Interrelated modification of excitatory and inhibitory
synapses in three-layer olivary-cerebellar neural network
Isabella Silkis *
Laboratory of‘Neurophysiology of Learning’,
Institute of Higher Ner6ous Acti6ity and Neurophysiology of the Russian Academy of Sciences,Butlero6a5a street, Moscow117865,Russia
Received 19 July 1999; accepted 20 October 1999
Abstract
The model of three-layer olivary-cerebellar neural network with modifiable excitatory and inhibitory connections between diverse elements is suggested. The same Hebbian modification rules are proposed for Purkinje cells, granule (input) cells, and deep cerebellar nuclei (output) cells. The inverse calcium-dependent modification rules for these cells and hippocampal/neocortical neurones or Golgi cells are conceivably the result of the involvement of cGMP and cAMP in postsynaptic processes. The sign of simultaneous modification of excitatory and inhibitory inputs to a cell is opposite and determined by the variations in pre- and/or postsynaptic cell activity. Modification of excitatory transmission between parallel fibers and Purkinje cells, mossy fibers and granule cells, and mossy fibers and deep cerebellar nuclei cells essentially depends on inhibition effected by stellate/basket cells, Golgi cells and Purkinje cells, respectively. The character of interrelated modifications of diverse synapses in all three layers of the network is influenced by olivary cell activity. In the absence (presence) of a signal from inferior olive, the long-term potentiation (depression) in the efficacy of a synapse between input mossy fiber and output cell can be induced. The results of the suggested model are in accordance with known experimental data. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords: Olivary-cerebellar network; Excitatory synapse; Inhibitory synapse; Long-term potentiation; Long-term depression; Modification rules
www.elsevier.com/locate/biosystems
1. Introduction
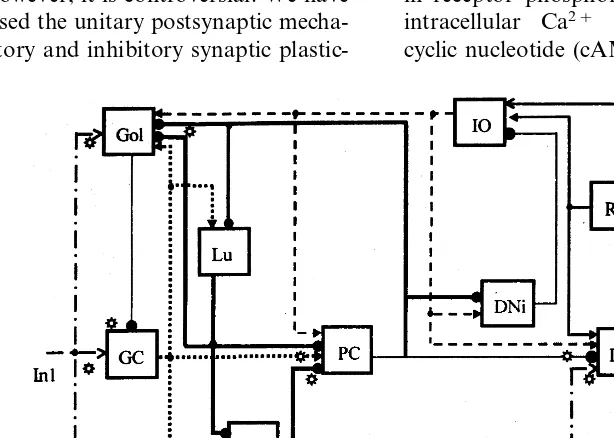
Cerebellum is the one of a few structures in the central nervous system wherein neural intercon-nections are known in detail (De Zeeuw et al.,
1998; Laine and Axelrad, 1998; Voogd and Glick-stein, 1998). These connections are schematically shown in Fig. 1. Neurones at different cerebellar layers receive information about peripheral events and central processes via mossy fibers (MFs) and climbing fibers (CFs) (Fig. 1). Output cerebellar elements, deep cerebellar nuclei cells (DCNCs), activate cerebral cortex via the thalamus. The
* Tel.: +7-95-334-4345; fax:+7-95-338-8500. E-mail address:[email protected] (I. Silkis)
knowledge of the role of cerebellum in motor learning allowed elaboration of a neuronal model of learning (Ito, 1984; Houk et al., 1996; Clark et al., 1997; Kenyon, 1997; Mauk and Donegan, 1997; Raymond and Lisberger, 1997). The Marr – Albus – Ito model of cerebellum-dependent learn-ing is based on only one type of synaptic plasticity: associative long-term depression of ex-citatory transmission (LTDe) in synaptic path-ways between parallel fibers (PFs) and Purkinje cells (PCs) (Marr, 1969; Albus, 1971; Ito, 1984). The induction of this LTDe requires a pairing of PFs and CF activation. Not only associative LTDe, but also homosynaptic LTDe and ho-mosynaptic long-term potentiation of excitatory transmission (LTPe) have been recently found in synapses formed by PFs on PCs (Hartell, 1994). In addition, long-term depression of inhibitory transmission (LTDi) between stellate or basket cells and PCs has been demonstrated (Llano et al., 1991; Kano et al., 1992). Only the mechanism of LTDe for PCs is described (Linden, 1994; Daniel et al., 1998); however, it is controversial. We have recently proposed the unitary postsynaptic mecha-nism of excitatory and inhibitory synaptic
plastic-ity for the neocortical, hippocampal and cerebellar Purkinje cells (Silkis, 1999). Using this mechanism, one can predict the character of mod-ification of excitatory and inhibitory synapses on diverse cells, if the types of postsynaptic recep-tors/channels and second messenger are known. Predicted results can be experimentally tested. The aim of this work has been to elucidate the possible mechanisms and character of simulta-neous modifications in the efficacy of excitatory and inhibitory synaptic inputs to different ele-ments of olivary-cerebellar neural network trig-gered by rhythmic activation of MFs and CF.
