www.elsevier.comrlocateranireprosci
Effects of high density lipoprotein containing high
or low
b
-carotene concentrations on progesterone
production and
b
-carotene uptake and depletion by
bovine luteal cells
S
¸
. Arikan
), R.G. Rodway
Department of Animal Physiology and Nutrition, UniÕersity of Leeds, Leeds LS2 9JT, UK
Received 5 August 1999; received in revised form 26 January 2000; accepted 23 February 2000
Abstract
Luteal cells were isolated from mid-luteal heifer ovaries by collagenase digestion. Cells were cultured with DMEMrHam’s F12 medium in serum pre-treated plastic culture dishes for periods
of up to 11 days. Asb-carotene is almost completely insoluble in all polar solvents, it was added
Ž . Ž .
to cultures in either dimethyl sulphoxide DMSO , tetrahydrofuran THF or as high-density
Ž .
lipoprotein HDL containing high or lowb-carotene concentrations. Medium was replaced after
24 h, thereafter medium was changed every 48 h. Treatment of cells with DMSO alone or with
Ž . Ž .
b-carotene 5mmolrl in DMSO both resulted in significant P-0.01 stimulation of
proges-Ž .
terone production.b-Carotene 5mmolrl in THF did not alter progesterone production but 50
Ž .
mmolrlb-carotene in THF resulted in significant inhibition P-0.02 of progesterone produc-tion on days 3 and 7. Cultures were also supplemented with bovine HDL preparaproduc-tions containing
Ž . Ž
equal concentrations of cholesterol 25mgrml but high or low b-carotene 12.4 or 0.44mgrmg
. Ž
of cholesterol . Both HDL preparations significantly stimulated progesterone production P
-. Ž .
0.001 but the high b-carotene HDL was significantly P-0.02 more effective than the low
Ž .
b-carotene HDL. However, when given together with bovine luteinizing hormone bLH or
Ž .
dibutyryl cAMP dbcAMP , the high b-carotene HDL stimulated progesterone production less
Ž .
than did the low HDL P-0.01 . Uptake and depletion ofb-carotene by luteal cells were also
examined in culture.b-Carotene supplementation increased luteal cellb-carotene from an initial
level of 373 ng per 106cells to 2030 ng per 106cells by day 6. In contrast, the levels in control
)Corresponding author. Faculty of Veterinary Medicine, University of Kirikkale, 71100-Kirikale, Turkey.
Tel.:q90-318-3573301; fax:q90-318-3573304.
Ž .
E-mail address: [email protected] S¸. Arikan .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
cells decreased to 14% of starting values during the same period. Cells treated with HDL
containing highb-carotene on day 1 or days 1 and 3 were then incubated with or without bLH or
dbcAMP for a further 2 days to investigate the effect of bLH and dbcAMP on depletion of b-carotene by luteal cells. b-Carotene depletion in the luteal cells was significantly higher
ŽP-0.05 in LH- and dbcAMP-treated cells than in the control cells in both groups. These results.
indicate that the use of solvents such as DMSO or THF may have undesirable effects due to alteration of cell membrane permeability. Supplementation with bLH or dbcAMP may increase the
metabolism of b-carotene in luteal cells. bLH or dbcAMP together with high b-carotene HDL
may, when combined with the effect of increased b-carotene metabolism, give less stimulation
than with lowb-carotene HDL.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Cattle-feeding and nutrition;b-Carotene; Luteal cells; Lipoprotein; Progesterone
1. Introduction
b-Carotene is present in extremely high concentrations in the bovine corpus luteum
ŽO’Fallon and Chew, 1984; Holt et al., 1995 , giving the CL its characteristic bright.
yellow colour. As well as acting as a precursor for vitamin A, there is increasing evidence that b-carotene may be necessary for optimal steroid production, possibly
Ž .
acting as an anti-oxidant Young et al., 1995 . It has been reported previously that
Ž
b-carotene may affect luteal cell steroid production in vitro Pethes et al., 1985;
. Ž .
