Clinical Update on Influenza
Dewi Dian Sukmawati
Division of Tropical and Infectious Disease Department of Internal Medicine
Udayana University School of Medicine-Sanglah Hospital Denpasar
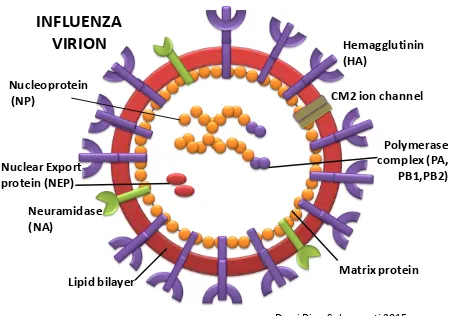
Influenza ia a viral infection commonly attack upper respiratory tract; nose, throat, bronchi and occasionally spread to lungs. The viral spread easily from person to person via droplets and small particles produced by infected person when they cough or sneeze. The virus come from Orthomyxoviridae family, differentiated into three strain: Influenza virus A, B and C with most virulent strains belongs to the type A. The classification of Influenza virus A further based on the antigenic differences on their major surface antigens: Hemagglutinins (H1 – H5) and Neuraminidase (N1 – N9) Figure 1.
Figure 1. Influenza A virion
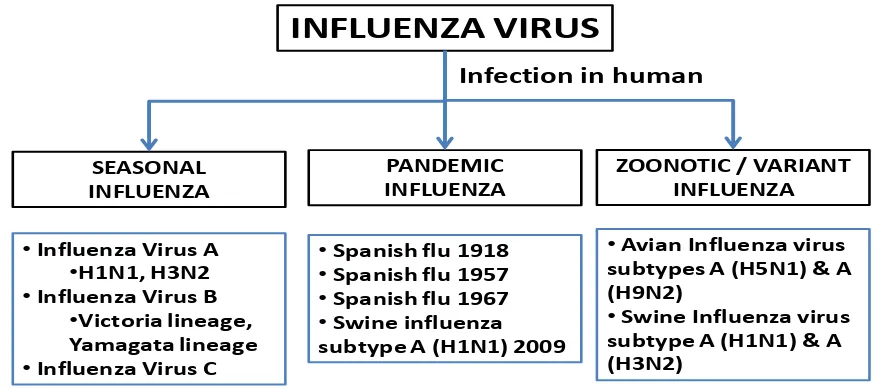
Some terms were use to describe the epidemiology of Influenza virus infection in human; seasonal influenza, pandemic influenza and zoonotic or variant influenza (Figure 2)
Seasonal influenza
Seasonal influenza viruses circulate and cause influenza in humans annually causing mild to severe illness and sometimes death, particularly in some high-risk individuals. Persons at increased risk for severe disease include pregnant women, infants and elderly, immune – compromised people, and people with co-morbidity chronic underlying medical conditions.
hemagglutinin or “H” protein and the neuraminidase or “N” protein. Currently, influenza A(H1N1) and A(H3N2) are the circulating seasonal influenza A virus subtypes. This seasonal A (H1N1) virus is the same virus that caused the 2009 swine influenza pandemic, as it is now circulating seasonally. In addition, there are two type B viruses that are also circulating as seasonal influenza viruses, which are named after the areas where they were first identified, Victoria lineage and Yamagata lineage. Type C influenza causes milder infections and is associated with sporadic cases and minor localized outbreaks. As influenza C poses much less of a disease burden than influenza A and B, only the latter two are included in seasonal influenza vaccines.
Pandemic Influenza
Pandemic influenza is a global outbreak. An influenza pandemic occurs when a new flu virus emerges for which humans have little or no immunity, which allows the virus to spread easily from person to person worldwide. These viruses may emerge, circulate and cause large outbreaks outside of the normal influenza season. As the majority of the population has no immunity to these viruses, the proportion of persons in a population getting infected may be quite large. Some pandemics may result in large numbers of severe infections while others will result in large numbers of milder infections, but the reasons behind these differences are not completely understood. Three influenza pandemics occurred during the 20th century: the 1918 – 1919 “Spanish flu,” the 1957 – 1958 “Asian flu,” and the 1968 – 1969 pandemic or “Hong Kong flu.” In 2009, the World Health Organization (WHO) declared a global pandemic of H1N1 flu “Swine Flu”. This action was a reflection of the spread of the new H1N1 virus, not the severity of illness caused by the virus.
Zoonotic or variant influenza
Figure 2. Influenza viruses in human
INFLUENZA VIRUS
SEASONAL INFLUENZA
PANDEMIC INFLUENZA
ZOONOTIC / VARIANT INFLUENZA Infection in human
•Influenza Virus A •H1N1, H3N2 •Influenza Virus B
•Victoria lineage, Yamagata lineage •Influenza Virus C
•Spanish flu 1918
•Spanish flu 1957
•Spanish flu 1967
•Swine influenza
subtype A (H1N1) 2009
•Avian Influenza virus subtypes A (H5N1) & A (H9N2)
•Swine Influenza virus subtype A (H1N1) & A (H3N2)
Influenza virus infections in humans. WHO 2014
Clinical Manifestations
The majority of person infected with influenza virus exhibit self limiting, uncomplicated, acute respiratory illness with fever or even asymptomatic (Figure 3).
The criterion standard for diagnosing influenza A and B is a viral culture of nasopharyngeal samples or throat samples. In elderly or high-risk patients with pulmonary symptoms, chest radiography should be performed to exclude pneumonia
Influenza Vaccine
Since the influenza seasons are unpredictable and influenza disease can have severe consequences, annual vaccination remains the best way to prevent the flu. Available vaccines including injectable and nasal spray form.
Seasonal Virus vaccine Trivalent types A and types B: • Injectable vaccine
– Afluria. CSL Limited.
– Agriflu. Novartis Vaccines and Diagnostics, Inc. – FluLaval. ID Biomedical Corporation of Quebec – Fluarix. GlaxoSmithKline Biologicals
– Flublok. Protein Sciences Corporation
– Flucefax. Novartis Vaccines and Diagnostics, Inc. – Fluvirin. Novartis Vaccines and Diagnostics Ltd.
– Fluzone, Fluzone highdose, Fluzone intradermal. Sanofi Pasteur, Inc. • Intranasal spray
– FluMist. MedImmune, LLC
Seasonal Virus vaccine Quadrivalent types A and types B: • Injectable vaccine
– Fluarix Quadrivalent. GlaxoSmithKline Biologicals – Fluzone Quadrivalent. Sanofi Pasteur, Inc.
– FluLaval Quadrivalent. ID Biomedical Corporation of Quebec • Intranasal spray
– FluMist Quadrivalent. MedImmune, LLC
Influenza virus vaccine for H5N1 (not available commercially, stock pile only used for outbreak):
• Influenza virus vaccine H5N1 Sanofi Pasteur, Inc
• Influenza A (H5N1) Virus Monovalent Vaccine Adjuvanted. ID Biomedical Corporation of Quebec
Antiviral Therapy
• Infected cases: Infected person will have milder sign and symptoms with shorter duration of illness. Antiviral medications work best when started within the first two days of flu onset.
• Exposed to the flu: antiviral medication can prevent Illness. But must be remember that antiviral drugs are not a substitute for the influenza vaccine.
Current approved antiviral for influenza including • Tamiflu (oseltamivir phosphate)
• Relenza (zanamivir) • Rapivab (peramivir)
Older drugs, such as amantadine and rimantadine possess resistance problem (including to H1N1 influenza) thus not widely be used anymore.