2. The proposed mechanism of synaptic plasticity for Purkinje cells
Synaptic plasticity in different nervous struc-tures is considered as the result of changes in the phosphorylation state of postsynaptic ionotropic AMPA, NMDA and GABAa receptors. Changes in receptor phosphorylation could be caused by intracellular Ca2+ enlargement, variations in cyclic nucleotide (cAMP or cGMP) concentration
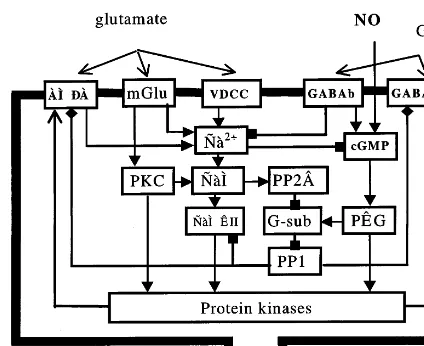
Fig. 2. The proposed consequence of post-tetanic processes, causing modification of synaptic inputs to cerebellar Purkinje cell, granule cell, deep cerebellar nuclei cell. VDCC, voltage-dependent Ca2+ channel. mGlu, metabotropic glutamate
re-ceptor; CaM, calmodulin; PKC, protein kinase C; PKG, protein kinase G; CaMKII, Ca2+/calmodulin-dependent
protein kinase II; PP2B, calcineurin; G-sub, G substrate-inhibitor of protein phosphatases 1, PP1.
causes cerebellar LTDe. However, this assump-tion is inconsistent with some of the experimental data (Daniel et al., 1998). Moreover, it means that properties of AMPA receptors on PCs and hippocampal/neocortical neurones are different, since LTDe in hippocampal/neocortical neurones is the result of AMPA receptor dephosphoryla-tion (Bear and Malenka, 1994). In PCs, LTDe and LTPi have been observed when Ca2+ level was high, while moderate Ca2+ rise resulted in LTPe and LTDi (Hirano, 1990; Hartell, 1994; Kasono and Hirano, 1994; Linden, 1994). The Ca2+ dependence of the character of synaptic modification in the neocortical/hippocampal neu-rones is opposite (Bear and Malenka, 1994; Ko-matsu, 1996). However, the commonly accepted idea of PC plasticity does not explain this distinction.
The suggested unitary postsynaptic mechanism of plasticity is based on our postulate that pro-perties of AMPA receptors (as well as GABAa receptors) are similar in PCs and hippocampal/ neocortical neurones (Silkis, 1999). Therefore, phosphorylation (dephosphorylation) of AMPA receptors leads to LTPe (LTDe) in PCs. The validity of this postulate is supported by the data that artificial activation of PKC, which must lead to additional phosphorylation of receptors, causes LTPe for the most part of PCs (Crepel and Jail-lard, 1991). Analogously, phosphorylation (dephosphorylation) of GABAa receptors results in LTDi (LTPi) in both, hippocampal/neocortical cells and PCs. This assumption is also experimen-tally supported (Pasqualotto et al., 1993). In addi-tion, we have proposed that activation of metabotropic GABAb receptors, which causes a changes in the concentration of intracellular Ca2+ and cyclic nucleotide, is required for input-specific modification of inhibitory synapses (Silkis, 1997). The necessity of GABAb receptors for the induc-tion of LTPi has been recently demonstrated in the neocortex (Komatsu, 1996).
Signal transduction processes proposed to par-ticipate in cerebellar plasticity are schematically presented in Fig. 2. We have assumed that the involvement of cGMP and cAMP in synaptic plasticity of PCs and hippocampal/neocortical and subsequent changes in the activity of protein
kinases and protein phosphatases (Ito and Kara-chot, 1992; Bear and Malenka, 1994; Linden, 1994; Daniel et al., 1998). Receptors can be phos-phorylated by cAMP-dependent protein kinase A (PKA), cGMP-dependent protein kinase G (PKG), protein kinase C (PKC) and Ca2+/ calmodulin dependent protein kinase II (CaMKII), and dephosphorylated by protein phosphatase 1. The rise of Ca2+ concentration is provided by its influx through voltage-dependent Ca2+ channels (VDCCs) after the depolarization of postsynaptic cell, and by its efflux from intra-cellular Ca2+ stores after metabotropic glutamate (mGlu) receptor activation (Bear and Malenka, 1994). Activation of mGlu receptors leads also to an increase in the activity of PKC and PKA (Linden, 1994; Breakwell et al., 1998).