O’Shaughnessy and Wathes, 1988 and in vivo Dembinski and Bronicki, 1994 . On the
Ž .
other hand, Graves-Hoagland et al. 1988 found that a positive relationship existed between in vitro bovine luteal cell progesterone production and plasma b-carotene during the winter when plasma b-carotene concentrations are low in dairy herds. They showed that during the summer when plasmab-carotene is increased, this relationship is lost. In addition, some studies in dairy cattle have failed to observe an effect of
Ž
b-carotene supplementation on plasma steroid hormone levels Folman et al., 1979;
.
Wang et al., 1982 .
Ž .
b-Carotene may also be required for normal ovarian function. Inaba et al. 1986 showed that the levels of plasmab-carotene are significantly lower in cows with ovarian cysts than those in normal cows, when they are raised on a feed with low b-carotene content. It has also been reported thatb-carotene supplemented cows have a much lower
Ž
incidence of ovarian cysts than unsupplemented cows Lotthammer and Ahlswede,
.
1977 .
There is evidence thatb-carotene may have an effect on follicular development in the
Ž .
bovine. Mayer et al. 1975 observed that ovulation occurred about 1 day after the onset of oestrus in theb-carotene supplemented group, but not until 2 days after the onset of
Ž .
oestrus in the deficient group. Their result was later confirmed by Schams et al. 1977 , who found that the time interval between the occurrence of the LH peak and ovulation was more than doubled in theb-carotene deficient animals They also reported that the maximum delay in ovulation after the occurrence of the LH peak was 72.5 and 49 h in b-carotene deficient and supplemented groups, respectively.
b-Carotene is normally transported to the ovary incorporated in the lipid component
Ž .
ŽLDL also supply other fat soluble substances such as cholesterol, vitamin A and.
Ž .
vitamin E to the corpus luteum Ribaya-Mercado et al., 1993; Aten et al., 1994 . However, several previous studies have used water miscible solvents such as THF
ŽBertram et al., 1991 and DMSO Young et al., 1995 to supply. Ž . b-carotene to cultured luteal cells. The aim of the present study was to compare the use of THF and DMSO with that of HDL as a mean of supplyingb-carotene to luteal cells, and to examine the interaction betweenb-carotene and LH and dbcAMP on progesterone production.
2. Materials and methods
2.1. Preparation of lipoproteins
A 10-day-old Holstein bull calf was fed twice daily with milk replacer containing 2.5
Ž .
g of b-carotene Roche, UK per feeding for 10 days. Blood was collected to obtain HDL after killing the animal. Serum was separated by centrifugation. HDL was isolated from serum between densities of 1.061 and 1.215 by sequential ultracentrifugation
ŽHavel et al., 1955 using potassium bromide and sodium chloride for density adjust-.
ment. Centrifugation was accomplished using a Beckman 70Ti fixed angle rotor for 48 h
Ž .
at 52,000 rpm. 200,000 gav and 158C in a Beckman Model LS-55 ultracentrifuge. HDL containing low b-carotene was prepared similarly from serum collected from an unsupplemented calf. Cholesterol in the lipoprotein fractions was determined using the
Ž
cholesterol-oxidase: peroxidase method. The assay was performed using a kit Roche
.
Diagnostic, NJ based on the oxidation of cholesterol to cholest-4-ene-3-one and hydrogen peroxide and the subsequent conversion of 4-aminoantipyrine to the dye quinoneimine. The absorbance was measured at 500 nm. HDL obtained from Sigma was also used for quality control. Theb-carotene concentrations in HDL isolated from serum containing high or lowb-carotene were 12.4 and 0.44mgrmg cholesterol, respectively. Lipoprotein fractions were sterilised by passage through a 0.22-mm millipore filter and stored aty408C until use.
2.2. Isolation and culture of boÕine luteal cells
Bovine ovaries were collected immediately after slaughter from the local abattoir and transported to the laboratory in ice-cold phosphate-buffered saline within 30 min. Ovaries were normally from non-pregnant Friesian Holstein cattle judged to be at
Ž .
mid-cycle by the criteria of Ireland et al. 1980 . All chemicals were obtained from
Ž .