Refferences
1. Fiore AE, Shay DK, Broder K, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57(RR-7):1–60.
2. D‟Heilly SJ, Janoff EN, Nichol P, Nichol KL. Rapid diagnosis of in- fluenza infection in older adults: influence on clinical care in a routine clinical setting. J Clin Virol 2008; 42:124–8
3. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014 Aug 14. 371(7):635-45
4. Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010 Jul 7. CD001269
Pulmonary Infection in HIV AIDS Patients
Anak Agung Ayu Yuli Gayatri, Tuti Parwati Merati
Division of Tropical and Infectious Disease Department of Internal Medicine
Udayana University School of Medicine-Sanglah Hospital Denpasar
Abstract
The immune dysregulation associated with HIV result in an increased incidence of respiratory infection at all CD4 T-cell count. The type of pulmonary condition to be developed by AIDS patients will depend on the stage of disease, which is generally determine based on the CD4 lymphocytes count. Some of the most common opportunistic infectious lung diseases seen in HIV-positive or AIDS patients are pneumocystis carinii pneumonia, tuberculosis and bacterial pneumonia. A simple patient risk assessment allows the clinician to determine the likelyhood that other opportunistic infections are the cause of severe respiratory disease and that further pathogens may need to be considered. Treatment is often started prior to laboratory confirmation of diagnosis. The intensity with which investigation is undertaken is usually determined by the patient risk assessment, severity of the illness and the resources available locally.
Keywords :
Introduction
Human Immunodeficiency virus (HIV) is the virus that causes acquired immunodeficiency syndrome (AIDS). HIV targets an important kind of white blood cell called CD-4 T Lymphocyte or T cells. T cells belong to the immune system and protect the body from germs and other disease-causing agents. Germs take this opportunity to invade the body and cause infections. People may be infected with HIV for years before developing AIDS. The lung is a major area of opportunistic infection in people living with HIV and AIDS. Some of the most common opportunistic infectious diseases seen in HIV-positive or AIDS patients are pneumocystis carinii pneumonia, tuberculosis and bacterial pneumonia. (Dockrell DH et al. 2011)
A simple patient risk assessment allows the clinician to determine the likelyhood that other opportunistic infections are the cause of severe respiratory disease and that further pathogens may need to be considered. Relevant factors include: (1) patient use of effective opportunistic infection prophylaxis or HAART; (2) from hospital or current hospital admission >5 days (nosocomial infections); (3) country/place of residence and travel history; (4) history of active injecting drug use, since these individuals are at increase risk of bacterial pneumonia and TB; (5) level of host immunity; (6) neutropenia; (7) use of prolong course of immune modulator (e.g. corticosteroids).
Pneumocystis carinii pneumonia (PCP)
Pneumocystis carinii pneumonia (PCP) is the first sign of illness in more than half of all persons with AIDS in the United States. This is caused by the Pneumocyctis jiroveci germ, formerly known as Pneumocystis carinii. Pneumocystis jirovecii is a fungus that causes infection specific to humans. The great majority occur in immunocompromised subjects and are associated with respiratory symptoms. Many people who are HIV positive take medication to prevent PCP; frequent blood tests help health care provider decided when such treatment should begin.
Presentation
Without preventive medicine, over 80% of people with HIV will likely get PCP. Common symptoms include coughing, fever and trouble breathing. Almost 90% of cases occur in HIV-seropositive persons with CD4 T-cell counts <200 cells/µl (or a CD4 T-cell percentage<14%). Other predictive factors for PCP in subjects not receiving effective HAART, include non-adherence to prophylaxis, oral candidiasis, oral hairy leukoplakia, unintentional weight loss, recurrent bacterial pneumonia, previous PCP and high plasma HIV load. (Dunleavy A et al. 2009)
apparent. Rarer presentation include a more rapid onset, haemoptysis and pleuritic chest pain. Physical examination reveals tachypnoea, normal breath sounds or less frequently, and-inspiratory crackles. Wheezing and sign of focal consolidation or pleural effusion are less common presentation. Spontaneous or infection-associated pneumothorax in an HIV-seropositive individual should prompt exclusion of PCP. Radiological findings in the chest include perihilar haze, interstitial infiltrates (characteristically sparing the apices and costo-phrenic angles), pneumatocoeles and pneumothoraces. Upper lobe infiltrates alone have been reported to occur in up to 39% of patient and should , there fore, not distract from pursuing the diagnosis of PCP clinically suspected. (Dockrell DH et al. 2011)
Diagnosis
There are no clinical features specific to PCP. Radiology and nuclear medicine test are not particularly sensitive or specific. Other opportunistic infections may mimic the typical radiological features of PCP. Demonstration of fall in oxygenation between rest and exercise has been validated as a reasonably specific test for PCP in cases with a normal or near-normal chest radiograph who have no previous history of PCP, but is not reliable enough to make a diagnosis without confirmatory microbiology. If induced sputum (IS) is routinely available, this can be performed initially(sensitivity 50-90%). If IS result are negative or inconclusive, then the patient should be assessed for bronchoscopy with bronco-alveolar lavage (BAL; diagnostic sensitivity>90%). Some may choose BAL as the first-line investigation employed. Open lung biopsy (diagnostic sensitivity 95-98%) is reserved for the occasional patient, with negative initial tests, and who is not improving on empirical treatment. Spontaneously expectorated sputum is not an adequate alveolar sample and should not be processed. (Beck. 2005; Dockrell DH et al. 2011)
Pneumocystis jirovecii cannot be cultured in vitro; diagnosis relies on visualization of the organism using either histochemical (typically with silver stains such as Grocott-Gomori methenamine silver stain) or immune-flourescent stains.
Treatment
duration; many switch individuals showing a good initial response to oral therapy at doses driven by either treatment-limiting toxicity or lack of efficacy. (Dunleavy A. et al. 2009)
Tabel 1. Stratification of disease severity in PCP (Dunleavy A.et al. 2009)
Mild Moderate Severe Note: for treatment purpose moderate severity is group with severe disease if
PaO2>9.3kPa
The choice of treatment is therefore determined by patient tolerance and ability to take either oral or iv medication. For severe PCP, the other treatment options are clindamycin 600-900mg qid/tid iv or 300-450 mg tid/qid po and primaquine 15-30 mg od or pentamidine 4 mg/kg od iv for 21 days. Many clinician favour clindamycin based therapy in view of the toxicity profile of iv pentamidine. Good bioavailability allows clindamycin to be given by the oral route unless the patient is unable to take oral medicines. A lower rate of methaemoglobinaemia means the 15 mg dose of primaquine recommended. (Dunleavy A. et al. 2009; Dockrell DH et al. 2011)
Prophylaxis
do not provide the additional protection provided by TMP-SMX against other infections and some are not as effective at low CD4 T-cell counts. Early initiation of HAART is favourable in individuals with PCP. The improvement in systemic and local immunity following continous use of HAART translate into very low risk of PCP if profilaxis is discontinued in population with CD4 T cell count >200 cells/µL for more than 3 months.
Tuberculosis
Tuberculosis (TB) infection in HIV patient remains a major global public health challenges, with estimated 1.4 million patients world wide. By the end of 2009, an estimated 2.6 million individuals had become newly infected with HIV and 1.8 million had died of AIDS in that year alone. TB is the most common opportunistic infection among HIV infected individuals, and co-infected individuals are at high risk of death. (Lawn S. et al. 2009)
TB may occur in any stage of HIV disease and is frequently the first recognized presentation of underlying HIV infection. As compared to people without HIV, people living with HIV (PLWH) have 20-fold higher risk of developing TB and the risk continues to increase as CD4 cell counts progressively decline. Although ART can reduced the incidence of TB both at the individual and population level, PLWH on ART still have higher TB incidence rates and a higher risk of dying from TB. This may be due to delayed initiation of ART or the fact that patients present with advance TB or both. Routine TB screening among PLWH offers the opportunity to identify those without TB, prevent TB by chemoprophylaxis as well as to diagnose and promptly treat TB. However co-administration of ART along with anti-TB therapy present several management challenges, including drug-drug interactions, overlapping drug toxicities and immune reconstitution syndrome. (Lawn S et al. 2009)
Presentation
Clinical symptoms of TB pulmonary disease are similar to those caused by other mycobacteria : fever, night sweats, weight loss, productive cough, dyspnea, central chest pain and sometimes hemoptysis.
