www.elsevier.com / locate / bres
Research report
Chronic stress regulates levels of mRNA transcripts encoding
b
subunits of the GABA receptor in the rat stress axis
Aa,b ,
*
aWilliam E. Cullinan
, Thomas J. Wolfe
a
Department of Biomedical Sciences, Marquette University, P.O. Box 1881, Milwaukee, WI 53201-1881, USA b
Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI 53226, USA Accepted 19 September 2000
Abstract
Semi-quantitative hybridization histochemical analyses were undertaken to determine expression levels of mRNA transcripts encoding the b1–3 subunits of the GABA receptor within the rat hypothalamic paraventricular nucleus (PVN) and hippocampal formationA following exposure to a chronic non-habituating stress protocol. After delivery of a battery of stressors on a randomized schedule over a 3-week period, expression levels of theb1 subunit of the GABA receptor were found to be decreased in the medial parvocellular PVNA (mpPVN) by 48.3% relative to control animals. Levels ofb2 mRNA following chronic stress were also found to be decreased in the mpPVN (29.8%), but increased in hippocampal subfields CA and CA (33.9 and 23.2%, respectively) and increased (24%) in the1 3 dentate gyrus. The results suggest that GABAA receptor subunit composition may be altered at a key regulatory site, and may have important implications for studies aimed at understanding GABAergic inhibitory influences upon the hypothalamic–pituitary–adreno-cortical (HPA) axis. Hypophysiotropic CRH neurons serve as the origin of the final common pathway for glucocorticoid secretion in response to stressful stimuli, and GABAergic afferents have been implicated in afferent control of these neurons. Regulation of GABAA receptors at these sites may alter the efficacy of a major inhibitory influence upon the stress axis, and thereby modulate stress-induced glucocorticoid secretion. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Hypothalamic–pituitary–adrenal axis
Keywords: GABA receptor subunit; Chronic stress; Hypothalamic paraventricular nucleus; Hippocampus; RatA
1. Introduction broad effects throughout the body, including immune,
metabolic and inflammatory actions, as well as profound The paraventricular nucleus of the hypothalamus (PVN) effects upon mood and behavior.
serves as the major integration site in the regulatory While the hypophysiotropic CRH neurons are known to control of the hypothalamic–pituitary–adrenocortical be under complex afferent regulatory control, an important (HPA) or stress axis. Stress-related inputs converge upon neurotransmitter in this regulation is the inhibitory amino the neurons of the medial parvocellular division of the acid GABA. Morphological evidence has indicated a PVN (mpPVN); cells within this region synthesize cor- prominent GABAergic innervation of the PVN, with ticotropin-releasing hormone (CRH), arginine vasopressin nearly half of all synapses within the mpPVN found to be (AVP) and other secretagogues, project to the median GABAergic [10]. These findings are consistent with phar-eminence, and release these substances into the portal macological studies performed both in vitro and in vivo vasculature, initiating a cascade of events resulting in the which have implicated GABAergic mechanisms in the adrenocorticotropin (ACTH)-mediated release of glucocor- inhibitory control of CRH secretion [3,19,26], as well as ticoids from the adrenal glands. Glucocorticoids have the finding that the GABAA pharmacological agents de-livered at the level of the PVN affect the corticosterone response to acute stress [5].
*Corresponding author. Tel.: 11-414-288-6764; fax: 1
1-414-288-The potential importance of GABAergic afferents in the
6564.
E-mail address: [email protected] (W.E. Cullinan). regulatory control of the HPA axis has prompted molecular
analyses of the subunit composition of this receptor with Control animals were handled for 1 min each time the respect to known stress circuitries. The GABA receptor isA experimental animals were subjected to stress.
formed by the assembly of five proteins from at least three On the day following the last stressor (day 22), animals different subunits; genes encoding such proteins have been were rapidly decapitated, with brains removed and frozen cloned and grouped into distinct classes (a, b, g, d, r) in isopentane at 2508C, and then stored at 2708C until which exist in multiple forms. The functional receptor is further processing. Sections (14 mm) were cut on a thought to be comprised of 2a, 2b, and a subunit from Reichert cryostat in the coronal plane, thaw-mounted onto either theg,d, orrclasses, and evidence has indicated that poly-L-lysine subbed slides, and then stored at2708C until
different combinations of subunits confer distinct kinetic processed for hybridization histochemistry. All experi-and pharmacological properties [12,14,21,22,27–29,31]. ments were performed in accordance with an approved For example, data advanced from transfection studies have institutional animal care protocol conforming to NIH and indicated that alterations in the subunit composition of the AALAC guidelines.