cells, respectively, underlies the opposite Ca2+ -de-pendent modification rules for these structures (Silkis, 1999). This assumption is based on the data that cGMP concentration is down-regulated by Ca2+
/calmodulin (Baltrons et al., 1997), while the increase in cAMP level is positively correlated with Ca2+ rise. We have proposed that the rise in cGMP level could be provided not only by NO action on soluble guanylate cyclase (Linden, 1994; Daniel et al., 1998), but also by the activation of membrane-bound guanylate cyclase via GABAb receptors (Silkis, 1998). Our preliminary experi-mental data support this hypothesis (Silkis et al., 1998).
To provide the fulfillment of the Hebbian rule, we have postulated that only receptors activated by a transmitter are modifiable. This postulate corresponds with the data that artificial rise of intracellular Ca2+ level or increase in protein kinase activity does not lead to a change in phos-phorylation state (to LTP or LTD) in the absence of synaptic activation (Nakazawa et al., 1995; Otsu et al., 1995; Wu et al., 1998). Using the computational model of postsynaptic processes, we have found that the Hebbian rule (the coinci-dence of pre- and postsynaptic cell activity) is the only necessary condition for synaptic plasticity. Modification of simultaneously activated excita-tory and inhibiexcita-tory inputs, such as LTPe together with LTDi, or LTDe together with LTPi can be obtained only due to variations in pre- and/or postsynaptic cell activity. The sign of modification
(LTP or LTD) is determined by the shift (positive or negative) in the ratio between active protein kinases and phosphatases in relation to the ratio produced by prior stimulation (Silkis, 1998).
3. Necessary conditions for the modifications of synapses on different cerebellar cells
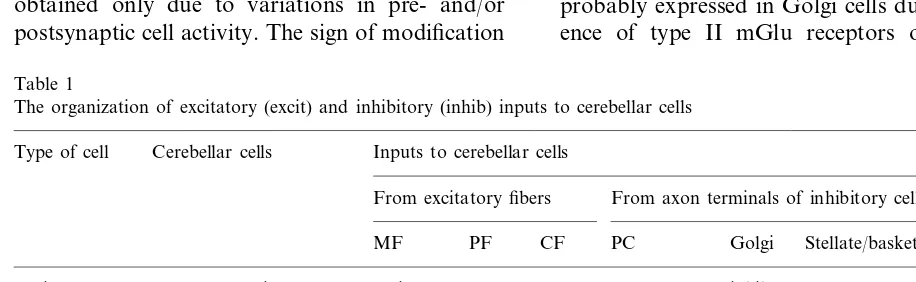
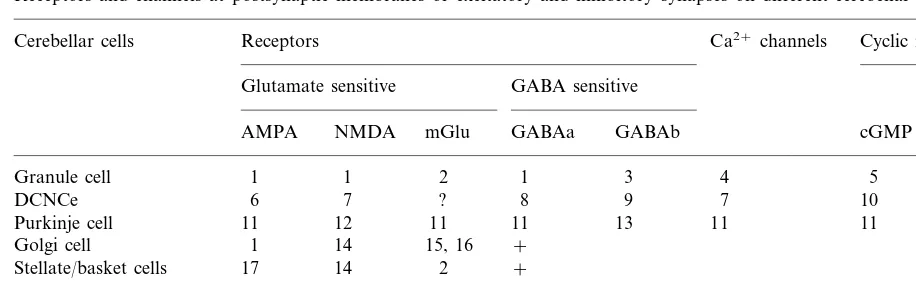
The arrangement of excitatory and inhibitory inputs to different cells of olivary-cerebellar net-works is summarized in Table 1. The examination of known experimental data have shown that postsynaptic membranes of excitatory synapses at different cerebellar cells contain NMDA recep-tors, mGlu receptors and AMPA receptors with high permeability for Ca2+ ions, while inhibitory synapses contain postsynaptic GABAa and GABAb receptors (Table 2). The presence of voltage-dependent Ca2+ channels on different cerebellar neurones was also demonstrated. Based on these data, we have assumed that necessary conditions for the changes in protein kinases and protein phosphatase activity, and subsequent synaptic modification, are fulfilled for all elements of olivary-cerebellar network. Since cGMP is ex-pressed in granule cells (GCs) and DCNCs, and since GABAb receptors are found on these cells, we used identical modification rules for PCs, GCs and DCNCs. Another cyclic nucleotide, cAMP, is probably expressed in Golgi cells due to the pres-ence of type II mGlu receptors on these cells
Table 1
The organization of excitatory (excit) and inhibitory (inhib) inputs to cerebellar cells
Type of cell Cerebellar cells Inputs to cerebellar cells
From excitatory fibers From axon terminals of inhibitory cells
MF PF CF PC Golgi Stellate/basket Lugaro
inhib excit
Granule cell (input) Excitatory
inhib excit excit
DCNCe (output)
excit
Inhibitory Purkinje cell excit inhib inhib
excit excit excit inhib inhib
Golgi cell
excit inhib inhib inhib
Stellate/basket cells excit
Lugaro cell excit inhib
Table 2
Receptors and channels at postsynaptic membranes of excitatory and inhibitory synapses on different cerebellar cellsa
Cerebellar cells Receptors Ca2+channels Cyclic nucleotides
GABA sensitive Glutamate sensitive
NMDA mGlu GABAa GABAb cGMP cAMP
AMPA
1 2 1
Granule cell 1 3 4 5
DCNCe 6 7 ? 8 9 7 10
Purkinje cell 11 12 11 11 13 11 11
14 15, 16 +
1
Golgi cell 16?