Sigma. Mixed luteal cells small and large were isolated from mid-luteal heifer ovaries
Ž .
by collagenase digestion as described by O’Shaughnessy and Wathes 1985 . Luteal
Ž .
cells were stained for 3b-hydroxysteroid dehydrogenase 3b-HSD as described by Bao
Ž .
et al. 1995 . Cells were counted using a hemocytometer and viability was estimated
Ž 5 5.
using trypan blue exclusion. Cells 1=10 y2=10 with a positive stain for 3b-HSD
Ž
were cultured in foetal bovine serum pre-treated six-well plastic culture dishes 35 mm,
. Ž
. Ž
experiments serum-free culture medium Dulbecco’s Modified Eagle’s and HAM’S
Ž . Ž .
F-12,1:1 vrv with 15 mmolr1 HEPES containing penicillin 100 unitsrml ,
strepto-Ž . Ž . Ž
mycin 100 mgrml , fungizone 2.5 mgrml and ITS premix 50 ngrml insulin, 50
.
ngrml transferrin and 50 pgrml selenium in a humidified incubator that contained 95% O and 5% CO . Medium was replaced after 24 h. Unless otherwise indicated, thereafter2 2 medium was changed every 48 h. Each treatment consisted of six separate cell wells. The protocol for each experiment is described in Section 3. Cells were incubated for a maximum of 11 days. Used medium was stored frozen at y208C until assayed for progesterone by radioimmunoassay. The mean extraction efficiency was 94.8%, the limit of sensitivity was 6.28 pgrtube and the intra- and inter-assay coefficients of variation were 7.48% and 8.73%, respectively.
2.3.b-Carotene analysis
2.3.1. Extraction of b-carotene from HDL, luteal tissue and cultured cells
Ž
To extractb-carotene from HDL, 2 ml ethanol containing 0.01% butylated
hydroxy-Ž . .
toluene BHT as an anti-oxidant was added to 100 or 250 ml HDL to precipitate protein. The mixture was vortexed and extracted three times with 3 ml of hexane. The combined supernatants were evaporated under a stream of N2 and the residue was redissolved in ethanol.
Ž .
Cells were removed from the culture dishes by trypsinization with 0.05% wrv
Ž .
trypsin in phosphate-buffered saline containing 0.02% wrv EDTA. Extraction of
Ž . Ž 6 .
b-carotene from luteal tissue 500 mg and incubated luteal cells 10 cells was
Ž .
performed as described by Schmitz et al. 1993 .
2.4. Chromatography
All solvents used were of HPLC grade. The HPLC system used for b-carotene analysis consisted of a Spectra-physic SP8700 solvent delivery system and an SP8750
Ž . Ž .
manual injector Spectra-physic, San Jose, CA, USA . Samples 50 ml were injected
Ž
onto a 10-mm, spherical, C-18 reverse-phase column 30 cm=3.9 mm; Resolve,
. Ž .
Millipore and eluted with a mobile phase consisting of 50:45:5 volrvolrvol
Ž .
methanol–acetonitrile–tetrahydrofuran Oliver and Kafwembe, 1992 , with a flow rate of 2 mlrmin. b-Carotene was detected at wavelength of 452 with a Spectraflow 757
Ž .
model variable wavelength detector Kratos Analytical Instruments, USA . Peak areas were compared to those of authentic standards for quantification.
2.5. Statistical analysis
Ž .
Different treatments were assessed by analysis of variance ANOVA and Duncan’s multiple range test. Significance was defined as P-0.05. All statistical analysis was
Ž .