GABAA receptor can have influences as dramatic as the
production of non-functional channels [31]. Recent work 2.2. In situ hybridization has begun to elucidate the subunit composition of GABAA
receptors expressed in the mpPVN. Theb subunit class is Riboprobes complementary to mRNA transcripts encod-of particular interest, as it is thought to contain the GABA ing the b1–3 subunits of the GABAA receptor were binding site;b1–3 subunits have recently been confirmed synthesized according to standard in vitro transcription to be expressed within hypophysiotropic CRH neurons [4]. methodology. Briefly, cRNA probes were generated in a In the present study, we sought to determine whether the reaction (total volume510 ml) containing 165 mCi of
35
GABAA receptor subunits b1–3 are regulated within the [ S]UTP (NEN), 1 ml each of 10 mM stocks of ATP, PVN, as well as within the hippocampal formation, a brain CTP, and GTP, 2 ml 5X transcription buffer (Epicentre structure prominently implicated in the regulatory control Technologies), 1 ml 0.1 M dithiothreitol, 1 ml linearized of the HPA axis. plasmid DNA (1 mg /ml), 1 ml dH O, 0.52 ml of RNase inhibitor (40 U /ml) and 1.5 ml of appropriate RNA polymerase (T7 or SP6; 25 U /ml). The reaction was allowed to proceed for 2 h, followed by separation of
2. Materials and methods labeled probe from reaction contents on a G50-sephadex
column. The GABA -receptor cDNAs were kindly pro-A
2.1. Animal treatments vided by Dr. A. Tobin (UCLA), and were subcloned and linearized to transcribe cRNA probes of the following Male Sprague–Dawley rats (Harlan Sprague–Dawley) sizes: b15668 nts;b25550 nts;b35750 nts.
weighing between 300 and 350 g were used in this study. Sections were removed from storage at 2708C and Animals were housed in pairs and placed on a 12-h placed in a 4% buffered paraformaldehyde solution for 1 h. light / dark cycle with access to food and water ad libitum Following rinsing in 23SSC (pH 7.4), slides were treated throughout the duration of the study. Animals were divided with proteinase K (0.2 mg / ml) for 8 min at 378C, rinsed into two groups (n55–6); the chronic stress group was 33in dH O, and placed in 0.1 M triethanolamine (pH 8.0)
2
subjected to intermittent stress twice daily on a randomized containing 0.25% acetic anhydride for 10 min at RT. schedule for period of 21 days, using the following battery Sections were then rinsed in 23SSC, and dehydrated in a of stressors. successive series of alcohols. For single (isotopic)
hybridi-35
zation, S-labeled probes were diluted in hybridization buffer (45% formamide; Amresco, Solon, OH) for a final
6
1. Cold exposure: animals were exposed to 408C for 2 h concentration of 1.5–2.0310 dpm per 35 ml. Tissue in home cages. sections were apposed to coverslips containing probe, and 2. Swim: rats were forced to swim in 358C water for stored in sealed, moistened chambers overnight at 558C. 20–30 min. The following day, coverslips were removed and rinsed 3. Vibration / rotation: rats were placed on a platform or extensively in 23 SSC, and treated with 200 mg / ml of orbital shaker for 1 h. RNase A in Tris buffer (pH 8.0) at 378C for 30 min. This 4. Restraint: animals were placed in plastic restraint was followed by several rinses in decreasing concen-holders for 30–60 min. trations of SSC (23, 13, 0.53, 0.13), and washing at 5. Cold swim: animals were forced to swim in 15–188C 658C in 0.13 SSC for 1 h. Sections containing were then water for 10 min. dehydrated in graded alcohols and exposed to Kodak 6. Isolation: animals were housed individually overnight. BioMax X-ray film for 24–48 h before development. All 7. Crowding: animals were placed six per cage overnight. sections for in situ hybridization histochemistry for a given 8. Ether exposure: animals were exposed to ether vapors probe (b1–3) were run in the same experiment.
were performed for all probes in preliminary hybridization studies.