14 2
Stellate/basket cells 17 + ?
2 15
+
Lugaro cell ?
DCNCi + +
a1, Maex and De Schutter (1998); 2, Grandes et al. (1994); 3, Barthel et al. (1996); 4, D’Angelo et al. (1997); 5, Poulopoulou and
Nowak (1998); 6, Audinat et al. (1990); 7, Sastry et al. (1997); 8, Morishita and Sastry (1993); 9, Mouginot and Gahwiler (1995); 10, Biggio and Guidotti (1976); 11, Daniel et al. (1998); 12, Yuzaki et al. (1996); 13, Batchelor and Garthwait (1992); 14, Scherzer et al. (1997); 15, Hamori et al. (1996); 16, Knoflach and Kemp (1998); 17, Bureau and Mulle (1998);+, the expression of receptors is defined by presence of presynaptic terminals.
(Neki et al., 1996). We assume that modification rules for inhibitory Golgi, stellate, basket and Lugaro interneurones can be taken identical to those of hippocampal/neocortical cells.
4. Interrelated modifications in the efficacy of excitatory and inhibitory synapses in
olivary-cerebellar network
It is significant that input signal incoming via MFs simultaneously activates GCs, input cells of cerebellar cortex (input layer of the network), and excitatory DCNCs (DCNCes), output cells of cere-bellum (output layer of the network) (Fig. 1). Being transformed by the GC signal affects PCs and numerous cerebellar inhibitory interneurones, such as Golgi, stellate, basket and Lugaro cells via PFs. Every cell of the network is not only excited, but also inhibited (Table 1). Usually, models of cerebellar network do not make any allowance for this fact. Thus, inhibition of Golgi cell by Lugaro interneuron was not considered in the model of the input layer of cerebellum (Maex and De Schutter, 1998). Cerebellar interneurones inhibit each other providing the basis for disinhibition. This effect was also left out of the account in models of learning.
It follows from suggested mechanism of plastic-ity that changes in the efficacy of simultaneously activated excitatory and inhibitory synaptic inputs to a cell are interrelated. The strengthening (atten-uation) of inhibition of PCs, GCs and DCNCs causes a decrease (increase) in postsynaptic Ca2+ level, increase (decrease) in PKG activity, receptor phosphorylation (dephosphorylation), and simul-taneous induction of LTPe together with LTDi (LTDe together with LTPi). On the contrary, the strengthening (attenuation) of inhibitory inputs to cerebellar interneurones causes a decrease (in-crease) in Ca2+
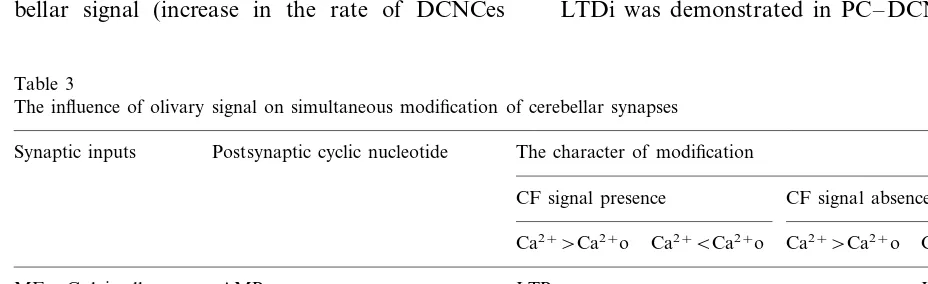
and DCNCs must affect synaptic efficacy. Thus, the character of simultaneous modification of synapses at different layers of cerebellar network depends on the presence of a signal from IO. Proposed long-term bidirectional changes in the efficacy of diverse cerebellar synapses are summa-rized in Table 3. In turn, the activity of neurons in IO is influenced by cerebellar cells (Fig. 1). Thus, DCNCis inhibit olivary neurones, while DCNCes (interpositus, for example) excite olivary cells via mesodiencefalic nuclei (red nucleus, for example). Cells of red nucleus activate also DCNCes, form-ing a reverberation loop and supportform-ing modifica-tion of synapses on DCNCes.