Diagnosis
Diagnosis of active TB disease in HIV- infected persons is difficult, because patients with HIV-associated TB have fewer bacilli in their sputum than do HIV-uninfected patients with pulmonary TB. In addition it has been observed that presence of a cough for > 3 weeks is not sensitive enough on its own as a symptom of TB in HIV-infected persons.
impact of methods that optimize the use of smear microscopy is not well understood for HIV-infected persons. Mycobacterial culture is the gold standard for TB diagnosis and is routinely recommended to assist the diagnosis of TB in HIV-infected persons, although it is frustratingly slow. HIV infection compromises the validity and effectiveness of chest radiography in the diagnosis of pulmonary TB in HIV-infected persons, and the findings could be normal for up to 14% of HIV- infected persons who have culture confirmed pulmonary TB. However, chest radiography remains an important adjunct in the diagnosis of TB, and its use must be expanded, including the use of advance and innovative technology such as digital imaging. (Padmapriyadarsini C et al. 2011)
The WHO recommends TB screening at the time that HIV infection is diagnosed, before the initiation of antiretroviral therapy and at regular interval during follow up. It was recommended that screening for TB should include asking questions about the present of any one of current cough, fever, night sweats or weight loss. In the hospital setting, bacteriological examination mostly failed to find Mycobacterium TB from sputum smear, and this is not simply just by difficulty to get the specimen. Because of the poor performance of sputum smear microscopy in HIV-infected patients, recently the WHO endorsed the used of GeneXpert-Rif for the rapid diagnosis of TB as well as rifampicin resistance among HIV-infected individuals with clinical suspicion of TB. (WHO report. 2010; Padmapriyadarsini Cet al 2011))
Chest X ray do not show typical feature of lung TB especially in patient with low CD 4 cell count. The spectrum of radiographic manifestation of Pulmonary TB is dependent on the relative level of immunodeficiency. During the early phase of HIV when individuals are not immunosupressed the radiographic pattern is similar to HIV un-infected individuals with more typical lesions-upper lobe infiltrates with or without cavities. With advancing immune-suppression, extra pulmonary involvement, intrathoraxic/ mediastinal lymphadenopathy, lower lobe infiltrates and milliary TB become more common.( Padmapriyadarsini C et al. 2011)
Treatment
symptoms and sign in one or two months after completing in six moths anti TB drugs treatment. (WHO report. 2010; Padmapriyadarsini C et al. 2011)
Bacterial Pneumonia
HIV related bacterial infection of the lower respiratory tract is common and occurs at levels of immunosupression. Risk factors for HIV- related bacterial pneumonia are declining CD4 lymphocyte count, cigarette smoking and injecting drug use. Incidence of bacterial pneumonia is five times greater in HIV positive population than in otherwise similar but HIV-negative population; the incidence of pneumococcal disease, including pneumonia, is 10 times greater and the development of pneumococcal septicemia is 100 times greater. (Beck. 2005)
Recurrent pneumonia (two or more episodes in a 12-month period) is classified as AIDS-defining. The etiology of community-acquired pneumonia (CAP) among HIV-seropositive individuals is similar to that of the general population with Streptococcus pneumonia and Haemophilus influenza predominating. Staphylococcus aureus has been reported at a greater frequency than in the general population. Pseudomonas aeroginosa has been noted more commonly at low CD4 T-cell counts. Although atypical pathogens such as Legionella pneumophila, Mycoplasma pneumonia and Chlamydophila (Chlamidia) pneumonia have not been frequently reported in HIV-related bacterial pneumonia, this may reflect diagnostic difficulties, and there are data to support that these occur at the same frequency in HIV-seropositive and HIV-seronegative populations. As with immunocompetent individuals . Gram-negative pathogens should be considered especially likely in those who develop pneumonia when hospitalized. (Dunleavy A. et al. 2009; Dockrell DH et al. 2011)
Presentation
Presenting symptoms are similar to HIV-seronegative individuals and typically have an acute onset (hours to days).The classical physical signs are those of lung consolidation, although there is an increased tendency to rapid progression: cavitation, parapnemonic effusion and empyema formation The peripheral white blood count (WBC) is usually elevated but may be low in more severe cases.
Diagnosis
suspected a chest radiograph should be obtained. Radiological features are similar to HIV-seronegative individuals. Patchy, lobar or segmental consolidation appears on plain radiograph, although an increased frequency of interstitial infiltrates has been recently reported; cavitation within consolidation, when the infection is cause by gram-negative organisms, as for example pseudomonas and multiple cavitating nodules in case of septic embolism especially in IVDU.
Blood cultures should be routinely performed in these patients because of the high incidence of bacteremia. (Dunleavy A. et al. 2009; Dockrell DH et al. 2011)
Treatment
While empirical therapy (usually directed against bacterial pathogens) may be appropriate for patients with CD4 counts> 200 cells/µL, every effort should be made to confirm a specific diagnosis in the more immunocompromised individual. Initial anti-microbial treatment is usually empirical and should be chosen according to; (a) pneumonia severity; (b)the likelyhood of particular pathogens as indicated by risk factors; (c) the potential for antibiotic resistant and (d) potential toxicities. A number of guidelines developed to guide the management of CAP in HIV-seronegative individuals exist and the possible regimens suggested in these guidelines are adapted from them. HIV-seropositive individuals with community-acquired pneumonia should be treated as per HIV-seronegative populations. Antibiotic prophylaxis is not indicated for bacterial pneumonia. The capsular polysaccharide vaccine protects against pneumococcal serotypes. The Departement of Health includes HIV-seropositive individuals amongst the “high- risk” groups for whom vaccination is recommended.
References
Beck J. Immunocompromised Host. Proc Am Thorax Soc 2005;2:423-7
Benson CA et al. Treating Opportunistic Infections Among HIV Infected Adults and Adolescents Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/ InfectiousDiseases Society of America. Available at: http: //www.cdc.gov/hiv/topics/treatment/index.htm
Dockrell DH, Breen R, Lipman M, Miller RF. Pulmonary Opportunistic infections. 2011. HIV Medicine. 12 (suppl.2) 25-42.
Dunleavy A, Lipman M, Miller R. Infection in the HIV compromised host. In: maskell N, Millar A, eds. Oxford Desk Reference Respiratory Medicine. Oxford, Oxford University Press. 2009: 210-217
Padmapriyadarsini C, Narendran G, Swaminathan S. Diagnosis & Treatment of Tuberculosis in HIV co-invected patients. 2011. Indian J Med Res 134;850-865
Tuberkulosis, Pedoman Diagnosis dan Penatalaksanaan di Indonesia. 2011. Perhimpunan Dokter Paru Indonesia.
Methicillin Resistant Staphylococcus Aureus (MRSA) and Extended Spectrum Beta-Lactamases (ESBL)
Anak Agung Ayu Yuli Gayatri, Tuti Parwati Merati
Division of Tropical and Infectious Disease Department of Internal Medicine
Udayana University School of Medicine-Sanglah Hospital Denpasar
Introduction
Resistance of pathogenic organisms to countenance antibiotics has become a world wide problem with serious consequences on the treatment of infectious diseases. The heightened use or misuse of antibiotics in human medicine, agriculture and veterinary is primarily contributing to the phenomenon. There is an alarming increase of antibiotic resistance in bacteria that cause either community infections or hospital acquired infections. Resistance genes borne on chromosomal, and increasingly, on transmissible extrachromosomal elements. The resulting resistant clones e.g. Meticillin-resistant Staphylococcus aureus (MRSA) USA 300, Escherichia coli ST 131 and Klebsiella ST258 are disseminated rapidly worldwide.