3. Results
3.1. Effects of b-carotene either in DMSO or THF on progesterone production
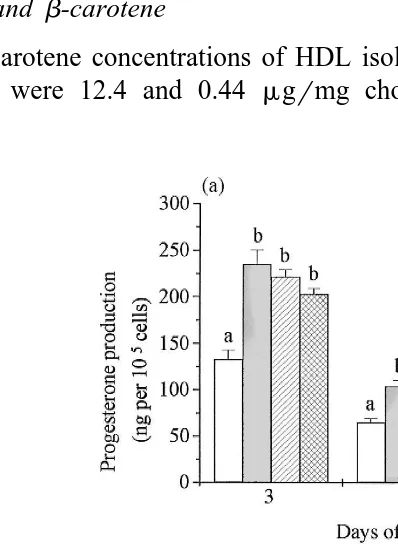
Luteal cells were cultured for 7 days in the absence or presence of b-carotene dissolved in organic solvents. Each treatment consisted of six separate cell wells.
Ž .
Treatment of cells with 1% DMSO alone or withb-carotene 5mmolrl in DMSO both
Ž .
resulted in significant P-0.01 stimulation. However, a higher concentration of
Ž . Ž .
b-carotene 50 mmolrl in DMSO did not affect progesterone production Fig. 1a . A 5-mmolrl b-carotene in THF did not alter progesterone production but 50 mmolrl in
Ž . Ž .
THF resulted in significant inhibition P-0.02 on days 3 and 7 Fig. 1b .
3.2. HDL and b-carotene
The b-carotene concentrations of HDL isolated from serum containing high or low b-carotene were 12.4 and 0.44 mgrmg cholesterol, respectively. Mean b-carotene
Ž .
Fig. 1. a Effect ofb-carotene dissolved in DMSO on progesterone production by luteal cells in culture. Cells
Ž . Ž . Ž
were cultured in medium under basal conditions I, with 1% DMSO dotted square , 5mmolrl square with
. Ž .
three jagged diagonal lines from top right to bottom left or 50mmolrl checkered square b-carotene in 1%
Ž .
DMSO. b Effect ofb-carotene dissolved in THF on progesterone production by luteal cells in culture. Cells
Ž . Ž
were cultured in medium under basal conditions I, with 0.1% THF square with three jagged diagonal lines
. Ž .
from top left to bottom right , 5mmolrl square with one vertical line in the middle or 50mmolrlb-carotene
Table 1
Uptake and depletion ofb-carotene by cultured luteal cells
Ž
The cells were incubated with or without HDL containing highb-carotene concentration 50mg cholesterolrml;
. Ž .
620mgb-carotenerml up to 6 days. Cells were treated with HDL on days 1 and 2 analysed on day 3 or 3
Ž .
and 5 analysed on day 6 . Results are the means"SEM of three independent experiments. Different
Ž .
superscripts denote significant differences between groups P-0.01 within columns.
6
Ž .
b-Carotene content of luteal cells ngr10 cells"SEM
Control HDL
a a
Day 0 373.1"83 373.1"83
b c
Day 3 151.4"39 1654.4"126
d e
Day 6 49.9"8.5 2030.3"171
Ž .
concentration of bovine corpus luteum was 52.13mgrg ns39 . b-Carotene content was quite different from animal to animal and ranged from 7.5 to 200 mgrg. This variation appeared to be correlated with time of year in which the samples were
Ž .
collected Arkan and Rodway, in preparation .
3.3. Uptake and depletion of b-carotene by luteal cells in culture
As seen in Table 1, uptake and depletion of b-carotene by luteal cells changed in parallel with increased incubation time. b-Carotene concentration in cells increased 5.5-fold by day 6 when compared with the initial level. In contrast, the concentration in
Ž .
control cells decreased to 14% of starting values during the same period P-0.01 .
3.4. Effect of b-carotene associated with HDL on progesterone production
Luteal cells were incubated for 11 days in medium supplemented with bovine HDL
Ž .
preparations containing equal concentrations of cholesterol 25mgrml but high or low
Fig. 2. Effect ofb-carotene associated with HDL on progesterone secretion by luteal cells in culture. Luteal
Ž . Ž . Ž .
cells were incubated without I or with HDL 25mg cholesterolrml containing low checkered square or
Ž .
high B b-carotene concentrations. Treatment was started on day 3. Within each day, groups with different
Ž .