2.3. Data analysis
Neuroanatomical subdivisions were made according to the atlas of Paxinos and Watson [24]. Semiquantitative analyses of hybridization films were performed on digit-ized images in the linear range of gray values obtained from an acquisition system (Fotodyne Variquest 100 light-box, Pulnix CCD camera model TM-745 connected via a Pulnix CCU-84 converter to a Power Macintosh 8500, captured with NIH Image v. 1.59 software). Signal pixels of the area of interest were defined as 3.5 standard deviations above the mean of a nearby cell-poor area; the number of pixels and average pixel values above the set background were then computed for each region, yielding an integrated densitometric measure. An average of 10–20 measurements per region were made from different sec-tions (five to 10), with values averaged to provide a single integrated density value / region for each animal. Slides undergoing hybridization, or in some cases an adjacent series of sections, were Nissl stained (cresyl violet) and used to aid in the determination of nuclear boundaries on digitized images, particularly within the PVN, where the division between parvocellular and magnocellular ter-ritories is readily discernible from Nissl-stained material. It should also be noted with respect to analysis of the hippocampal formation that (1) CA3 measurements were taken from the regio inferior and did not include portions of the transitional angle between superior and inferior regions (i.e., the generally heavily labeled more superior part of CA3, and (2) all measurements from the dentate gyrus were made from the inferior blade. Statistical analyses were performed using multiple unpaired Student’s t-tests with the Bonferroni correction method.
3. Results
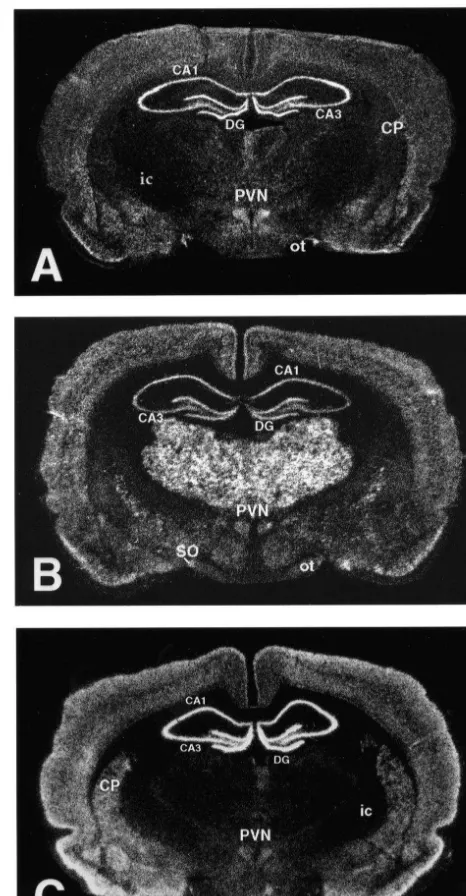
Fig. 1. Darkfield micrographs of coronal brain sections taken at the level of the hypothalamic paraventricular nucleus illustrate the distribution
3.1. Physiological impact of chronic stress pattern of mRNA transcripts encoding theb1 (A),b2 (B), andb3 (C)
subunits of the GABA receptor. Regions identified include the caudateA putamen (CP), internal capsule (ic), optic tract (ot), hypothalamic
Table 1 summarizes the effects of the chronic
intermit-paraventricular nucleus (PVN), supraoptic nucleus (SO), and the CA1
tent stress paradigm upon thymus and adrenal weights,
and CA3 subfields of the hippocampus and the dentate gyrus (DG).
reflecting the long-term impact of the procedure.
De-Table 1
Group Thymus weight Thymus weight Adrenal weight Adrenal weight
(mg) (mg / 100 g) (mg) (mg / 100 g)
Control 449 (617) 119.3 (65.6) 52.7 (62.6) 14.0 (60.8)
Chronic stress 372 (615)* 106.7 (63.2) 64.8 (62.8)* 18.4 (60.8)*
creased raw thymus weight was detected (t(10)53.56; pal subfields investigated: CA1(33.9%) (t(10)510.98; P,
P50.005), although when corrected for body weight the 0.01), CA3 (22.3%) (t(10)56.15; P,0.01), and dentate difference approached but did not reach statistical signifi- gyrus (24.0%) (t(10)56.34; P,0.01) (Figs. 3 and 5). b3 cance (t(10)51.97; P50.077). Adrenal hypertrophy was subunit mRNA expression levels were not different be-confirmed both by increased raw adrenal weight (t(10)5 tween experimental and control groups in any regions 12.17; P,0.01) and adrenal weight / 100 g body weight examined (Fig. 6).
(t(10)54.1; P50.002).
