253 (2000) 243–251
www.elsevier.nl / locate / jembe
Benthic predator–prey interactions: evidence that adult
Monoporeia affinis (Amphipoda) eat postlarval Macoma
balthica (Bivalvia)
* ´
Gunilla Ejdung , Lars Byren, Ragnar Elmgren
Department of Systems Ecology, Stockholm University, SE-106 91 Stockholm, Sweden Received 28 April 2000; received in revised form 17 July 2000; accepted 24 July 2000
Abstract
Predation by adults of the amphipod Monoporeia affinis on the plantigrade postlarval stage of the bivalve Macoma balthica was studied in the laboratory. We confirmed that M. affinis consumes
14
small M. balthica. Amphipods offered C-labelled postlarvae took up the radioactive tracer, while those presented Rhodamine B-stained postlarvae acquired gut contents fluorescing strongly in orange, whereas control amphipods did not. Both labelling methods proved convenient to use in laboratory experiments, and are particularly useful when organisms lack structures that can be easily identified after being ingested, or when cross-over reactions may bias the results of immunoassays. The results reported here support the conclusion from earlier studies that predation by M. affinis on M. balthica can affect population dynamics of M. balthica and is likely to be an important structuring factor in the low-diversity benthic macrofauna community of the Baltic Sea.
2000 Elsevier Science B.V. All rights reserved.
Keywords: Predator–Prey interaction; Baltic Sea; Macoma–Pontoporeia interaction; Post-larval bivalves; Predation
1. Introduction
Marine soft bottoms are the habitat on earth with the greatest areal extension. The recruitment success of benthic invertebrates is of crucial importance for the structure of the marine soft-bottom communities (Bachelet, 1990). Post-settlement mortality is very high (.90%) among benthic marine invertebrate taxa (Thorson, 1966; Gosselin and Qian, 1997, and references therein), a majority of which have a two-phase larval life
*Corresponding author. Tel.: 146-8-161-744; fax: 146-8-158-417.
E-mail address: [email protected] (G. Ejdung).
cycle (Thorson, 1950). After some time in the plankton the larvae settle at the bottom. The period immediately following settlement is generally considered to be the most critical for a just settled postlarva (e.g., Thorson, 1966). Both the newly settled postlarvae and their predators occupy the surface layer of the sediment, exposing the postlarvae to a great risk of being eaten. The losses to predation in this temporary meiofauna (sensu McIntyre, 1964) are size-dependent and change as the postlarvae
˚
outgrow some potential predators (Segerstrale, 1962). Survival of postlarvae is greatly influenced by inter- and intraspecific interactions among resident adult macro- and
´ meiofauna, and juvenile macrofauna (Elmgren et al., 1986; Luckenbach, 1987; Olafsson et al., 1994 and references therein; Danovaro et al., 1995; Cummings et al., 1996; Beukema et al., 1998; Ejdung and Elmgren, 1998). Predation, the killing and consump-tion of one organism by another, is thought to be the most important cause of post-settlement juvenile mortality (Thorson, 1966; Gosselin and Qian, 1997), influencing and regulating abundance, distribution and reproduction of benthic populations and the composition of communities.
In the Baltic Sea, the bivalve Macoma balthica (L.) and the amphipods Monoporeia ¨
affinis (Lindstrom) (syn. Pontoporeia affinis, see Bousfield, 1989) and Pontoporeia
¨
femorata Kroyer dominate large areas, both in abundance and biomass (Ankar and
Elmgren, 1976; Ankar, 1977; Cederwall, 1996; Rumohr et al., 1996; Laine et al., 1997; Cederwall, 1999). In areas with dense amphipod populations, M. balthica is generally less common. Hessle (1924) hypothesized that predation or competition for food caused poor recruitment of M. balthica in areas densely populated by M. affinis. Elmgren et al. (1986) showed that newly settled (,400mm) M. balthica plantigrade-stage postlarvae (cf. Baker and Mann, 1997) are killed by adult M. affinis due to a direct physical contact. Juvenile M. affinis and adult P. femorata also kill the plantigrades, but their impact is less drastic than that of adult M. affinis (Ejdung and Elmgren, 1998). While showing that both adult and juvenile amphipods can kill the bivalves, the two last-mentioned studies did not present conclusive evidence that the amphipods also ingest the killed bivalve postlarvae, although this was considered highly likely. Normally M. affinis and P. femorata in the Baltic Sea are food limited (Elmgren, 1978; Uitto and Sarvala, 1990), except for short periods after sedimentation of algal blooms. The amphipods feed mainly on surface sediment (Lopez and Elmgren, 1989) and utilise settled phytoplankton and detrital organic matter as their main food source, but bacteria, and probably meiofauna are also included in the diet (Elmgren, 1978; Uitto and Sarvala, 1990; Goedkoop and Johnson, 1994; Lehtonen, 1996).