This spread is fascilitated by interspecies gene transmission, poor sanitation and hygiene in communities and hospitals and the increasing frequency of global, travel, trade and disease transmission. (Alekshun et al. 2007)
Treatment of these multiple drug resistant organisms, pose unique challenges to clinicians, clinical microbiologists, infection control professionals and antibacterial-discovery scienties. It is generally recognized that patients infected with MRSA and ESBL-producing organisms are at risk for poor outcome if they are treated with anti bacterials to which the organisms exhibits high level resistance. The mortality rate in these susceptibility/ mismatched patients has ranged from 42-100%. (Chong Yet al. 2011)
1. Methicillin- Resistant Staphylococcus Aureus (MRSA)
MRSA was first described in 1961, almost immediately after the agent was introduced into clinical practice. MRSA are a type of staphylococcus bacteria that are resistant to many antibiotics. MRSA bacteria are more likely to develop when antibiotics are used too often or are not used correctly. Staphylococcus bacteria only become a problem when they cause infection. For some people especially those who are weak or ill, these infections can become serious.
MRSA in an opportunistic bacterium which may colonize and grow readily on the skin and mucous membranes of a person without harm to that person. It competes with other microorganisms found on the skin surface and is commonly found in the nose, groin, perineum or any other warm, moist sites. The human skin is constantly shedding skin scales-MRSA is shed with the skin as it falls from the human body. The greater the number of MRSA colonies on a person, the greater the potential for contamination of the environment and the transmission of MRSA to others.
Infected and colonized patients are the reservoir of MRSA both in hospital and the community with transmission generally being via contact with health workers. Effective, rapid laboratory diagnosis and susceptibility testing is critical in treating, managing any preventing MRSA infections.
1.1. MRSA Infections in Hospital
on hemodialysis, iii. Receive cancer treatment or medicines that weaken their immune system, iv. Inject illegal drugs, v. Had surgery in the past year.
The prevention of horizontal transmission of MRSA has become increasingly important as the prevalence of this pathogen increases. Oral carriage of MRSA may serve as a reservoir for re-colonization of other body sites or for cross-infection to other patients or health care workers. Therefore, it is important that consideration be given to the oral cavity if eradication of colonization by MRSA is clinically appropriate. Eradication of throat carriage of MRSA has been achieve with use of topical chlorhexidine (0.2%) in addition to normal control measures of patient isolation, nasal mupirocin and chlorhexidine body washes.
1.2. MRSA infection in the Community
MRSA infections can also occur in healthy people who have not recently been in the hospital. The strains were labeled community-associated MRSA (CA-MRSA) if they had been isolated within 48 hours of hospitalization from patients who had not been in any hospital for >1 year. CA-MRSA resistance is usually limited to β-lactams and the strains remain susceptible to clindamycin, gentamycin, sulfamethoxazole-trimethoprim, vancimycin, rifampin, tetracycline and linezolide. Most CA-MRSA strains characterized by carry the Panton-Valentine leukocidin genes, leading to leukocyte destruction, skin abcesses and necrotizing pneumonitis, and also presence of staphylococcal chromosome cassette mec (SCCmec) IVa, a novel smaller variant of the methicillin-resistant locus. Other S aureus strains that are not resistant to methicillin will be referred to as methicillin- sensitive Staphylococcus aureus (MSSA). Most of CA-MRSA infections are on the skin or less commonly lung infections. These infections can occur among people who are likely to have cuts or wounds and who have close contact with one another, such as members of sports teams. People who may be at risk are: i. Atlletes and other people who may share items such as towels or razors, ii. Children in day-care. iii. Members of military, iv. People who have gotten tattoos. In some cases these organisms can cause invasive infection such as septic arthritis, bacteriemia, or community-acquired necrotising pneumonia. An early skin infection often has the initial appearance of an insect bite. These infections often develop into cellulitis, furuncles, large boils or clusters of boils (up to 10 cm in diameter) and deep-seated abscesses often in the thighs or buttocks. If the bacteria gain access to the lungs, fortunately a rare event, a devastating pneumonia that kills more than 40% of patients can result.
Table 1. Comparison between healthcare-associated and community-acquired Methicillin resistant Staphylococcus aureus. ( Sievert et al. 2013)
CA-MRSA HA-MRSA
Clinical spectrum
Skin and soft tissue infections Wound infections, urinary tarct infections and bacteraemia
Sensitive to multiple antibiotics Resistant to multiple antibiotic
Toxin production
May produce PVL toxin Not yet reported produce PVL toxin
1.3. MRSA testing
MRSA screening may be undertaken for the following reasons:
1. Screening requirement determined from the multidrug Resistant Organism (MDRO) Risk Assesment
2. If found positive after admission from a clinical sample 3. As part of Outbreak Management
MRSA specimens
A purple bacterial swab is use to sample the following sites: 1. Nasal swab (one swab for both nostrils)
2. Groin swab (one swab for both sides) 3. Perineum swab
4. Wound swab-including decubitus ulcer (pressure sore) or surgical wound and device insertion e.g. IV tracheostomy, drain, suprapubic
5. Additional site:
Placement of patients in single room
Treatment procedure applies to all patients and staff who may or may not be currently receiving systemic antibiotic treatment for MRSA infection
Hand hygiene with antimicrobial liquid soap or alcohol- based hand rub
Dedicated patient-care equipment or disinfect between use if shared with other patients e.g. blood pressure and oximetry equipment
Remove unnecessary equipment from the isolation room and ensure supplies are not overstocked within the room
If no Ensuite shower is available the patient showers last in the communal shower and the shower is disinfected after use
Mupirocin (Bactroban) is to be applied to the anterior nasal nares three times a day
Tridosan 1% is to be used for daily washing of skin and bathing. Cetrimide shampoo for hair washing twice weekly
Treatment is to be for an initial period of seven days
Visitors do not wear PPE but are encourage to perform hand hygiene after visiting the patient
Where possible, permanent staff should be used
Treatment
Patients who are severely ill or have a rapidly progressing infection should be referred to the hospital for consideration of intravenous antibiotics. Intravenous vancomycin is the most commonly used antibiotic for this indication. Vancomycin can have serious side effects, especially in elderly persons. These side effects could include ototoxicity (loss of hearing or other auditory damage), nephrotoxicity (damage to the kidneys or renal system), and allergic reactions such as fever and rash. Infusion of vancomycin, especially when to rapid, can result in flushing, hypotension, and tachycardia known as the “red man syndrome”. Vancomycin given by mouth is not absorbed and is not effective against MRSAEmergence of vancomycin-intermediate and vancomycin-resistant MRSA (VISA and VRSA, respectively) has been reported, but are uncommon (Moran. 2006)). Linezolid may offer an alternative to intravenous vancomycin. Recent studies have shown that the adverse event rate of linezolid is not significantly different than that for vancomycin and that linezolid is an effective agent for SSTIs including those caused by MRSA. Daptomycin has been used effectively in cases of complicated SSTI and for treatment of CA-MRSA bacteremia. Quinupristin/dalfopristin is active against MRSA, but is rarely used due to an adverse-effect profile and potential cross-resistance with clindamycin-resistant strains. Tigecycline is active against MRSA and it has FDA approval for the treatment of skin and soft tissue infections (Rybak. 2005; Moran.2006).
Access to oral linezolid may be limited due to formulary restrictions and other cost related issues. Some infectious disease specialists save linezolid for use in infections due to organisms resistant to other agents. This conservative approach is supported by reports of the emergence of linezolid-resistant organisms in healthcare settings (Kelly, 2006). Topical, rather than oral, antibiotics can be used to treat superficial lesions. For instance, topical mupirocin TID for ~7 days has been utilized for treatment of limited impetigo (Stevens, 2005; Swartz 2005). Of note, resistance to mupirocin may develop, but this usually occurs in the setting of prolonged usage. For multiple or larger CA-MRSA lesions, oral antibiotics are recommended.