3.2. Chronic stress (Figs. 1 –6) 4. Discussion
The pattern of expression ofb subunits of the GABAA The results of the present study indicate that mRNA receptor, as illustrated in Fig. 1, was found to be in accord levels for theb1 andb2 subunits of the GABA receptorA
with previous reports [4,13,25,30,32]. Among regions are down-regulated in the mpPVN following the chronic examined, a down-regulation (48.3%) of b1 subunit non-habituating stress protocol employed. As the subunit transcripts was found in the mpPVN (t(9)5210.12; P, containing the GABA binding site, thebsubunit may be of 0.01) (Fig. 2A,B), with no changes detected within the particular interest with respect to regulation of GABAergic pmPVN or within any of the subfields of the hippocampal inhibitory neurotransmission. Previous evidence has shown formation (Figs. 2A,B and 4). A decrease in b2 subunit that b subunits of this receptor are expressed within the mRNA expression (29.8%) was also detected in the CRH-containing neurons of the mpPVN; transcripts encod-mpPVN (t(10)5211.14; P,0.01) (Fig. 2C,D), also with ing the b1 and b3 subunits were found to be expressed no change detected within the magnocellular division of within nearly all CRH neurons, whereas those expressing the nucleus (Figs. 2C–D, 5). However, increases in b2 b2 were detected in approximately two-thirds of CRH-transcript expression levels were detected in all hippocam- containing cells [4]. The regulatory change detected in this
Fig. 5. Relative expression levels ofb2 mRNA in the medial parvocellu-lar (mpPVN) and posterior magnocelluparvocellu-lar (pmPVN) divisions of the hypothalamic paraventricular nucleus and in CA1 and CA3 hippocampal subfields and the dentate gyrus (DG) in chronically stressed and control groups (n56 / group). **P,0.01.
thalamic–pituitary–adrenocortical (HPA) axis, given that the CRH neurons of the mpPVN are known to be the
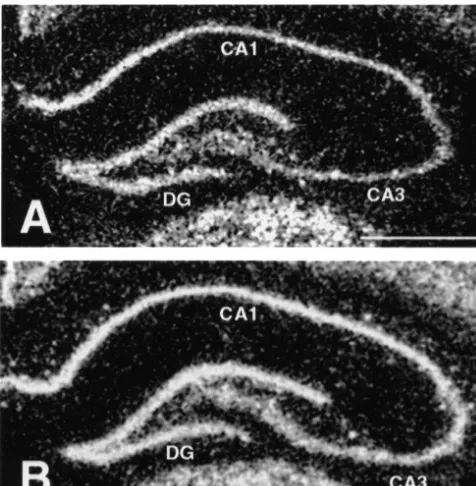
Fig. 3. Darkfield photomicrographs indicate examples of expression origin of the final common pathway of stress-induced levels of mRNA for theb2 subunit of the GABAA receptor within the glucocorticoid secretion. With regard to the possibility of hippocampal formation in control (A) and chronic stress (B) conditions.
altered receptor efficacy, it is worth noting that data from
Scale bar in A (for A and B)51.0 mm. [Note: the images used to
transfection studies have shown that GABAA receptors
generate this figure were produced from X-ray films scanned to a
containing the b2 subunit displayed greater maximal
PowerMac 8500 computer using a Polaroid Sprintscan Scanner. Images
were imported into Adobe Photoshop 5.0 where they were inverted, current amplitudes than those containing otherb subunits,
sharpened (equally) and labeled, and subsequently exported to a Kodak although the greatest overall GABA receptor sensitivity
A
8670 dye-sublimation printer.] Abbreviations: DG, dentate gyrus; CA1
appeared to be conferred by theb3 subunit [12]. While no
and CA3 hippocampal subfields.
