] Catapano AL. Activation of lipoprotein lipase by apolipo-protein C-II is modulated by the COOH terminal region of apolipoprotein C-III. Chem Phys Lipids 1987;45:39 – 47. [46] Kamboh MI, Albers JJ, Majumder PP, Ferrell RE. Genetic
studies of human apolipoproteins. IX. Apolipoprotein D poly-morphism and its relation to serum lipoprotein lipid levels. Am J Hum Genet 1989;45:147 – 54.
[47] Schindler PA, Settineri CA, Collett X, Fielding CJ, Burlingame AL. Site-specific detection and structural characterization of the glycosylation of human plasma proteins lecithin: cholesterol acyltransferase and apolipoprotein D using HPLC/electrospray mass spectrometry and sequential glycosidase digestion. Protein Sci 1995;4:791 – 803.
[48] Ghiselli G, Beigel Y, Soma M, Gotto AM, Jr. Plasma catabolism of human apolipoprotein E isoforms: lack of con-version of the doubly sialylated form to the asialo form. Metabolism 1986;35:399 – 403.
[49] Marmillot P, Rao MN, Liu QH, Lakshman MR. Desialylation of human apolipoprotein E decreases its binding to human high-density lipoprotein and its ability to deliver esterified cholesterol to the liver. Metabolism 1999;48:1184 – 92. [50] Ghosh P, Chirtel SJ, Lakshman MR. Effect of chronic ethanol
on apolipoprotein (Apo) E synthesis and glycosylation in rats. Alcohol Clin Exp Res 1991;15:725 – 9.
[51] Ghosh P, Hale EA, Lakshman R. Long-term ethanol exposure alters the sialylation index of plasma apolipoprotein J (Apo J) in rats. Alcohol Clin Exp Res 1999;23:720 – 5.
[52] Koschinsky ML, Tomlinson JE, Zioncheck TF, Schwartz K, Eaton DL, Lawn RM. Apolipoprotein(a): expression and char-acterization of a recombinant form of the protein in mam-malian cells. Biochemistry 1991;30:5044 – 51.
[53] Frank S, Krasznai K, Durovic S, Lobentanz E-M, Dieplinger H, Wagner E, Zatlouka K, Cotton M, Utermann G, Kostner GM, Zechner R. High-level expression of various apolipo-protein(a) isoforms by ‘‘transferrinfection’’: the role of kringle IV sequences in the exracellular association with low-density lipoprotein. Biochemistry 1994;33:12329 – 39.
[54] Stack MS, Pizzo SV, Gonzalez-Gronow M. Effect of desialyla-tion on the biological properties of human plasminogen. Biochem J 1992;284:81 – 6.
[55] Seifert PS, Roth I, Zioncheck TF. The apolipoprotein(a) moiety of lipoprotein(a) interacts with the complement activation frag-ment iC3b but does not functionally affect C3 activation or degradation. Atherosclerosis 1992;93:209 – 16.
[56] Svennerholm L. The gangliosides. J Lipid Res 1964;5:145 – 55. [57] Senn HJ, Sellin S, Fitzke E, Stehle T, Haussinger D, Wieland H, Gerok W. Biosynthesis and excretion of gangliosides by the isolated perfused rat liver. Eur J Biochem 1992;205:809 – 14. [58] Senn HJ, Orth M, Fitzke E, Wieland H, Gerok W.
Gan-gliosides in normal human serum. Concentration, pattern and transport by lipoproteins. Eur J Biochem 1989;181:657 – 62. [59] Rebba A, Portoukalian J. Distribution of exogenously added
gangliosides in serum proteins depends on the relative affinity of albumin and lipoproteins. J Lipid Res 1995;36:564 – 72. [60] Ruan S, Raj BK, Lloyd KO. Relationship of
glycosyltrans-ferases and mRNA levels to ganglioside expression in neurob-lastoma and melanoma cells. J Neurochem 1999;72:514 – 21. [61] Tsuji S. Molecular cloning and functional analysis of
sialyl-transferases. J Biochem 1996;120:1 – 13.
[62] Filipovic I, Schwarzmann G, Mraz W, Wiegandt H, Buddecke E. Sialic-acid content of low-density lipoproteins controls their binding and uptake by cultured cells. Eur J Biochem 1979;93:51 – 5.