These drugs generally have activity against CA-MRSA:
1. Vancomycin (Vancocyn®) 15 mg/kg IV q12 hours
2. Daptomycin (Cubicin®) 4 mg/kg IV daily (higher dosages are used for bacteremia/endocarditis) Intravenous or Oral Antibiotics
3. Linezolid (Zyvox®) 600 mg IV or PO twice daily
4. Clindamycin (Cleocin®) 900 mg IV q8 hours or 300–450 mg PO QID 5. Oral Antibiotics
• Tetracyclines
• Doxycycline (Vibramycin®) 100 mg PO twice daily • Minocycline (Minocin®) 100 mg PO twice daily
• Trimethoprim-sulfamethoxazole (Bactrim®, Septra®) 1 double-strength with 160 TMP/800 SMX tablet twice daily
6. Rifampin (Rifadin®) 300 mg PO twice daily 7. Topical Antibiotics
• Topical mupirocin (Bactroban®) apply to each nares twice daily
• Chlorhexidine body soaps, shower with soap daily (used for decolonization purposes, not treatment)
* Dosages listed assume normal kidney and liver function; for patients with abnormal values, drug dosage adjustments may be needed. Some antibiotics listed are not recommended in children or during pregnancy.
1.5. MRSA Treatment in the Setting of Highly Active Antiretroviral Therapy (HAART)
warned to watch for and report any signs or symptoms suggestive of the serotonin syndrome which include hyperthermia, agitation, tremors, myoclonus, altered mental status, and/or diaphoresis (Rybak, 2006).
Drug-drug interactions limit the co-administration of rifampin with several antiretroviral agents including protease inhibitors and non-nucleoside reverse transcriptase inhibitors. Rifampin is a powerful inducer of the cytochrome P450 enzyme system, leading to a decrease in antiretroviral plasma levels below the inhibitory concentration of 50% (IC50) of the latter and potentially leading to viral rebound. Rifampin should be avoided in patients receiving protease inhibitors and an alternate antibiotic, such as clindamycin, linezolid or tetracycline should be selected.
2. Extende Spectrum β-Lactamase (ESBL)
There is no consensus of the precise definition of ESBLs. β-lactamases are bacterial enzymes that ineffective β-lactam antibiotics by hydrolysis, which results in ineffective compounds. One group of β-lactamases, extended-spectrum β-lactamases (ESBLs) have the ability to hydrolyse and cause resistance to various types of the newer β-lactam antibiotics, including the expanded-spectrum (or third generation) cephalosporins (eg, cefotaxime, ceftriaxone, ceftazidime) and monobactams (eg, aztreonam), but not the cephamycins (eg, cefoxitin and cefotetan) and carbapenem (eg, imipenem, meropenem and ertapenem). ESBL has generally been defined as transmissible β- lactamases that can be inhibited by clavulanic acid, tazobactam or sulbactam, and or which are encoded by genes that can be exchanged between bacteria. Most ESBLs can be devided into three groups: TEM, SVH and CTX-M types. The currently most common genetic variant of ESBL is CTX-M.( Paterson DL et al. 2005)
2.1. Mechanisms of Transmission
A review of the literature on mechanism of transmission of MDR-GNB was problematic for three main reasons; the low number of studies; the low availability of high quality studies and the high heterogeneity of definitions, settings and pathogens. Patient-to patient transmission was frequently thought to be the most important route of transmission whenever several patients shared clonally related isolates. This is based on the hypothesis that colonized or infected patients are the only reservoir for the microorganism. However, intermediate vectors for spread between patients, including contaminated hands of healthcare workers (HCWs), environment, and visitors should also be taken into consideration for the prevention and control of healthcare-associated MDR-GNB transmission.
2.2. Extended Spectrum β- lactamase Escherichia coli (ESBL E-coli)
feature of these strains is that they carry specific genes that enable them to produce enzymes that destroy a large number of common antibiotics, making the infection they cause very difficult to treat. In many instances, only two oral and a very limited group of intravenous antibiotics remain effective. ESBL-producing strains E coli were first noted in 2003 when East and West Midlands region of England reported.
E. coli as the constant influx of community isolates colonizing patients at hospital admission is highly significant in the epidemiology of these organisms within hospitals, understanding the complex epidemiological behavior of E. coli in the community is a key to adequate interpretation of studies addressing the epidemiology of E. coli in hospitalized patients. The extra-intestinal pathogenic strain are the predominant strains in 20% of individual and harbor the typical virulence factors causing extra-intestinal infections when reaching the appropriate site from the bowel, which serve as their primary reservoir. Transmission of extra-intestinal pathogenic E. coli in the community is thought to occur by person to person transmission, either through direct contact or by means of faecal-oral route through or by contaminated food and/or water. (Johnson JR et al. 2010)
2.3. Klebsiella species
There have been several rescent studies of the epidemiology of K. pneumonia as a nosocomial pathogen. Cross-transmission via HCWs‟hands seems to be important in the nosocomial spread of K pneumonia strains. However in a recent study, an outbreak caused by contaminated food was described, indicating that transmission may also occur via the food chain.
2.4. Detection
The clinical laboratory acts as an early warning system, alerting the medical community to new resistance mechanism present in clinically important bacteria. The methods for detection of ESBLs can be broadly devided into two groups: phenotypic methods that use non-molecular techniques, which detect the ability of the ESBL enzymes to hydrolyse different cephalosporins and genotypic method, which use molecular techniques to detect the gene responsible for the production of the ESBL. Clinical diagnostic laboratories use mostly phenotypic methods because these tests are easy to do, are cost effective and have been incorporated in most automated susceptibility systems, making them widely accessible.
2.5. Treatment
when selecting an empirical regimen is to choose an agent that has adequate activity against the infecting organism(s). Empirical antibiotic choices should be individualized based on institutional antibiograms, which tend to be quite different from hospital to hospital, from city to city, and from country to country. ( Pitout J.D., et al. 2008)
The next issue surrounding the therapy of ESBL-producing infections is that even if an agent is selected that has activity against the bacteria in vitro, clinical efficacy in patients is not always guaranteed. Although in vitro tests ESBLs are inhibited by ß-lactamase inhibitors such as clavulanic acid, other study has shown the activity of ß-lactam/ß-lactamase inhibitor combination agent (e.g. piperacillin-tazobactam) is influenced by the bacterial inoculums, dose administration regimen and specific type of ESBL present. The other consideration treatment of ESBL-producing bacteria are cephamycins (e.g. cefoxitin, cefotetan) and Cefepime. This is widely believe to occur as a result of the so-called inoculums effect that occurs when the minimum inhibitory concentration of the antibiotic rises (i.e. the antibiotic looses activity) with the increasing size of the inoculums (or number) of bacteria tested. This effect has been described for cephalosporins, β-lactam-β lactamase inhibitor combinations (piperacillin, tazobactam) and to a lesser extent with the quinolones. Tigecycline is also one of the drugs in the pipeline which can be considered for treatment. (Pitout J.D., et al. 2008; Perez et al. 2007)