The aim of this study was to clarify whether the M. balthica postlarvae killed by M.
affinis are also eaten and thus used as food by the amphipod. We used two methods,
14
C-labelling and Rhodamine B fluorescent staining of plantigrade-stage postlarval M.
balthica.
2. Materials and methods
¨
178389E), on the Swedish east coast, north-western Baltic Sea proper, in a room, with near natural light- and temperature conditions. The daily light / dark cycle, regulated with
2
a faint green light, was set to 16 h / 8 h. We used plastic 105-ml aquaria (13 cm sediment area; sediment depth, 1.9–2.1 cm) (Ejdung and Elmgren, 1998), each supplied
21
with 200 ml h cold (6.060.58C, mean6S.E.M.), filtered seawater (5.960.1 PSU). A few days before the experiment, sediment and animals were collected with a benthic sled (Blomqvist and Lundgren, 1996), from 30 to 40 m (amphipods) and 4 to 16 m depth (postlarval M. balthica). Before the experiment, they were stored in a room with the same light and temperature conditions as in the experiments.
Sediment from the amphipod habitat with a loss on ignition of 4%, and sieved through a 100-mm mesh sieve, was used in the experiments. The postlarvae, concentrated
14
according to Elmgren et al. (1986), were picked in batches and labeled with C or stained with Rhodamine B. In order to replace dead or injured individuals, all M. affinis and M. balthica were checked under a stereo-microscope before being added to the experimental aquaria. Amphipods were left without food for 5 days before the start of the experiments. Animals were measured with an image analyser. The length of the straightened out amphipod was measured from the tip of the rostrum to the end of the last urosome segment, while for M. balthica the maximum length and height of the shell were measured.
14
2.1. C-Labelled Macoma balthica postlarvae and Monoporeia affinis
A 9-day experiment with four treatments, (1) M. affinis control, (2) M. affinis and
14 14
C-labelled M. balthica, (3) C-labelled M. balthica, (4) unlabelled M. balthica (control), each replicated 8 times, was started on July 5. For experimental details see Table 1.
14
Newly settled M. balthica were C-labelled by adding a 2-ml suspension of
14 21
C-labelled diatoms (Skeletonema costatum, 760 000 dpm ml ) to small (90 ml) aquaria without sediment. Each aquarium contained 100 M. balthica, and the total volume of sea water and added algae was 50 ml. The diatoms had been cultured in a
14
medium of artificial sea water and NaH CO3 (Kester et al., 1967). After 5 days,
14
thoroughly rinsed C-labelled M. balthica, and unmarked M. balthica were picked in
14
separate batches of 10. Five randomly chosen batches of M. balthica, either C-labelled or unlabelled, were added to each aquarium (Table 1). Thereafter five M. affinis were
14
added to both the C-labelled M. balthica and M. affinis treatment, and the M. affinis control.
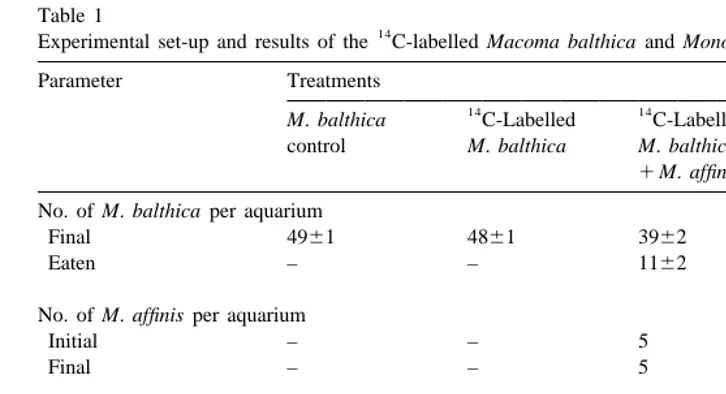
Table 1
14 a
Experimental set-up and results of the C-labelled Macoma balthica and Monoporeia affinis experiment Parameter Treatments
14 14
M. balthica C-Labelled C-Labelled M. affinis control M. balthica M. balthica control
1M. affinis No. of M. balthica per aquarium
Final 4961 4861 3962 –
Eaten – – 1162 –
No. of M. affinis per aquarium
Initial – – 5 5
Final – – 5 5
Radioactivity (dpm per aquarium)
M. balthica 0 72006200 57006300 –
M. affinis – – 7506120 0
M. affinis fecal pellets – – 170650 461
Sediment depth (cm) 2.060.1 2.060.1 2.160.1 2.060.1
a
The initial number of M. balthica was 50 specimens per aquarium. The radioactivity was measured on surviving specimens, pooled from each aquarium, and dpm values reported have been corrected by subtraction of the radioactivity of the scintillation fluid. The experiment lasted 5–14 July 1999. All treatments had eight replicates. Mean6standard error of mean are shown.