differences were found inb3 mRNA levels in the mpPVN between chronically stressed and control animals in the study, if indeed confirmed at the protein level, may
present study, changes in b1 and b2 subunits may be suggest: (1) altered GABAA receptor efficacy resulting
capable of altering the response properties of CRH neurons from changes in subunit composition; or (2) a decrease in
to GABA. The present findings might also reflect a the total number of functional GABA receptors in CRH-A
reduction in the total number of functional GABA re-containing cells. Such possibilities may have important
ceptors on CRH neurons. The absence of compensatory implications for the regulatory control of the
hypo-transcriptional regulation ofb3 subunits may support such
a notion, although regulation at the level of the receptor GABAergic neurons (e.g., bed nucleus of the stria ter-protein could clearly be involved, requiring further in- minalis and hypothalamus) [7,8]. In this manner an excitat-vestigation to resolve. In either scenario, a reduction in the ory (glutamatergic) hippocampal output signal may be efficacy of or capacity for GABA transmission at this key converted to inhibition at the level of the mpPVN. regulatory site might explain the previously reported Interestingly, within the hippocampal formation, b2 re-elevations of stress-induced glucocorticoid secretion fol- ceptor subunit expression is predominantly found within lowing imposition of chronic stress [2,9]. interneuronal populations expressing the a1 /b2 /g2 A further issue regarding the observed down-regulation subunit combination [15] and known to receive GABAer-ofb1 andb2 GABA subunits in the mpPVN is whetherA gic terminals. In contrast, pyramidal neurons innervated by or not the effects might be mediated directly by glucocor- these interneuronal populations display a different subunit ticoids. Previous evidence for corticosteroid regulation of composition profile (a2 /b3 /g2). An increase in hippocam-GABAA receptor subunits in brain has been advanced, pal b2 subunit expression, if reflective of increases in including regulation ofbsubunits [23], although the issue functional receptors, might represent a compensatory has not been investigated within the PVN. While steroid mechanism aimed at limiting glucocorticoid secretion, levels were not determined in the present study, the whereby a relatively selective inhibition of hippocampal changes noted in thymus and adrenal weight are consistent interneuronal populations produces disinhibition of hip-with elevation in circulating glucocorticoid levels in pocampal (pyramidal cell) outflow, and ultimately, greater response to chronic stress. The fact that the present effects inhibition at the level of the mpPVN. Clearly, further were confined to the parvocellular but not magnocellular investigation will be required to determine if such a division of the PVN may also support a direct steroid mechanism is-induced, as well as the extent to which effect, as only the former region is thought to express the alterations in the pharmacological properties and / or num-glucocorticoid receptor type (GR; type II) that is exten- bers of GABAA receptors play a prominent role in sively occupied at stress levels of the steroids [11,16]. modulating neural circuits which mediate HPA activity. Alternatively, the observed decreases in b subunit
tran-scription may reflect a regulatory response to hyperactivity
of GABAergic afferent systems engaged following chronic Acknowledgements
stress. This idea is consistent with a growing body of
evidence suggesting that a local GABAergic network is The authors wish to thank Ms. Lisa Treiber and Ms. positioned to provide an inhibitory brake on HPA activity Stephanie Skubal for their expert technical assistance with across a wide spectrum of stressful stimuli [1,6,7,17], and this study. Supported by MH56577 and NARSAD. further supported by the finding that levels of the 65-kDa
isoform of the GABA synthesizing enzyme (GAD;
References
glutamic acid decarboxylase) are up-regulated following chronic non-habituating stress in brain regions containing
[1] C. Boudaba, K. Szabo, J.G. Tasker, Physiological mapping of local
sources of GABAergic input to the mpPVN [2]. While inhibitory inputs to the hypothalamic paraventricular nucleus, J. further study will be required to distinguish among the Neurosci. 16 (1996) 7151–7160.
possibilities identified, it is recognized that the situation is [2] G.T. Bowers, W.E. Cullinan, J.P. Herman, Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central
complicated by the fact that GABAA receptor subunit
stress circuits, J. Neuroscience 18 (1998) 5938–5947.
regulation in the mpPVN may result from glucocorticoid
[3] A.E. Calogero, W.T. Galluci, G.P. Chrousos, P.W. Gold, Interaction
actions upon sources of GABAergic inputs to the nucleus. between GABAergic neurotransmission and rat hypothalamic cor-The present findings indicate that b2 subunit mRNA ticotropin-releasing hormone secretion in vitro, Brain Res. 463
levels were up-regulated in the three hippocampal regions (1988) 28–36.
[4] W.E. Cullinan, GABA receptor subunit expression within
hypo-investigated. Similar to the situation in the mpPVN, it is A
physiotropic CRH neurons: a dual hybridization histochemical
unclear whether these findings reflect the effects of stress,
study, J. Comp. Neurol 419 (2000) 344–351.
the feedback actions of glucocorticoids, or both. Interest- [5] W.E. Cullinan, Evidence for a PVN site of action of GABA in the ingly, a previous study in which rats were treated for 10 regulatory control of the rat stress axis, Physiologist (Suppl.) 41 (5)
days with stress levels of corticosterone revealed increases (1998) 353.
[6] W.E. Cullinan, D.L. Helmreich, S.J. Watson, Swim stress-induced
ofb2 expression similar to those reported here CA1, CA3
Fos expression in forebrain afferents to the hypothalamic
paraven-and the dentate gyrus [23].
tricular nucleus, J. Comp. Neurol. 368 (1996) 88–99.