A signal from IO is considered as a training signal. It could teach PCs to recognize specific pattern of input signals (Marr, 1969) or train PCs to reduce error (Albus, 1971). With respect to conventional opinion, the rise of output cerebellar signal requires CF activity, since this rise is the consequence of associative LTDe in the PF – PC pathway and disinhibition of DCNCes. However, such an effect is inconsistent with experimentally found data that activity of olivary cells is inhib-ited during the reaching and all phases of the behavior (Horn et al., 1998). Unlike, according to our model, induction of LTPe in PF – PC synapses that occurs in the absence of a CF activity, this could cause an increase of inhibition of DCNCes, and advance LTP of excitatory inputs from MFs to DCNCes. Thus, a potentiation of output cere-bellar signal (increase in the rate of DCNCes
discharges) could be induced only during the si-lence of olivary neurones. This result is in a good accordance with the aforementioned experimental data (Horn et al., 1998).
Neural networks with numerous interconnected inhibitory cells have been implicated in the gener-ation of high-frequency oscillgener-ations (Whittington et al. 1995). The olivary-cerebellar network in-cludes different reciprocally connected inhibitory neurones (Fig. 1). Frequency of oscillations pro-duced by this network tends to shift to lower frequencies after an inhibition of GABAa recep-tors on IO cells (decrease of inhibition of IO cells) (Lang et al., 1996). This result leads us to the assumption that external stimulation and/or DC-NCis discharges that cause inhibition of IO cells could result in a rise in the frequency of oscillations.
5. The comparison with known experimental data
The necessity of two distinctive modifiable synapses was recently proposed in some models of cerebellar learning (Fiala et al., 1996; Kenyon, 1997; Raymond and Lisberger, 1997; Thompson et al. 1997). The modification of different cerebel-lar synapses was revealed in only a few experi-ments. LTPe was found in the MF – GC pathway (Racine et al., 1986; D’Angelo et al., 1999) and the MF – DCNC pathway (Rossi et al., 1996). LTDi was demonstrated in PC – DCNC synapses
Table 3
The influence of olivary signal on simultaneous modification of cerebellar synapses
Synaptic inputs Postsynaptic cyclic nucleotide The character of modification
CF signal presence CF signal absence
Ca2+BCa2+o
Ca2+\Ca2+o
Ca2+BCa2+o
Ca2+\Ca2+o
cAMP LTPe
MFGolgi cell LTDe
cAMP LTPe
PFGolgi cell LTDe
cGMP
MFGranule cell LTPe LTDe
cGMP LTDi LTPi
GolgiGranule cell
PFPC cGMP LTDe LTPe
LTPi
PCDCNCe cGMP LTDi
LTPe LTDe
(Morishita and Sastry, 1993), and Golgi cell – GC synapses (Robello et al., 1996; Amico et al., 1998). The character of synaptic modification is in the accordance with the suggested mechanism of cere-bellar plasticity. Thus, LTDi in PC – DCNC synapses was found in the absence of excitation (Morishita and Sastry, 1993). Owing to the ab-sence of glutamate, mGlu receptors could not be activated, and PKC was inactive. Neither depolar-ization of DCNC nor intracellular Ca2+ rise was observed in this study. In the absence of Ca2+ ions, the activation of protein phosphatase 1 and CaMKII must be excluded (Fig. 2). NO synthase is absent in PC axon terminals (Ross et al., 1990), therefore it cannot participate in cGMP produc-tion. However, cGMP levels could increase due to the action of GABA at GABAb receptors, and LTDi could be induced as a result of phosphory-lation of GABAa receptors by PKG.
In terms of our model, experimentally observed LTPe in MF – GC synapses (Racine et al., 1986) and LTDi in Golgi cell – GC synapses (Amico et al., 1998) indicates that inhibitory action of Golgi cell at GC is usually strong. This inhibition pro-motes activation of PKG, and phosphorylation of AMPA and GABAa receptors on GC. Subse-quent long-term strengthening of an input signal by GC can be restricted by disinhibitory action of the Lugaro cell that inhibits the Golgi cell. It follows from our model that LTPe in MF – GC synapses can be also induced in the absence of inhibition from Golgi cell. In this case, LTPe can be the result of activation of mGlu receptors and phosphorylation of AMPA receptors by PKC, while PKG is inactive. Such an effect has been obtained in GCs after MF stimulation that caused excitatory postsynaptic potential (EPSP) but not discharges of GCs (D’Angelo et al., 1999). Possi-bly, this stimulation of MFs has also been too weak to fire Golgi cells directly.