2.2. Rhodamine B-stained Macoma balthica postlarvae and Monoporeia affinis
A 5-ppm mixture of the vital fluorescent stain Rhodamine B and filtered brackish water was used to stain M. balthica postlarvae. The postlarvae were exposed to the stain for 3 days in 90 ml aquaria without sediment. They were then rinsed thoroughly in filtered cooled brackish water and picked out in batches of 10. Randomly selected batches giving a total of 100 M. balthica were added to each of three 105-ml aquaria with a sediment layer. Ten M. affinis were added to two of these. Another two aquaria, each with 10 M. affinis in sieved sediment, served as amphipod controls. After 3 days the content of each aquarium was sieved through a 100-mm sieve, and surviving bivalves and amphipods counted. The amphipod exoskeleton is transparent, allowing the gut and parts of the gut content to be observed under a fluorescence microscope without dissection. Rhodamine B fluoresces in orange when exposed to UV-light and remains of ingested stained bivalves could be detected as fluorescing spots in the amphipod gut. One control amphipod and one offered stained M. balthica, were randomly placed in pairs on a microscope slide, and observed under the fluorescence microscope. Two persons, neither of whom knew which of the amphipods had been offered stained M.
balthica, independently decided which individual in each pair showed the strongest
2.3. Statistics
The Mann–Whitney U-test, the sign test, and regression analysis were performed with Statistica 5 for PC.
3. Results
14
3.1. C-Labelled Macoma balthica postlarvae and Monoporeia affinis
14
C-Labelled M. balthica survived better alone than in the presence of M. affinis (P,0.002, Mann–Whitney U-test) (Table 1). The initial size of M. balthica was 426613 mm (average6S.E.M.), and the survivors in the control treatment were on average 502625 mm. All amphipods (7.360.1 mm) survived in both amphipod treatments. Control amphipods had a significantly lower radioactivity than amphipods
14
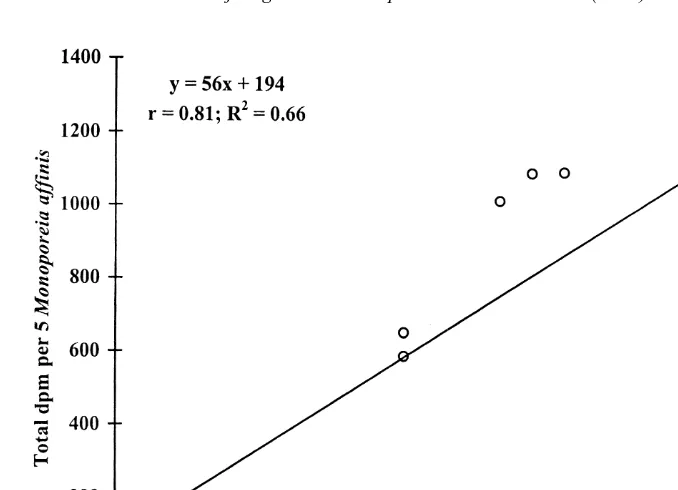
that had been offered C-labeled M. balthica (P,0.001, Mann–Whitney U-test) (Table 1). Regression analysis revealed a significant relationship between the number of labelled M. balthica missing and the amount of radioactivity in M. affinis (P,0.05) (Fig. 1). The radioactivity of the amphipod fecal pellets was likewise much higher in the labelled bivalve treatment (P,0.001, Mann–Whitney U-test). Pieces of damaged shells were found in the sieve residues from both treatments with amphipods and small bivalves, as found by Elmgren et al. (1986), and Ejdung and Elmgren (1998).