Ž .
b-carotene 12.4 or 0.44 mgrmg of cholesterol . Both HDLs significantly stimulated
Ž .
progesterone production P-0.01 but the high b-carotene HDL was significantly
ŽP-0.02 more effective than the low. b-carotene HDL by day 11 Fig. 2 . BothŽ . Ž .
dbcAMP and bLH alone significantly P-0.01 stimulated progesterone production. This stimulation was increased when either high or lowb-carotene HDL was included.
Ž .
However, the high b-carotene HDL gave significantly P-0.01 less additional
Ž .
stimulation than did the low Fig. 3 .
3.5. Effect of bLH and dbcAMP on depletion of b-carotene by luteal cells
Ž .
Depletion of b-carotene in the luteal cells was significantly higher P-0.05 in
Ž .
bLH- and dbcAMP-treated cells than the control cells in both groups Table 2 . However, depletion was faster in bLH-treated cells than dbcAMP-treated cells.
b-Caro-Ž .
Fig. 3. a Effect ofb-carotene with or without bLH on progesterone production by luteal cells. Cells were
Ž . Ž . Ž . Ž .
incubated without I or with bLH 100 ngrml dotted square , bLHqHDL 25 mg cholesterolrml
Ž . Ž
containing low square with three jagged diagonal lines from top right to bottom left or high checkered
. Ž . Ž . Ž . Ž
square b-carotene concentrations. b Cells were incubated without I or with dbcAMP 1 mmolrl square
. Ž
with three jagged diagonal lines from top left to bottom right , dbcAMPqHDL containing low square with
. Ž .
one horizonal line in the middle or high B b-carotene concentrations. Treatment was started on day 3.
Ž .
Table 2
Effect of bLH and dbcAMP on depletion ofb-carotene by luteal cells
Ž .
Cells were pre-cultured with HDL containing high b-carotene concentrations 50 mg cholesterolrml for
Ž . Ž .
either 2 or 4 days incubated then with or without dbcAMP 1 mmolrl or LH 100 ngrml for a further 2 days.b-Carotene content of cells was measured by HPLC. Results are the means"SEM of three independent
Ž .
experiments. Different superscripts denote significant differences between groups P-0.05 within columns.
6
Ž .
b-Carotene content of luteal cells ng per 10 cells"SEM Days ofb-carotene treatment
2 4
a c
Control 939.18"29 1174.41"52
ab a
dbcAMP 807.26"71 934.75"43
b ab
LH 713.85"28 831.03"71
tene content was also effected by treatment time. Content of b-carotene was higher in
Ž .
the control group treated for 4 days than the control group treated for 2 days P-0.02 .
4. Discussion
The present study has examined the hypothesis that increasing theb-carotene content of bovine luteal cells in culture will increase progesterone production. Progesterone production was maintained for at least 11 days in culture, although the rate declined with time. No attempt was made to separate small from large luteal cells, although the former
Ž .
may affect progesterone synthesis by the latter Girsh et al., 1988 .
Asb-carotene is almost completely insoluble in solvents such as ethanol and acetone, several previous studies have used water-miscible solvents such as DMSO and THF
ŽYoung et al., 1995; Cooney et al., 1993 to deliver the. b-carotene to the cells. The experiments reported here demonstrate that neither b-carotene dissolved in DMSO or b-carotene dissolved in THF stimulates progesterone production. Indeed, the solvent type used can, itself, affect steroidogenesis, with DMSO alone stimulating progesterone production and THF alone having no effect.
Since the normal transport mechanism for b-carotene in the bovine circulation is in
Ž .
high density lipoproteins HDL and luteal cells have been shown to have HDL
Ž .
receptors on their cell membranes Ferreri et al., 1992 , this was, therefore, used as route of administration of b-carotene to luteal cells. The two bovine HDL preparations used both gave significant stimulation of progesterone production, but the higher b-carotene preparation gave a consistently greater response than the lower one, although this did not become significant until day 9 of incubation. As cholesterol is the precursor of progesterone, cholesterol supply is obviously a factor in the control of the rate of steroidogenesis. Care was therefore taken to ensure that both HDL preparations con-tained identical concentrations of cholesterol.
is possibly due to the fact that the endogenous b-carotene within the luteal cells becomes depleted with time. Over the course of a 6-day culture period, theb-carotene content decreased by over 80% in the absence of supplementary b-carotene. On the other hand, when the cells were incubated with the high b-carotene HDL preparation, the content within the cells increased by over 700% over the 6-day incubation. As the endogenousb-carotene becomes depleted, it therefore seems reasonable to assume that the effects of supplementary b-carotene increase.