6. Conclusion
Based on known experimental data and the suggested unitary mechanism of synaptic plastic-ity, we assume that the efficacy of excitatory and inhibitory connections between diverse elements
of cerebellar network can be modified. The sign of simultaneous modification of excitatory and in-hibitory inputs to the same cell is opposite and determined by the variations in pre- and/or post-synaptic cell activity. The same modification rules must be used for Purkinje cells, granule (input) cells, and deep cerebellar nuclei (output) cells of cerebellar network, while modification rules for Golgi cells are distinctive. The sign of modifica-tion (LTPe or LTDe) of excitatory transmission between parallel fibers and Purkinje cells, mossy fibers and granule cells, and mossy fibers and deep cerebellar nuclei cells essentially depends on the strength of inhibition effected by stellate/basket cells, Golgi cells and Purkinje cells, respectively. The character of interrelated modifications of synapses at all three layers of the network is influenced by olivary cells activity. According to the suggested model, LTPe (LTDe) in the path-way between input mossy fibers and output deep cerebellar nuclei cells could be induced in the absence (presence) of a signal from inferior olive. The results of the model are in agreement with known experimental data. Suggested olivary-cere-bellar neural network with numerous modifiable synapses can be used for the further complication of existing neuronal models of learning and un-derstanding of learning mechanisms.
Acknowledgements
This work is supported by the Russian Founda-tion of Fundamental Research, grant 98-04-48368.
References
Albus, J.S., 1971. A theory of cerebellar function. Math. Biosci. 10, 25 – 61.
Amico, C., Cupello, A., Fossati, C., Robello, M., 1998. In-volvement of phosphatase activities in the run-down of GABA(A) receptor function in rat cerebellar granule cells in culture. Neuroscience 84, 529 – 535.
Baltrons, M.A., Saadoun, S., Agullo, L., Garcia, A., 1997. Regulation by calcium of the nitric oxide/cyclic cGMP system in cerebellar granule cells and astroglia in culture. J. Neurosci. Res. 49, 333 – 341.
Barthel, F., Kienlen-Campard, P., Demeneix, B.A., Feltz, P., Loeffler, P., 1996. GABAB receptors negatively regulate
transcription in cerebellar granular neurons through cyclic AMP responsive element binding protein-dependent mech-anisms. Neuroscience 70, 417 – 427.
Batchelor, A.M., Garthwait, J., 1992. GABAb receptors in the parallel fiber pathways of rat cerebellum. Eur. J. Neurosci. 4, 1059 – 1064.
Bear, M.F., Malenka, R.C., 1994. Synaptic plasticity: LTPe and LTDe. Curr. Opin. Neurobiol. 4, 389 – 399.
Biggio, G., Guidotti, A., 1976. Climbing fiber activation and 3%,5%-cyclic guanozine monophaspate (cGMP) content in cortex and deep nuclei of cerebellum. Brain Res. 117, 365 – 373.
Breakwell, N.A., Rowan, M.J., Anwyl, R., 1998. (+)-MCPG blocks induction of LTP in CA1 of rat hippocampus via agonist action at an mGluR group II receptors. J. Neuro-physiol. 79, 1270 – 1276.
Bureau, I., Mulle, C., 1998. Potentiation of GABAergic synap-tic transmission by AMPA receptors in mouse cerebellar stellate cells: changes during development. J. Physiol. (Lond.) 509, P817 – P831.
Clark, R.E., Gohl, E.B., Lavond, D.G., 1997. The learning-re-lated activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the inter-positus nucleus. Learn. Memory 3, 532 – 544.
Crepel, F., Jaillard, D., 1991. Pairing of pre- and postsynaptic activities in cerebellar Purkinje cells induces long-term changes in synaptic efficacy. An in vitro study. J. Physiol. (Lond.) 432, 123 – 141.
D’Angelo, E., De-Filippi, G., Rossi, P., Taglietti, V., 1997. Synaptic activation of Ca2+action potentials in immature
rat cerebellar granule cells in situ. J. Neurophysiol. 78, 1631 – 1642.
D’Angelo, E., Rossi, P., Armano, S., Taglietti, V., 1999. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J. Neurophysiol. 81, 277 – 287. Daniel, H., Levenes, C., Crepel, F., 1998. Cellular mechanisms
of cerebellar LTD. Trends Neurosci. 21, 401 – 407. De Zeeuw, C.I., Simpson, J.I., Hoogenraad, C.C., Galjart, N.,
Koekkoek, S.K.E., Ruigrok, T.J.H., 1998. Microcircuitry and function of the inferior olive. Trends Neurosci. 21, 391 – 400.