3.2. Rhodamine B-stained Macoma balthica postlarvae and Monoporeia affinis
Only one of the initially 100 M. balthica postlarvae died in the aquarium without amphipods. In the presence of the amphipods, 38 and 36 bivalves were missing, 10 and nine amphipods survived, respectively, in the two aquaria, while all control amphipods survived. Damaged pieces of bivalve shells were retained on the 100-mm sieve in amphipod treatments. The average initial and final sizes of postlarvae were 45861 and 449613 mm, respectively. Seventeen of the 19 surviving amphipods were rated for fluorescence in comparison with a randomly picked control amphipod, that had not been offered Rhodamine B-stained bivalves. Control amphipods showed a weak fluorescence. A stronger fluorescence was found in the amphipods offered stained M. balthica in 16 cases by one observer and in 15 cases by the other (two sign tests, each giving a P value of ,0.05).
4. Discussion
14
The result of the C experiment clearly demonstrates that plantigrade M. balthica are consumed by M. affinis, a result supported by the Rhodamine B stain found in the gut of
Fig. 1. Total radioactivity, dpm (disintegrations per minute) in Monoporeia affinis plotted against the number
Macoma balthica missing from initial number in each aquarium.
that the amphipods also eat the radioactive and stained bivalves, hence the bivalve mortality was caused by amphipod predation. We cannot judge whether all of the soft parts were ingested, some may have been scattered when the amphipod was eating. We have observed that decomposition is slower for shells than for soft parts, and takes 5–10 days for small naturally dead M. balthica (Elmgren et al., 1986). As in earlier studies (Elmgren et al., 1986; Ejdung and Elmgren, 1998), shell remains and damaged shells of
M. balthica were found only where M. affinis had been present.
The mortality of M. balthica in the northern Baltic Sea proper, estimated over the first 5 months after settlement, was ca. 95% at 10 m depth, where M. affinis and P. femorata were absent (Ankar, 1980). During their first months of life at the bottom, the plantigrades are vulnerable to predation by adult M. affinis, P. femorata, and juvenile M.
affinis (Elmgren et al., 1986; Ejdung and Elmgren, 1998). A M. balthica cohort, settling at 37 m in the northern Baltic Sea proper in July 1989, was reduced by 92% by October
22
´
(Olafsson and Elmgren, 1997) at an M. affinis abundance of 2000 m (C. Hill in ˚
only predator present in high densities at the time of settling was juvenile shrimp, C.
crangon. In a laboratory study, parts of small stained bivalves were recovered in their
gut. We find the evidence convincing that predation from M. affinis reduces the recruitment of M. balthica in our study area, as suggested by Ejdung and Elmgren (1998), and that such predatory processes also affect the M. balthica population negatively in the other areas discussed above. Environmental factors, e.g., oxygen condition, currents, sediment quality, and biotic factors, e.g., predation by benthos-eating fishes, and intraspecific interactions, described in a conceptual model for shallow sandy bottoms by Bonsdorff et al. (1995), may also directly or indirectly affect recruitment and influence the success of M. balthica populations, also on deeper sediment bottoms.
In a food-limited environment, every morsel of food, even if much smaller than the daily energy needs, is valuable for future survival, growth, and reproduction. According to optimal foraging theory, an organism should include all food items in its diet, when food is scarce. In accordance with this theory, the deposit-feeding M. affinis ingested small M. balthica, even though the bivalve is a scarce food item compared to the much greater amount of sediment in the aquaria. The carbon content of a 300-mm long M.
balthica with a shell-free dry weight of 0.25mg, is 0.11mg C (estimated from Cederwall and Jermakovs, unpublished data), while the same dry weight of the sediment used in our experiment contained 0.002mg C (estimated from loss on ignition, using algorithm in Carman and Cederwall, in press). Thus the energy content of the bivalve is ca 50 times that of the sediment. Consumption of M. balthica will also provide M. affinis with valuable dietary components, such as protein, lipids and glycogen (Beukema and de Bruin, 1977). However, a 1-year-old, 7.7 mm M. affinis probably gets ,2% of its daily energy requirements by eating one postlarval M. balthica per day (Elmgren et al., 1986). To increase the understanding of how soft-bottom communities are structured, it is important to demonstrate the mechanisms that produce observed community and population patterns. We need to assess and separate effects of predation, disturbance, emigration, bioturbation, competition and chemically induced avoidance of areas. In the field, mechanistic studies are often impossible to perform for logistic reasons, or may be biased due to difficulties of distinguishing among effects, whereas in the laboratory separate factors can be controlled. It was not possible for us to demonstrate the mechanism of interaction between the predator M. affinis and its prey M. balthica in the field or from field material since appropriate methods were not available. At ca. 30 m depth, the night-active amphipods live mainly in the upper 5 cm of the sediment, and the bivalve postlarvae dwell in the top millimetres of the sediment. Thus their activities could not be followed by in situ video-recordings. Gut analysis, successful for prey organisms possessing identifiable hard parts or for immunoassay analysis, cannot be used when prey is dismembered and masticated or lysed by the predator, or when the potential predator does not eat the species-specific structures. M. affinis breaks the shell of M. balthica, and eats the soft parts, which are impossible to distinguish visually from other items ingested, whereas nematode cuticles have been found in Pontoporeid guts (Elmgren, 1976).