The mechanism by which b-carotene may affect luteal cell function at the cellular level is unclear at present, however there are several possible mechanisms.b-Carotene is a powerful anti-oxidant with the capacity to act as a quencher of singlet oxygen and
Ž .
hydroxyl radicals Stahl et al., 1997 , which cause lipid peroxidation and cross-linking of membrane lipids, and these effects can decrease steroidogenic cytochrome P450 and
Ž
cholesterol side-chain cleavage activity in adrenal and ovarian tissue Hornsby, 1980;
.
Young et al., 1995 .b-Carotene can also stimulate gap junction formation between cells
Ž
in culture. This has been demonstrated in several different cell lines. Bertram and
.
Bortkiewicz, 1995; Stahl et al., 1997 . b-Carotene stimulates transcription of the connexin 43 gene, which codes for a transmembrane protein, which increases gap junction formation; thereby increasing cell to cell communication in human dermal
Ž .
fibroblast cells Zhang et al., 1991 . Gap junction formation may be important in the
Ž
coordination of luteal cell function Redmer et al., 1991; Khan-Dawood et al., 1996;
.
Grazul-Bilska et al., 1996 and this may, therefore, be important in the mechanism by whichb-carotene stimulate steroidogenesis.
The experiments with LH and dibutyryl cAMP gave somewhat unexpected results. When given alone, both stimulated progesterone secretion as expected. When HDL, either with high or low b-carotene was given in combination with LH or dibutyryl cAMP, both gave a two- to three-fold stimulation above that given by LH or dibutyryl cAMP alone. However, the additional stimulation given by the highb-carotene prepara-tion was significantly less than that given by the low preparaprepara-tion. LH has been shown to increase gap junction formation between luteal cells and it is thought that this gap junctional communication may be important in the regulation not only of steroidogenesis
Ž .
but also of luteolysis Khan-Dawood et al., 1996 . As b-carotene has been shown in other cell types to increase connexin 43 and gap junction formation, it is possible that the combination of high LH andb-carotene may act to stimulate gap junction formation to such an extent that apoptosis and luteolysis may occur. This may explain why in the presence of both these factors progesterone synthesis was reduced whenb-carotene was in its highest concentration.
Experiments in which the cellular content ofb-carotene was measured showed that in the presence of the high b-carotene HDL, the accumulation of b-carotene was signifi-cantly less in cells treated with either LH or dibutyryl cAMP than in control cultures. This suggests that stimulation of progesterone synthesis results in increased utilisation of b-carotene and that this may be due to the increased requirement for anti-oxidant capacity. It has been known for many years that another anti-oxidant, ascorbic acid, is depleted in both the ovary and the adrenal when either LH or ACTH is administered
ŽParlow, 1972, Sayers et al., 1948 . It is, therefore, possible that the requirement for.
In conclusion, we have shown that spurious results may be obtained by using solvents such as DMSO and THF to deliver b-carotene to luteal cells, but that b-carotene in HDL will stimulate progesterone production.b-Carotene concentration in luteal cells is depleted over several days in culture, but this can be overcome by supplementing the culture medium with b-carotene in bovine HDL. In the presence of LH or dibutryl cAMP, high concentrations of b-carotene reduces the stimulation of progesterone production possibly due to a luteolytic effect of high concentrations of b-carotene. Utilisation of b-carotene also occurs more rapidly in luteal cells in the presence of LH or dibutyryl cAMP.
Acknowledgements
We would like to thank Dr. A.F. Parlow, Pituitary Hormones and Antisera Centre, for providing bovine LH hormone.