J.S.Millar/Atherosclerosis154 (2001) 1 – 13 12
[64] Thomas PD, Poznansky MJ. Cholesterol transfer between lipid vesicles. Effect of phospholipids and gangliosides. Biochem J 1988;251:55 – 61.
[65] Sobenin IA, Tertov VV, Orekhov AN. Optimization of the assay for sialic acid determination in low density lipoprotein. J Lipid Res 1998;39:2293 – 9.
[66] Zannis VI, vanderSpek J, Silverman D. Intracellular modifica-tion of human apolipoprotein E. J Biol Chem 1986;261:13415 – 21.
[67] Basu SK, Ho YK, Brown MS, Bilheimer DW, Anderson RGW, Goldstein JL. Biochemical and genetic studies of the apolipo-protein E secreted by mouse macrophages and human mono-cytes. J Biol Chem 1982;257:9788 – 95.
[68] Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta 1987;917:148 – 61.
[69] Elovson J, Chatterton JE, Bell GT, Schumaker VN, Reuben MA, Puppione DL, Reeve JR, Jr., Young NL. Plasma very low density lipoproteins contain a single molecule of apolipoprotein B. J Lipid Res 1988;29:1461 – 73.
[70] Chan L. Apolipoprotein B, the major protein component of triglyceride-rich and low density lipoproteins. J Biol Chem 1992;267:25621 – 4.
[71] Gotto AM, Jr., Pownall HJ, Havel RJ. Introduction to lipo-proteins. In: Segrest JJ, Albers JJ, editors. Methods of enzymol-ogy. London: Academic Press, 1986:3 – 41.
[72] Camato R, Marcel YL, Milne RW, Lusser-Cacan S, Weech PK. Protein polymorphism of a human plasma apolipoprotein D antigenic epitope. J Lipid Res 1989;30:865 – 75.
[73] Lussier-Cacan S, Bard J-M, Boulet L, Nestruck AC, Grothe A-M, Fruchart J-C, Davignon J. Lipoprotein composition changes induced by fenofibrate in dysbetalipoproteinemia type III. Atherosclerosis 1989;78:167 – 82.
[74] Via DP, Massey JB, Vignale S, Kundu SK, Marcus DM, Pownall HJ, Gotto AM, Jr. Spontaneous and plasma factor-mediated transfer of pyrenyl cerebrosides between model and native lipoproteins. Biochim Biophys Acta 1985;837:27 – 34. [75] Zannis VI, McPherson J, Goldberger G, Karathanasis SK,
Breslow JL. Synthesis, intracellular processing, and signal pep-ticle of human apolipoprotein E. J Biol Chem 1984;259:5495 – 9. [76] Orekhov AN, Tertov VV, Mukhin DN. Desialylated low den-sity lipoprotein-naturally occurring modified lipoprotein with atherogenic potency. Atherosclerosis 1991;86:153 – 61. [77] Schauer R, Veh RW, Wember M. Demonstration of
neu-raminidase activity in human blood serum and human milk using a modified radioactively labeled 1-glycoprotein as sub-strate. Hoppe-Seyler’s Z Physiol Chem 1976;357:559 – 66. [78] Hanson VA, Shettigar UR, Loungani RR, Nadijcka MD.
Plasma sialidase activity in acute myocardial infarction. Am Heart J 1987;114:59 – 63.
[79] Hof L, Loegering DJ. Increase in plasma neuraminidase activ-ity in experimental peritonitis. Proc Soc Exp Biol Med 1982;169:501 – 5.
[80] Nestel PJ, Fidge NH. Apoprotein C catabolism in man. Adv Lipid Res 1982;19:55 – 83.
[81] Malmendier CL, Delcroix C, Fontaine M. Effect of sialic acid removal on human low density lipoprotein catabolism in vivo. Atherosclerosis 1980;37:277 – 84.
[82] Attie AD, Weinstein DB, Freeze HH, Pittman RC, Steinberg D. Unaltered catabolism of desialylated low-density lipoprotein in the pig and in cultured rat hepatocytes. Biochem J 1979;180:647 – 54.
[83] Hatton MWC, Regoeczi E, Kaur H. Bovine transferrin gly-copeptide: the relevance of its structure with the mammalian hepatic lectin that binds asialoglycoproteins. Can J Biochem 1978;56:339 – 44.
[84] Morell AG, Gregoriadis A, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glyco-proteins in the circulation. J Biol Chem 1971;246:1461 – 7. [85] Grewal T, Bartlett A, Burgess JW, Packer NH, Stanley KK.