This study, when combined with earlier laboratory experiments (Elmgren et al., 1986; ´
balthica and can thus be an important structuring factor in the low diversity benthic
macrofauna community of the Baltic Sea.
Acknowledgements
¨
We thank Ylva Lilliemarck for advice on vital staining, Goran Malmberg for instruction on the image analysing system, Sven Ankar and Hans Cederwall for valuable ¨ discussions and comments on an earlier draft of the manuscript, the staff of the Asko laboratory for assistance in field and laboratory, and Rolf Carman for access to unpublished results. This study was supported by grants from the Swedish Natural Research Council to R.E, and from the Stockholm Centre for Marine Research to G.E and L.B. [RW]
References
Ankar, S., 1977. The soft bottom ecosystem of the northern Baltic proper with special reference to the ¨
macrofauna. Contrib. Asko Lab. Univ. Stockholm 19, 1–62.
Ankar, S., 1980. Growth and production of Macoma balthica (L.) in a northern Baltic soft bottom. Ophelia (Suppl.) 1, 31–48.
¨
Ankar, S., Elmgren, R., 1976. The benthic macro- and meiofauna of the Asko-Landsort area — a stratified ¨
random sampling survey. Contrib. Asko Lab. Univ. Stockholm 11, 1–115.
Bachelet, G., 1990. Recruitment of soft-sediment infaunal invertebrates: the importance of juvenile benthic stages. La Mer 28, 199–210.
Baker, P., Mann, R., 1997. The postlarval phase of bivalve mollusks: a review of functional ecology and new records of postlarval drifting of Chesapeake Bay bivalves. Bull. Mar. Sci. 61, 409–430.
Beukema, J.J., de Bruin, W., 1977. Seasonal changes in dry weight and chemical composition of the soft parts of the tellinid bivalve Macoma balthica in the Dutch Wadden Sea. Neth. J. Sea Res. 11, 42–55. Beukema, J.J., Honkoop, P.J.C., Dekker, R., 1998. Recruitment in Macoma balthica after mild and cold
winters and its possible control by egg production and shrimp predation. Hydrobiologia 375–376, 23–34. Blomqvist, S., Lundgren, L., 1996. A benthic sled for sampling soft bottoms. Helgol. Meeresunters. 50,
453–456.
¨
Bonsdorff, E., Norkko, A., Bostrom, C., 1995. Recruitment and population maintenance of the bivalve
Macoma balthica (L.) — factors affecting settling success and early survival on shallow sandy bottoms. In:
Eleftheriou, A., Ansell, A.D., Smith, C.J. (Eds.), Proc. 28th Eur. Mar. Biol. Symp. Olsen and Olsen, Fredensborg, Denmark, pp. 253–260.
Bousfield, E.L., 1989. Revised morphological relationships within the amphipod genera Pontoporeia and Gammaracanthus and the ‘glacial relict’ significance of their postglacial distributions. Can. J. Fish. Aquat. Sci. 46, 1714–1725.
Carman, R., Cederwall, H., Sediments and macrofauna in the Baltic Sea–characteristics, nutrient contents and distribution. In: Wulff, F., Rahm, L., Larsson, P. (Eds.), A Systems Analysis of the Baltic Sea. Springer, Berlin, in press.
¨ ¨ ˚ ˚ ¨ ¨
Cederwall, H., 1996. Mjukbottenfauna. Ostersjo ’95. Arsrapport fran den marina miljoovervakningen juni 1996. Annual Report (ISSN 1104-9243), pp. 32–35 (in Swedish).
Cederwall, H., 1999. Bottenvatten och mjukbottenfauna (English summary: bottom water and soft bottom
¨ ¨
Cummings, V.J., Pridmore, R.D., Thrush, S.F., Hewitt, J.E., 1996. Effect of the spionid polychaete Boccardia
syrtis on the distribution and survival of juvenile Macoma liliana (Bivalvia: Tellinacea). Mar. Biol. 126,
91–98.