Fiala, J.C., Grossberg, S., Bullock, D., 1996. Metabotropic glutamate receptor activation in cerebellar Purkinje cells as substrate for adaptive timing of the classically conditioned eye-blink response. J. Neurosci. 16, 3760 – 3774.
Grandes, P., Mateos, J.M., Ruegg, D., Kuhn, R., Knopfel, T., 1994. Differential cellular localization of three splice vari-ants of the mGluR1 metabotropic glutamate receptor in rat cerebellum. Neuroreport 5, 2249 – 2252.
Hamori, J., Takacs, J., Gorcs, T.J., 1996. Immunocytochemi-cal loImmunocytochemi-calization of mGluR1a metabotropic glutamate re-ceptor in inhibitory interneurons of the cerebellar cortex. Acta Biol. Hung. 47, 181 – 194.
Hartell, N.A., 1994. Induction of cerebellar long-term depres-sion requires activation of glutamate metabotropic recep-tors. Neuroreport 5, 913 – 916.
Hirano, T., 1990. Depression and potentiation of the synaptic transmission between a granule cell and a Purkinje cell in rat cerebellar culture. Neurosci. Lett. 119, 141 – 144. Horn, K.M., Hamm, T.M., Gibson, A.R., 1998. Red nucleus
stimulation inhibits within the inferior olive. J. Neurophys-iol. 80, 3127 – 3136.
Houk, J.C., Buckingham, J.T., Barto, A.G., 1996. Models of cerebellum and motor learning. Behav. Brain Sci. 19, 368 – 383.
Ito, M., 1984. The Cerebellum and Neural Control. Raven Press, New York.
Ito, M., Karachot, L., 1992. Protein kinases and phosphatase inhibitors mediating long-term desensitization of glutamate receptors in cerebellar Purkinje cells. Neurosci. Res. 14, 27 – 38.
Kano, M., Rexhausen, U., Dreessen, J., Konnerth, A., 1992. Synaptic excitation produces a long-lasting rebound poten-tiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature 356, 601 – 604.
Kasono, K., Hirano, T., 1994. Critical role of postsynaptic calcium in cerebellar long-term depression. Neuroreport 6, 17 – 20.
Kenyon, G.T., 1997. A model of long-term memory storage in the cerebellar cortex: a possible role for plasticity at paral-lel fiber synapses onto stellate/basket interneurons. Proc. Natl. Acad. Sci. USA 94, 14200 – 14205.
Knoflach, F., Kemp, J.A., 1998. Metabotropic glutamate group II receptors activate a G protein-coupled inwardly rectifying K+current in neurones of the rat cerebellum. J.
Physiol. (Lond.) 509, 347 – 354.
Komatsu, Y., 1996. GABAb receptors, monoamine receptors, and postsynaptic inositol triphosphate induced Ca2+
re-lease are involved in the induction of long-term potentia-tion at visual cortical inhibitory synapses. J. Neurosci. 16, 6342 – 6352.
Laine, J., Axelrad, H., 1998. Lugaro cells target basket and stellate cells in the cerebellar cortex. Neuroreport 9, 2399 – 2403.
Lang, E.J., Sugihara, I., Llinas, R., 1996. GABA-ergic modu-lation of complex spike activity by the cerebellar nucleooli-vary pathway in rat. J. Neurophysiol. 76, 255 – 275. Linden, D.J., 1994. Long-term synaptic depression of the
mammalian brain. Neuron 12, 457 – 472.
Llano, I., Leresche, N., Marty, A., 1991. Calcium entry in-creases the sensitivity of cerebellar Purkinje cells to applied GABA and decrease inhibitory synaptic current. Neuron 6, 565 – 574.
Marr, D., 1969. A theory of cerebellar cortex. J. Physiol. 202, 437 – 470.
Mauk, M.D., Donegan, N.H., 1997. A model of pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn. Memory 4, 130 – 158.
Morishita, W., Sastry, B.R., 1993. Long-term depression of IPSPs in rat deep cerebellar nuclei. Neuroreport 4, 719 – 722.
Mouginot, D., Gahwiler, B.H., 1995. Characterization of synaptic connections between cortex and deep nuclei of the rat cerebellum in vitro. Neuroscience 64, 699 – 712. Nakazawa, K., Mikawa, S., Hashikawa, T., Ito, M., 1995.
Transient and persistent phosphorylation of AMPA-type glutamate receptor subunits in cerebellar Purkinje cells. Neuron 15, 697 – 709.