The carbapenems ( imipenem, meropenem, ertapenem, doripenem) are still the first choice of treatment for serious infections with ESBL-producing E coli and K pneumonia. It has been reported that >98% of the ESBL-producing E coli, K pneumonia and P. mirabilis are still susceptible to these drugs. This agents are highly stable to hydrolysis by ESBLs, are distributed into body tissues in high concentration and there is no inoculums effect. (Perez et al, 2007; Pitout J.D., et al. 2008). But with the emergence of the carbapenem – resistant Enterobateriaceae, the “magic bullet” is actually difficult to find. There are some older drugs which can be used to treat the ESBL-producing E. coli or K. pneumonia infections. Fosfomycin was reported of having admirable in vitro activity against the ESBL-producing E. coli or K. pneumonia. In HongKong, most of the ESBL-ESBL-producing E coli isolates were reported to be sensitive to fosfomycin. ( Ho et al., 2010). Colistin is another choice which we can consider for the treatment of these organisms. Although once considered as quite a toxic antibiotic, it is a last resort that we can consider at the present moment as there is no new anti gram negative antibiotics available for the treatment of these multidrug resistant organisms. ( Perz et al. 2007)
REFERENCES
Alekshun,MN., Levy, SB., 2007. Molecular mechanisms of antibacterial multidrug resistance. Cell 128. 1027-1050
Chong Y, Yakushiji H, Ito Y, Kamimura T. Clinical and molecular epidemiology of Extended-spectrum ß-lactamase-producingg Escherechia coli and Klebsiella pneumonia in a long-term study from Japan. Eur J Clin Microbiol Infect Dis 2011;30 :83-7
Falagas ME, Karageorgopoulos DE. Extended-spectrum ß-lactamase-producing organisms. Journal of Hospital Infection 2009;73:345-354
Gayatri Y, Merati TP. 2013. Clinical Features and Antibiotics Resistant Pattern of MRSA Infection in Sanglah Hospital. Free oral presentation in Petri XIX Aceh
Ho, P.L., Yip, K.S., Chow,K.H., Lo, J.Y., Que, T.L., Yuen, K.Y., 2010. Antimicrobial resistance among uropathogens that cause acute uncomplicated cystitis in womaen in HongKong: a prospective multicenter study in 2006 to 2008. Diagn. Microbiol. Infect, Dis. 66, 87-93
Johnson, J.R., Menard, M. Johnston, B., Kuskowski, M.A., Castanheira, M. 2008. Escherichia coli sequence type ST 131 as the major cause of serious multidrug-resisntant E.coli infections in the United States. Clin Infect Dis;51:286-294
Kelly S., Collins J., David M., Gowing C., Murphy PG. 2006. Linezolid resistance in coagulase negative staphylococci. J Antimicrob Chemother 58: 898-899
Moran GJ., Krishnadasan A., Gorwithz RJ et al. 2006. EMERGEncy ID Net Study Group. Methicillin-resistant S aureus Infection among patients in the emergency department; 355: 666-674
Paterson DL., Bonomo RA. Extended-spectrum ß-lactamase: a clinical update. Clin Microbiol Rev 2005, 18: 657-86
Perez, F., Endimiani, A., Hujer, K.M., Bonomo, R.A., 2007. The continuing challenge of ESBLs. Curr. OPin. Pharmacol. 7, 459-469
Pitout JD, Loupland KB. 2008. Extended-Spectrum ß-lactamase-producing Enterobacteriaceae :an emerging public-health concern.Lancet Infect Dis ;8:159-66
Rybak MJ., LaPlente KL. 2005. Community acquired methicillin resistant Staphylococcus aureus: a review. Pharmacotherapy; 25: 74-85
Diagnosis and Treatment of Dengue Infection
Dewi Dian Sukmawati
Division of Tropical and Infectious Disease Department of Internal Medicine
Udayana University School of Medicine-Sanglah Hospital Denpasar
Dengue infection, a mosquito – borne flavivirus, is caused by dengue virus transmitted mainly by vector Aedes aegypti and Aedes albopictus, other species (Aedes polynesiensis and Aedes scutellaris) play the role of less common vector. There are five serotypes, DEN-1, 2, 3, 4 and 5. Each episode of infection induces a life-long protective immunity to the homologous serotype which confers partial and transient protection against subsequent infection by the other serotypes. Secondary infection (by another serotype) is a major risk factor for DHF, mainly due to antibody induced enhancement. Epidemiologic studies have identified young age, female sex, high body-mass index, virus strain or virulence and genetics of the human host e.g. major histocompatibility complex class I related sequence B and phospholipase C epsilon 1 genes as risk factors for severe dengue. All five serotypes may be circulating in the population at any one time but from the experience in the south-east Asia it appears that the predominant circulating dengue virus will show a sinusoidal pattern – with a peak to peak interval of 7 – 9 years. It is likely that this interval allows a buildup of immune – naïve population of children.1,2,3,4
1. Course of Dengue Infection
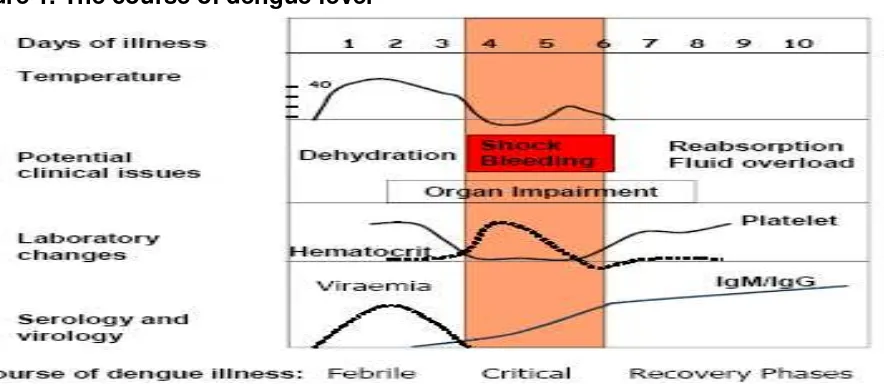
Figure 1. The course of dengue fever
IgM = immunoglobulin M; IgG = immunoglobulin G. Temperature is given in degrees Celsius (°C)
Source: adapted from Yip, 1980 (6)
2. Dengue Case Classification
The recent WHO guidelines7 use three categories for case management namely A, B and C based on case classification that follows after the patient fulfilled the criteria for probable dengue (Figure 2).
Figure 2. Dengue case classification by severity
3. Dengue Diagnostics
In areas of the world where dengue disease is endemic, including Indonesia, all the cases of acute febrile illness, presenting with nonspecific features, would have dengue viral infection included in the list of differential diagnosis. The steps in assessing a case including prompt anamnesis, physical examination, laboratory and imaging studies. Laboratory investigations can be classified as disease monitoring laboratory test or diagnostics tests.7 – 10
3.1. Disease monitoring laboratory tests
Complete Blood Count (CBC).White blood count in the beginning febrile phase usually normal, and rapidly decrease as the disease progress and shows relative lymphocytosis. The presence of non specific leucopenia should raise suspicion of possible dengue infection in appropriate setting. The rise of Hematocrit best known as Hemoconcentration as a result of plasma leakage, differentiate between dengue fever and dengue hemorrhagic fever. Early fluid replacement or blood loss due to bleeding manifestation may mask the Hemoconcentration. It is off a great importance to obtain a baseline Hematocrit in early febrile phase so it can be used to detect early recognition of plasma leakage. Thrombocytopenia might be the most common and prominent laboratory feature in dengue cases. In the early febrile phase, platelet count is usually within normal range and will decrease rapidly as the disease progresses to the late febrile phase or at defervescence. The thrombocyte may continue to remain low for the first few days of recovery phase. There is a significant negative correlation between disease severity and platelet count but it is not predictive of bleeding.
Liver profile evaluation. Abnormal transaminates is commonly seen in dengue infection. The elevation of AST usually greater compared to AST. The degree of transaminates usually higher in DHF compared to DF.
3.2. Diagnostic tests
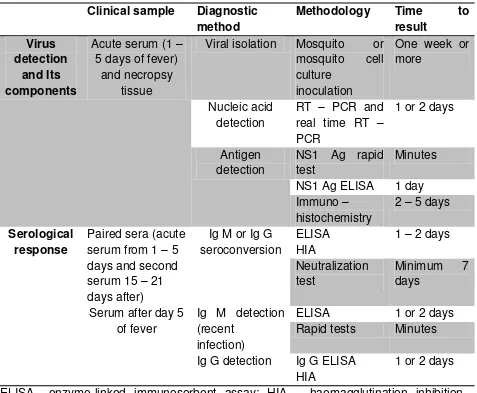
Table 1. Dengue diagnostic and sample characteristics
ELISA= enzyme-linked immunosorbent assay; HIA = haemagglutination inhibition assay; IgG = immunoglobulin G; IgM = immunoglobulin M; NS1 Ag = Non - structural protein 1 antigen; RT – PCR = reverse transcriptase polymerase chain reaction. Source: WHO – TDR 2012 (10)
Three main aspects should be considered for an adequate dengue diagnosis: • Virological and serological markers in relation to the time of dengue infection; • Type of diagnostic method in relation to clinical illness;
• Characteristics of the clinical samples
A. Virological and serological markers in relation to the time of dengue infection
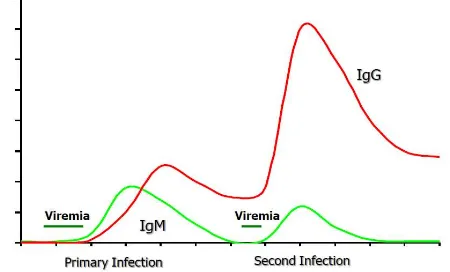
increasing but low levels of dengue-specific IgG, becoming elevated at days 9 – 10. Low IgG levels persist for decades, an indication of a past dengue infection. A totally different picture is observed during a secondary infection, with a rapid and higher increase of anti-dengue specific IgG antibodies and slower and lower levels of IgM. High IgG levels remain for 30 – 40 days. A short-lasting but higher viremia level characterizes the secondary infection compared to the primary infection (Figure 3).