References
Aten, R.F., Kolodecik, T.R., Behrman, H.R., 1994. Ovarian vitamin E accumulation: evidence a role of lipoproteins. Endocrinology 135, 533–539.
Bao, B., Thomas, M.G., Griffith, M.K., Burghardt, R.C., Williams, G.L., 1995. Steroidogenic activity, insulin-like growth factor-I production, and proliferation of granulosa and theca cells obtained from dominant preovulatory and nonovulatory follicles during the bovine oestrous cycle: effects of low-density and high-density lipoproteins. Biol. Reprod. 53, 1271–1279.
Bertram, J.S., Bortkiewicz, H., 1995. Dietary carotenoids inhibit neoplastic transformation and modulate gene expression in mouse and human cells. Am. J. Clin. Nutr. 62, 1327S–1336S.
Bertram, J.S., Pung, A., Churley, M., Kappock, T.J., Wilkins, L.R., Cooney, R.V., 1991. Diverse carotenoids protect against chemically induced neoplastic transformation. Carcinogenesis 12, 671–678.
Cooney, R.V., Kappock, T.J., Pung, A., Bertram, J.S., 1993. Solubilization, cellular uptake, and activity of b-carotene and other carotenoids as inhibitors of neoplastic transformation in cultured cells. Methods Enzymol. 214, 55–68.
Dembinski, Z., Bronicki, M., 1994. Progesterone P-4 level in blood and the values of selected fertility indexes in cows fed various doses of carotenes. Bull. Vet. Inst. Pulawy 38, 115–118.
Ferreri, K., Talavera, F., Menon, K.M.J., 1992. Increased cellular cholesterol upregulates high density lipoprotein binding to rat luteal cells. Endocrinology 131, 2059–2064.
Folman, Y., Ascarelli, I., Herz, Z., Rosenberg, M., Davidson, M., Halevi, A., 1979. Fertility of dairy heifers given a commercial diet free ofb-carotene. Br. J. Nutr. 41, 353–359.
Girsh, E., Wang, W., Mamluk, R., Arditi, F., Friedman, A., Milvae, R.A., Meidan, R., 1988. Regulation of endothelin-1 expression in the bovine corpus luteum: elevation by prostaglandin F 2 alpha. Endocrinology 137, 5191–5196.
Graves-Hoagland, R.L., Hoagland, T.A., Woody, C.O., 1988. Effect of b-carotene and vitamin A on progesterone production by bovine luteal cells. J. Dairy Sci. 71, 1058–1062.
Grazul-Bilska, A.T., Reynolds, L.P., Kirsch, J.D., Redmer, D.A., 1996. Gap Junctional intercellular communi-cation of bovine luteal cells from several stages of the estrous cycle: effects of cyclic adenosine 3,5-monophosphate. Biol. Reprod. 54, 538–545.
Havel, R.J., Eder, H.A., Bragdon, J.H., 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34, 1345–1353.
Hornsby, P.J., 1980. Regulation of cytochrome-P450-supported 11 b-hydroxylation of deoxycortisol by steroids, oxygen and antioxidants in adrenocortical cells. J. Biol. Chem. 255, 4020–4027.
Inaba, T., Mezan, M., Shimizu, R., Nakano, Y., Mori, J., 1986. Plasma concentrations of b-carotene and vitamin A in cows with ovarian cyst. Jpn. J. Vet. Sci. 48, 1275–1278.
w
Ireland, J.J., Murphee, R.L., Coulson, P.B., 1980. Accuracy of predicting stages of bovine oestrus cycle by x
gross appearance of the corpus luteum J. Dairy Sci. 63, 155–160.
Khan-Dawood, F.S., Jang, J., Dawood, Y., 1996. Expression of gap junction protein connexin-43 in the human
Ž .
and baboon Papio anubis corpus luteum. J. Clin. Endocrinol. Metab. 81, 835–842.
Lotthammer, K.-H., Ahlswede, L., 1977. Undersuchungen uber eine spezifische, Vitamin-A-unabhangige¨ ¨ Wirkung des b-Carotins auf die Fertilitat des Rindes: 3. Mitteilung: Blutserumuntersuchungen. Dtsch.¨ Tieraerztl. Wochenschr. 84, 220–226.