Neki, A., Ohishi, H., Kaneko, T., Shigemoto, R., Nakanishi, S., Mizuno, N., 1996. Metabotropic glutamate receptors mGluR2 and mGluR5 are expressed in two non-overlap-ping populations of Golgi cells in the rat cerebellum. Neuroscience 75, 815 – 826.
Otsu, Y., Kimura, F., Tsumoto, T., 1995. Hebbian induction of LTP in visual cortex: perforated patch-clamp study in cultured neurons. J. Neurophysiol. 74, 2439 – 2444. Pasqualotto, B.A., Lanius, R.A., Shaw, C.A., 1993.
Regula-tion of GABAa and AMPA receptors by agonist and depolarizing stimulation requires phosphatase or kinase activity. Neuroreport 4, 47 – 450.
Poulopoulou, C., Nowak, L.M., 1998. Extracellular 3%,5%cyclic guanosine monophosphate inhibits kainate-activated re-sponses in cultured mouse cerebellar neurons. J. Pharma-col. Exp. Ther. 286, 99 – 109.
Racine, R.J., Wilson, D.A., Gingell, R., Sunderland, D., 1986. Long-term potentiation in the interpositus and vestibular nuclei in the rat. Exp. Brain Res. 63, 158 – 162.
Raymond, J.L., Lisberger, S.G., 1997. Multiple subclasses of Purkinje cells in the primate floccular complex provide similar signals to guide learning in the vestibulo-ocular reflex. Learn. Memory 3, 503 – 518.
Robello, M., Amico, C., Bucossi, G., Cupello, A., Rapallino, M.V., Thellung, S., 1996. Nitric oxide and GABAA recep-tor function in the rat cerebral cortex and cerebellar gran-ule cells. Neuroscience 74, P99 – P105.
Ross, C.A., Bredt, D., Snyder, S.H., 1990. Messenger molecules in the cerebellum. Trends Neurosci. 13, 216 – 222.
Rossi, P., D’Angelo, E., Taglietti, V., 1996. Differential
long-lasting potentiation of the NMDA and non-NMDA synap-tic currents induced by metabotropic and NMDA receptor coactivation in cerebellar granule cells. Eur. J. Neurosci. 8, 1182 – 1189.
Sastry, B.R., Morishita, W., Yip, S., Shew, T., 1997. GABA-ergic transmission in deep cerebellar nuclei. Prog. Neuro-biol. 53, 259 – 271.
Scherzer, C.R., Landwehrmeyer, G.B., Kerner, J.A., Stan-daert, D.G., Hollingsworth, Z.R., Daggett L.P., Silkis, I.G., 1997. The unitary principles of synaptic plasticity in the neocortex, hippocampus and cerebellum. In: Pavlov, I.P. (Ed.), Journal of Higher Nervous Activity, English version for XXXIII International IUPS Congress, Nauka, Moscow, pp.109 – 122.
Silkis, I.G., 1998. The unitary modification rules for neural networks with excitatory and inhibitory synaptic plasticity. Biosystems 48, 205 – 213.
Silkis, I.G., 1999. Unitary postsynaptic mechanisms of LTP and LTD in the neocortex, hippocampus, cerebellum. In: Miller, R., Ivanitsky, A.M., Balaban, P.M. (Eds), Complex Brain Functions: Conceptual Advances in Russian Neuro-science. Harwood Press, UK, pp. 21 – 51 (in press). Silkis, I.G., Egorova, L.K., Markevich, V.A., Gylyaeva, N.V.,
1998. The involvement of GABAb receptors in cGMP production in cerebellar cortex. Neurochimia 15, 335 – 400 (in Russian).
Thompson, R.F., Bao, S., Chen, L., Cipriano, B.D., Grethe, J.S., Kim, J.J., Thompson, J.K., Tracy, J.A., Weninger, M.S., Krupa, D.J., 1997. Associative learning. Int. Rev. Neurobiol. 41, 151 – 189.
Voogd, J., Glickstein, M., 1998. The anatomy of the cerebel-lum. Trends Neurosci. 21, 370 – 375.
Whittington, M.A., Traub, R.D., Jefferys, J.G., 1995. Syn-chronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature 373, 612 – 615.
Wu, J., Wang, Y., Rowan, M.J., Anwyl, R., 1998. Evidence for involvement of the cGMP-protein kinase G signaling system in the induction of long-term depression, but not long-term potentiation, in the dentate gyrus in vitro. J. Neurosci. 186, 3589 – 3596.
Yuzaki, M., Forrest, D., Verselis, L.M., Sun, S.C., Curran, T., Connor, J.A., 1996. Functional NMDA receptors are tran-siently active and support the survival of Purkinje cells in culture. J. Neurosci. 16, 4651 – 4661.