Figure 3. Virological and serological markers of dengue infection according to time of illness
; IgG = immunoglobulin G; IgM = immunoglobulin M
B. Type of diagnostic method in relation to clinical illness
The diagnostic method to confirm an acute infection depends on the time of clinical illness: the febrile phase is coincident with the presence of viremia, some viral components and replication products in blood; the critical and convalescent phases coincide with the development of antibodies, as summarized in Table 1.
Febrile phase (day 1 to day 4 – 5 of fever), diagnostic test best using isolation of infective virus thus allow detection of serotype of dengue virus. Virus genome detection using RT – PCR confirms the diagnosis during this phase. NS1 is a marker of acute dengue infection. Critical and convalescent phase (after day 4 – 5 of fever), specific IgM is the best marker for recent dengue infection. High levels of IgG collected early after fever onset also suggested a recent infection
C. Characteristics of the clinical samples
The pitfalls in diagnostic test for dengue infection
Serological test for dengue have been known to cross – react with: other flaviviridae (Japanese Encephalitis), Non – Flavivirides infections (malaria, leptospirosis, toxoplasmosis, syphilis), connective tissue disease (rheumatoid arthritis).11,12,13
Secretion of the NS1 protein is a hallmark of flavivirus infection on mammalian cells and can be found in dengue infection as well as in yellow fever and West Nilevirus infection. This antigen is present in high concentrations in the serum during early phase of the disease. The detection rate is much better in acute sera of primary infection (75%- 97.3%) compared to the acute sera of secondary infection (60% 70%). The sensitivity of NS1 antigen detection starts to drop off from the day 4-5 of illness and is usually undetectable in the convalescence phase.
Laboratory confirmation of a dengue case
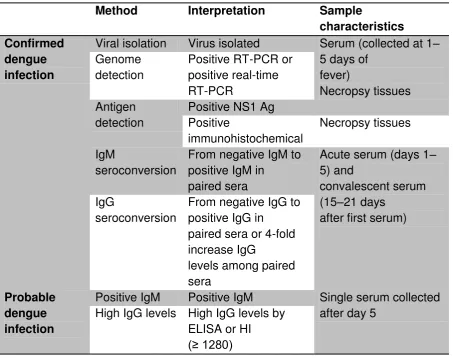
A diagnosis of dengue infection is confirmed by the detection of the virus, the viral genome or NS1 Ag, or seroconversion of IgM or IgG (from negative to positive IgM/IgG or four-fold increase in the specific antibody titre) in paired sera (Table 2).
Table 2. Confirmed and probable dengue diagnosis, interpretation of results and sample characteristics
Viral isolation Virus isolated Serum (collected at 1– 5 days of
Positive IgM Positive IgM Single serum collected after day 5
ELISA= enzyme-linked immunosorbent assay; HIA = haemagglutination inhibition assay; IgG = immunoglobulin G; IgM = immunoglobulin M; NS1 Ag = Non - structural protein 1 antigen; RT – PCR = reverse transcriptase polymerase chain reaction. Source: WHO – TDR 2012 (10)
4. Recommendation for Clinical Management
There is no currently available anti viral medication against the dengue virus after the host invasion. The of dengue infection management stays symptomatic and supportive. Dengue is a dynamic disease and management issues vary according to the three phases of the clinical course. It is crucial to recognize plasma leakage and shock at an early stage, to guard against severe organ impairment. This can only be achieved through frequent clinical and laboratory monitoring.
4.1 Clinical Management
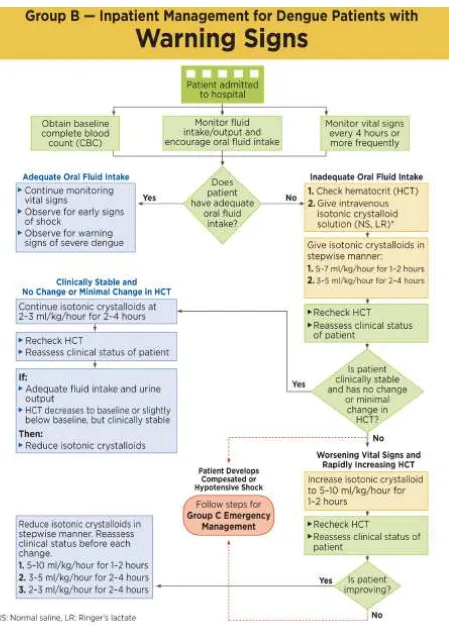
The stepwise approach for management of dengue can be used to assessing dengue case investigation (Table 3). Figure 4, Figure 5, Figure 6, Figure 7 and and Figure 6 show the summary of management decision.
Table 3. A stepwise approach to the management of dengue
Step I – Overall assessment
I.1 History, including symptoms, family history and past medical history
I.2 Physical examination, including full physical and mental asessement
I.3 Investigation, including routine laboratory tests and dengue-specific laboratory tests
Step II − Diagnosis, assessment of disease phase and severity
Based on evaluations from history, physical examination +/- CBC and HCT, the clinicians should be able to determine:
1. Diagnosis of Dengue Fever (suspected, probable or confirmed) 2. The phase of illness (febrile/critical/convalescent or recovery) 3. The hydration and hemodynamic status of patient
4. Whether the patient requires admission
Step III – Management III.1 Disease notification
III.2 Management decisions. Depending on the clinical manifestations and other circumstances, patients may:
- be sent home (Group A)
- be referred for in-hospital management (Group B)
4.2 When to stop intravenous fluid therapy
Recognizing when to decrease or stop intravenous fluids as part of the treatment of severe
dengue is crucial to prevent fluid overload. When any of the following signs are present, intravenous fluids should be reduced or discontinued:
• signs of cessation of plasma leakage; • stable BP, pulse and peripheral perfusion;
• haematocrit decreases in the presence of a good pulse volume; • apyrexia (without the use of antipyretics) for more than 24–48 hours; • resolving bowel/abdominal symptoms;
• improving urine output.
Continuing intravenous fluid therapy beyond the 48 hours of the critical phase will put the patient at risk of pulmonary edema and other complications such as thrombophlebitis.
4.3. Management of hemorrhagic complication
Mucosal bleeding may occur in any patient with dengue but if the patient remains stable with fluid resuscitation/replacement, this should be considered as a minor issue. The bleeding usually improves rapidly during the recovery phase. In patients with profound thrombocytopenia, ensure strict bed rest and protection from trauma. No evidence exists that prophylactic platelet transfusions are beneficial in haemodynamically stable patients. If major bleeding occurs it is usually from the gastrointestinal tract, and/or hypermenorrhoea. Internal bleeding may not become apparent for many hours until the first black stool is passed.
Patients at risk of severe bleeding are those who: • have profound/prolonged/refractory shock;
• have hypotensive shock and multi-organ failure or severe and persistent metabolic acidosis;
• are given non-steroidal anti-inflammatory agents; • have pre-existing peptic ulcer disease;
• are on anticoagulant therapy;
• have any form of trauma, including intramuscular injection.