Desialylated LDL uptake in human and mouse macrophages can be mediated by a lectin receptor. Atherosclerosis 1996;121:151 – 63.
[86] Tertov VV, Kaplun VV, Sobenin IA, Orekhov AN. Low-den-sity lipoprotein modification occurring in human plasma possi-ble mechanism of in vivo lipoprotein desialylation as a primary step of atherogenic modification. Atherosclerosis 1998;138:183 – 95.
[87] Tanaka K, Tokumaru S, Kojo S. Possible involvement of radical reactions in desialylation of LDL. FEBS Lett 1997;413:202 – 4.
[88] Van Lenten L, Ashwell G. Studies on the chemical and enzy-matic modification of glycoproteins. J Biol Chem 1971;246:1889 – 94.
[89] Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem 1959;234:1971 – 5.
[90] Svennerholm L. Quantitative estimation of sialic acids. Acta Chem Scand 1958;12:547 – 54.
[91] Gross HJ, Brossmer R. Characterization of human plasma sialyltransferase using a novel fluorometric assay. Clin Chim Acta 1991;197:237 – 48.
[92] Bijl VD, Reman FC. Human very low density lipoproteins: loss of electrophoretic mobility on enzymatic removal of sialic acid residues. Clin Chim Acta 1975;60:191 – 5.
[93] Lindbohm N, Gylling H, Miettinen TE, Miettinen TA. Sialic acid content of LDL and lipoprotein metabolism in combined hyperlipidemia and primary moderate hypercholesterolemia. Clin Chim Acta 1999;285:69 – 84.
[94] Camejo G, Lopez A, Lopez F, Quinones J. Interaction of low density lipoproteins with arterial proteoglycans. The role of charge and sialic acid content. Atherosclerosis 1985;55:93 – 105. [95] Vedie B, Jeunemaitre X, Megnien JL, Myara I, Trebden H, Simon A, Moatti N. Charge heterogeneity of LDL in asymp-tomatic hypercholesterolemic men is related to lipid parameters and variations in the ApoB and C-III genes. Arterioscler Thromb Vasc Biol 1998;18:1780 – 9.
[96] Fontaine M, Malmendier CL. The effect of fasting on the interactions between human low density lipoproteins and cal-cium, heparin and chondroitin sulfate. Clin Chim Acta 1979;94:201 – 5.
[97] Tertov W, Orekhov AN. Effect of lipoprotein(a) on lipid metabolism of cultured human intimal aortic cells. Chem Phys Lipids 1994;67 – 68:161 – 6.
[98] Goldstein S, Chapman MJ. Role of the carbohydrate moiety in the antigenic site(s) of human serum low-density lipoprotein. Biochemistry 1981;20:1025 – 32.
[99] Shireman RB, Fisher WR. The absence of a role for the carbohydrate moiety in the binding of apolipoprotein B to the low density lipoprotein receptor. Biochim Biophys Acta 1979;572:537 – 40.
[100] Yoshida H, Kondratenko N, Green S, Steinberg D, Quehen-berger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J 1998;334:9 – 13. [101] Bezouska K, Vlahas G, Horvth O, Jinochova G, Fiserova A,
[103] Gaubatz JW, Chad MV, Nava ML, Guyton JR, Morrisett JD. Isolation and characterization of the two major apoproteins in human lipoprotein(a). J Lipid Res 1987;28:69 – 79.
[104] Negroni E, Chigorno V, Tettamanti G, Sonnino S. Evaluation of the efficiency of an assay procedure for gangliosides in human serum. Glycoconj J 1996;13:347 – 52.
[105] Leenders RGG, de Jong JGN, Wevers RA. Extraction and purification of gangliosides from plasma and fibroblasts before
analysis by thin layer chromatography. Ann Clin Biochem 1995;32:68 – 73.
[106] deSilva HV, Stuart WD, Duvic CR, Wetterau JR, Ray MJ, Ferguson DG, Albers HW, Smith WR, Harmony JAK. A 70-kDa apolipoprotein designated apoJ is a marker for sub-classes of human plasma high density lipoproteins. J Biol Chem 1990;265:13240 – 7.
[107] Klezovich O, Scanu AM. Heterogeneity of lipoprotein (a): growing complexities. Curr Opin Lipidol 1995;6:223 – 8.