Mayer, H., Ahlswede, L., Lotthammer, K.-H., 1975. Undersuchungen uber eine spezifische, Vitamin-A-un-¨ abhangige Wirkung des b-Carotins auf die Fertilitat des Rindes: I. Mitteilung: Versuchsantellung, Koperentwicklung und Eierstockfunktion. Dtsch. Tieraerztl. Wochenschr. 82, 444–449.¨
O’Fallon, J.V., Chew, B.P., 1984. The subcellular distribution ofb-carotene in bovine corpus luteum. Proc. Soc. Exp. Biol. Med. 177, 406–411.
O’Shaughnessy, P.J., Wathes, D.C., 1985. Characteristics of bovine luteal cells in culture: morphology, proliferation and progesterone secretion in different media and effects of LH, dibutyryl cyclic AMP, antioxidants and insulin. J. Endocrinol. 104, 355–361.
O’Shaughnessy, P.J., Wathes, D.C., 1988. Bovine luteal cell activity in culture: maintenance of steroidogenesis by high density lipoprotein containing high or low beta-carotene concentrations. Anim. Reprod. Sci. 17, 165–176.
Oliver, R.W.A., Kafwembe, E.M., 1992. A new spectrophotometric assay for the determination of vitamin A and related compounds in serum. Int. J. Vitam. Nutr. Res. 62, 221–227.
Parlow, A.F., 1972. Influence of difference in the persistence of luteinizing hormones in blood on their potency in the ovarian ascorbic acid depletion bioassay. Endocrinology 91, 1109–1112.
Pethes, G., Horvath, E., Kulcsar, M., Huszenicza, G., Somorjai, G., Varga, B., Haraszti, J., 1985. In vitro progesterone production of corpus luteum cells of cows fed low and high levels of beta-carotene. Zentralbl. Veterinaermed., Reihe A. 32, 289–296.
Redmer, D.A., Grazul-Bilska, A.T., Reynolds, L.P., 1991. Contact-dependent intercellular communication of bovine luteal cells in culture. Endocrinology 129, 2757–2766.
Ribaya-Mercado, J.D., Lopez-Miranda, J., Ordovas, J.M., Blanco, M.C., Fox, J.G., Russell, R.M., 1993. Distribution ofb-carotene and vitamin A in lipoprotein fractions of ferret serum. Ann. N. Y. Acad. Sci. 691, 232–236.
Sayers, M.A., Sayers, G., Woodbury, L.A., 1948. The assay of adrenocorticotrophic hormone by the adrenal ascorbic acid-depletion method. Endocrinology 42, 380–393.
Schams, D., Hoffmann, B., Ahlswede, L., 1977. Untersuchungen uber eine spezifische Vitamin-A-unabhangige¨ ¨ Wirkung desb-carotins auf die Fertilitat des Rindes: 4. Mitteilung: Auswirkung auf hormonale Parameter¨ wahrenddes Zyklus. Dtsch. Tieraerztl. Wochenschr. 84, 307–310.¨
Schmitz, H.H., Poor, C.L., Gugger, E.T., Erdman, J.W., 1993. Analysis of carotenoids in human and animal tissues. Methods Enzymol. 214, 102–116.
Stahl, W., Nicolai, S., Briviba, K., Hanusch, M., Broszeit, G., Peters, M., Martin, H.-D., Sies, H., 1997. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis 18, 89–92.
Wang, J.Y., Larson, L.L., Owen, F.G., 1982. Effect of beta-carotene supplementation on reproductive performance of dairy heifers. Theriogenology 18, 461–473.
Young, F.M., Luderer, W.B., Rodgers, R.J., 1995. The antioxidantb-carotene prevents covalent cross-linking between cholesterol side-chain cleavage cytochrome P450 and its electron donor, adrenodoxin, in bovine luteal cells. Mol. Cell. Endocrinol. 109, 113–118.