Severe bleeding should be recognized in the following situations:
• persistent and/or severe overt bleeding in the presence of unstable haemodynamic status, regardless of the haematocrit level;
• a decrease in haematocrit after boluses of fluid resuscitation together with unstable haemodynamic status;
• refractory shock that fails to respond to consecutive fluid resuscitation of 40– 60 ml/kg;
• hypotensive shock with inappropriately low/normal haematocrit;
• persistent or worsening metabolic acidosis in patients with a well-maintained systolic BP, especially in those with severe abdominal tenderness and distension. Blood transfusion is life-saving and should be given as soon as severe bleeding is suspected or recognized. However, blood transfusion must be given with care because of the risk of fluid overload. Do not wait for the haematocrit to drop too low before deciding on blood transfusion. Note that for the reasons stated above a haematocrit of < 30% as a trigger for blood transfusion, as recommended in the Surviving Sepsis Campaign Guideline, is not applicable to dengue.
The action plan for the treatment of haemorrhagic complications is as follows:
• If possible, attempts should be made to stop bleeding if the source of bleeding is identified e.g. severe epistaxis may be controlled by nasal adrenaline packing. • If blood loss can be quantified, this should be replaced. If not, give aliquots of 5−10
ml/kg of fresh -packed red cells or 10−20 ml/kg of fresh or fairly fresh whole blood (FWB) at an appropriate rate and observe the clinical response. It is important that fresh whole blood or fresh red cells are given. Oxygen delivery at tissue level is optimal with high levels of 2,3 diphosphoglycerate (2,3 DPG). Stored erythrocytes lose 2,3 DPG, low levels of which impede the oxygen-releasing capacity of haemoglobin, resulting in functional tissue hypoxia. A good clinical response includes improving haemodynamic status and acid-base balance.
• Consider repeating the blood transfusion if there is further overt blood loss or no appropriate rise in haematocrit after blood transfusion in an unstable patient.
• There is no evidence that supports the practice of transfusing platelet concentrates and/or fresh-frozen plasma for severe bleeding in dengue. Observational studies show that transfusions of platelet concentrates and fresh frozen plasma in dengue were not able to sustain the platelet counts and coagulation profile. However, in the case of massive bleeding, they often exacerbate the fluid overload.
• Nevertheless, in certain situations such as obstetrical deliveries or other surgeries, transfusions of platelet concentrates with or without fresh frozen plasma should be considered in anticipation of severe bleeding.
• In gastrointestinal bleeding, H-2 antagonist and proton pump inhibitors have been used, but their efficacy have not been studied.
the trauma during insertion. Insertion of central venous catheters should be done with ultra-sound guidance or by an experienced person.
• It is essential to remember that blood transfusion is only indicated in dengue patients with severe bleeding. Unnecessary blood transfusions cause the haematocrit to rise sharply, thus giving a false impression of haemoconcentration and severe plasma leakage leading to unwarranted fluid therapy.
4.4. Discharge criteria
Discharge criteria for patients admitted with dengue fever as follow: No fever for 48 hours, improvement in clinical pictures, increasing trends of platelets count, stable Hematocrit without intravenous fluid and without respiratory distress.
5. Dengue in special condition
5.1. Dengue in elderly
Clinical manifestations of dengue in the elderly are similar to those of younger adults. However,rash, hepatomegaly and mucocutaneous haemorrhage are less frequent but gastrointestinal tract bleeding and microhaematuria are more common. The elderly have significantly lower incidences of fever (10% without fever), abdominal pain, bone pain and rashes but higher frequencies of concurrent bacteraemia, gastrointestinal bleeding, acute renal failure, and pleural effusion, higher incidence of prolonged prothrombin time and lower mean haemoglobin levels than younger adult patients. The elderly has higher risk of severe dengue and case fatality rate.
5.2. Dengue with pre existing hypertension
Challenges when managing patients with pre-existing hypertension:
Interpretation of BP
Hypotension is a late sign of shock. However, in patients with uncontrolled hypertension a BP reading that is
considered normal for age may, in reality, be low for patients with uncontrolled hypertension. Similarly, what is considered
as “mild” hypotension may in fact be profound. Patients with
chronic hypertension should be considered to be
hypotensive when the mean arterial pressure (MAP) declines by 40 mmHg from the baseline, even if it still exceeds 60 mmHg. (For example, if the
baseline MAP is 110 mmHg, a MAP reading of 65 mmHg should be considered as significant hypotension.) Look for other manifestations of shock
The heart rate response
It is essential to know the specific antihypertensive agent a patient is taking for the following reasons.
in patients on ß-blockers.
•Tachycardia: Antihypertensive agents such as calcium channel blockers may cause tachycardia. Tachycardia in these patients may not
indicate hypovolemia. Knowing the baseline heart rate before the dengue illness is helpful in the haemodynamic assessment.
The impact on hypotension:
The continuation of antihypertensive agents during the acute dengue illness should be evaluated carefully during the plasma leaking phase. The BP lowering effects of these agents and diuretic therapy may exacerbate the hypotension and
hypoperfusion of intravascular volume depletion
End-organ damage from chronic
hypertension:
Heart failure and renal failure are common complications of chronic uncontrolled hypertension. Clinicians should be aware if there is pre-existing or new onset of endorgan damage.
Interpretation of urine output as a marker of renal perfusion has to be revoked in these situations
5.3. Dengue with pre – existing Diabetes Mellitus
Hyperglycaemia
Just like other acute infections, dengue can precipitate diabetic ketoacidosis or hyperosmolar hyperglycaemia, the two major acute metabolic complications in diabetics.
•Osmotic diuresis
Hyperglycaemia results in osmotic diuresis and worsens intravascular
hypovolaemia. Not correcting the hyperglycaemic state exacerbates the shock state
•Increased risk of
concomitant sepsis:
Hyperglycaemia also puts patients at risk of bacterial infection
•DKA & HHS
Clinical manifestations of diabetic ketoacidosis and hyperosmolar hyperglycaemia (nausea, vomiting and abdominal pain) are
similar to the warning signs of severe dengue. It is not uncommon for dengue shock to be misdiagnosed as diabetic ketoacidosis.
Hypoglycaemia: Hypoglycaemia may occur in those patients taking oral
hypoglycaemic agents (e.g. long-acting sulphonylurea), but who had poor oral intake. Hypoglycaemia could be aggravated by severe hepatitis from dengue
Oral
hypoglycaemic agents
5.4. Dengue with chronic renal failure
Morbidity & mortality risk
Dengue patients with chronic renal failure (CRF) have a significantly higher risk of severe
dengue and mortality. The outcome correlates with the renal function
The warning signs of severe dengue are similar to those of uraemia in CRF. Ascites and/or pleural effusion, and signs of plasma leakage in dengue, are not uncommon findings in patients with CRF and fluid retention. The ambiguity of these symptoms and signs could delay the recognition of plasma leakage and severe dengue.
Hematocrit & platelet
Low baseline haematocrit and platelet count. This could be another challenge in the early recognition of dengue infection. Platelet dysfunction, well recognized in CRF together with severe thrombocytopenia ± coagulopathy, predispose the dengue patient to severe bleeding that may be difficult to control
Challenge in fluid management Narrow window
of fluid
tolerance
Patients with CRF have limited fluid tolerance. Frequent assessments of the haemodynamic state and frequent fluid regime adjustments are mandatory to avoid fluid overload or under-fill. Adequate fluid replacement is necessary to prevent worsening of renal function during the critical phase
Urine output The urine output should not be used as an indicator of the
intravascular volume status because patients with CRF can have either low or high urine-output renal failure. Low urine output in CRF contributes to the risk of fluid overload whereas high urine output may aggravate hypovolaemia.
Limited effect of diuretics
Diuretics have a limited effect in CRF, making patients more susceptible to fluid overload. Dialysis may be required Acid base &
electrolyte balance
Patients with CRF are at risk of metabolic acidosis and electrolyte imbalance which will become worse during dengue shock. If these persist after adequate fluid replacement, dialysis may be considered after haemodynamic stability is achieved
5.5. Dengue with chronic heart disease with or without heart failure