Danovaro, R., Fraschetti, S., Belgrano, A., Vincx, M., Curini-Galletti, M., Albertelli, G., Fabiano, M., 1995. The potential impact of meiofauna on the recruitment of macrobenthos in a subtidal coastal benthic community of the Ligurian Sea (north-western Mediterranean): a field result. In: Eleftheriou, A., Ansell, A.D., Smith, C.J. (Eds.), Proc. 28th Eur. Mar. Biol. Symp. Olsen and Olsen, Fredensborg, Denmark, pp. 115–122.
Ejdung, G., Elmgren, R., 1998. Predation on newly settled bivalves by deposit-feeding amphipods: a Baltic Sea case study. Mar. Ecol. Prog. Ser. 168, 87–94.
¨
Elmgren, R., 1976. Baltic benthos communities and the role of the meiofauna. Contrib. Asko Lab. Univ. Stockholm 14, 1–31.
Elmgren, R., 1978. Structure and dynamics of Baltic benthos communities, with particular reference to the relationship between macro- and meiofauna. Kieler Meeresforsch. Sonderb. 4, 1–22.
Elmgren, R., Ankar, A., Marteleur, B., Ejdung, G., 1986. Adult interference with postlarvae in soft sediments: the Pontoporeia-Macoma example. Ecology 67, 827–836.
Goedkoop, W., Johnson, R.K., 1994. Exploitation of sediment bacterial carbon by juveniles of the amphipod
Monoporeia affinis. Freshwat. Biol. 32, 553–563.
Gosselin, L.A., Qian, P.-Y., 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282.
¨ ¨
Hessle, C., 1924. Bottenboniteringar i inre Ostersjon. Medd. K. Lantbruksstyrelsen 250, 1–52.
Kester, D.R., Duedall, I.W., Connors, D.N., Pytkowicz, R.M., 1967. Preparation of artificial seawater. Limnol. Oceanogr. 12, 176–179.
Laine, A.O., Sandler, H., Andersin, A.-B., Stigzelius, J., 1997. Long-term changes of macrozoobenthos in the Eastern Gotland Basin and the Gulf of Finland (Baltic Sea) in relation to the hydrographical regime. J. Sea Res. 38, 135–159.
Lehtonen, K.K., 1996. Ecophysiology of the benthic amphipod Monoporeia affinis in an open-sea area of the northern Baltic Sea: seasonal variations in body composition, with bioenergetic considerations. Mar. Ecol. Prog. Ser. 143, 87–98.
Lopez, G., Elmgren, R., 1989. Feeding depths and organic absorption for the deposit-feeding benthic amphipods Pontoporeia affinis and Pontoporeia femorata. Limnol. Oceanogr. 34, 982–991.
Luckenbach, M.W., 1987. Effects of adult infauna on new recruits: implications for the role of biogenic refuges. J. Exp. Mar. Biol. Ecol. 105, 197–206.
McIntyre, A.D., 1964. Meiobenthos of sublittoral muds. J. Mar. Biol. Assoc. UK 44, 665–674. ´
Olafsson, E., Elmgren, R., 1997. Seasonal dynamics of sublittoral meiobenthos in relation to phytoplankton sedimentation in the Baltic Sea. Estuar. Coast. Shelf Sci. 45, 149–164.
´
Olafsson, E.B., Peterson, C.H., Ambrose Jr, W.G., 1994. Does recruitment limitation structure populations and communities of macro-invertebrates in marine soft sediments: the relative significance of pre- and post-settlement processes. Oceanogr. Mar. Biol. Annu. Rev. 32, 65–109.
Rumohr, H., Bonsdorff, E., Pearson, T.H., 1996. Zoobenthic succession in Baltic sedimentary sediments. Arch. Fish. Mar. Res. 44, 179–214.
˚
Segerstrale, S.G., 1962. Investigations on Baltic populations of the bivalve Macoma baltica L. Part II. What are the reasons for the periodic failure of recruitment and the scarcity of Macoma in the deeper waters of the inner Baltic? Commentat. Biol., Soc. Sci. Fenn. 24, 1–26.
Thorson, G., 1950. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 25, 1–45. Thorson, G., 1966. Some factors influencing the recruitment and establishment of marine benthic communities.
Neth. J. Sea Res. 3, 267–293.