The significance of hippocampal GABA receptor regu-A [7] W.E. Cullinan, J.P. Herman, D.L. Helmreich, S.J. Watson, A
lation in the context of stress responsiveness remains to be neuroanatomy of stress, in: M.J. Friedman, D.S. Charney, A.Y.
determined. A large body of evidence has implicated the Deutch (Eds.), Neurobiological and Clinical Consequences of Stress: From Normal Adaptation to PTSD, Raven Press, New York, 1995,
hippocampus in the regulatory control of the HPA axis The
pp. 3–26.
majority of studies have emphasized an inhibitory
relation-[8] W.E. Cullinan, J.P. Herman, S.J. Watson, Ventral subicular
inter-ship between hippocampal outflow and glucocorticoid action with the hypothalamic paraventricular nucleus: evidence for a secretion [18,20], occurring via putative relays between the relay in the bed nucleus of the stria terminalis, J. Comp. Neurol. 332
[9] M.F. Dallman, S.F. Akana, N. Levin, C.D. Walker, M.J. Bradbury, [21] P. Malherbe, E. Sigel, R. Baur, E. Persohn, J.G. Richards, H. S. Suemara, K.S. Scribner, Corticosteroids and control of function in Mohler, Functional characteristics and sites of gene expression of the hypothalamic-pituitary-adrenal (HPA) axis, Ann. NY Acad Sci. thea1,b1,g2-isoform of the rat GABA receptor, J. Neurosci. 10A
746 (1994) 22–31. (1990) 2330–2337.
[10] C. Decavel, A.N. van den Pol, GABA: a dominant neurotransmitter [22] R.M. McKernan, P.J. Whiting, Which GABAA receptor subtypes in the hypothalamus, J. Comp. Neurol. 302 (1990) 1019–1037. really occur in the brain?, Trends Neurosci. 19 (1996) 139–143. [11] E.R. De Kloet, Brain corticosteroid receptor balance and homeo- [23] M. Orchinik, N.G. Weiland, B.S. McEwen, Chronic exposure to
static control, Front. Neuroendocrinol. 12 (1991) 95–164. stress levels of corticosterone alters GABAA receptor subunit [12] I. Ducic, H.J. Caruncho, W.J. Zhu, S. Vicini, E. Costa,g-Amino- mRNA levels in rat hippocampus, Mol. Brain Res. 34 (1995)
2
butyric acid gating of Cl channels in recombinant GABAA 29–37.
receptors, J. Pharmacol. Exp. Ther. 272 (1995) 438–445. [24] G. Paxinos, C. Watson, in: The Rat Brain in Stereotaxic Coordinates, [13] V.S. Fenelon, W. Sieghart, A.E. Herbison, Cellular localization of Academic Press, Orlando, FL, 1986.
differential distribution of GABAA receptor subunit proteins and [25] E. Persohn, P. Malherbe, J.G. Richards, Comparative molecular messenger RNAs within hypothalamic magnocellular neurons, neuroanatomy of cloned GABA receptor subunits in the rat CNS, J.A Neuroscience 64 (1995) 1129–1143. Comp. Neurol. 326 (1992) 193–216.
[14] J.M. Fritschy, H. Mohler, GABA -receptor heterogeneity in theA [26] P.M. Plotsky, S. Otto, S. Sutton, Neurotransmitter modulation of adult rat brain: differential regional and cellular distribution of seven corticotropin-releasing factor secretion into the hypophyseal-portal major subunits, J. Comp. Neurol. 359 (1995) 154–194. circulation, Life Sci. 41 (1987) 1311–1317.
[15] B. Gao, J.M. Fritschy, Selective allocation of GABAA receptors [27] D.B. Pritchett, P.H. Seeburg, g-Aminobutyric acidA receptor a 5-containing the a1 subunit to neurochemically distinct subpopula- subunits creates novel type II benzodiazepine receptor pharma-tions of rat hippocampal interneurons, Eur. J. Neurosci. 6 (1994) cology, J. Neurochem. 54 (1990) 1802–1804.
837–853. [28] D.B. Pritchett, H. Luddens, P.H. Seeberg, Type I and type II [16] J.P. Herman, Regulation of adrenocorticosteroid receptor mRNA GABA -benzodiazepine receptors produced in transfected cells,A
expression in the central nervous system, Cell. Mol. Neurobiol. 13 Science 245 (1989) 1389–1392.
(1994) 349–372. [29] T.A. Verdoorn, Formation of heteromeric g-aminobutyric acid type [17] J.P. Herman, W.E. Cullinan, Neurocircuitry of stress: Central control A receptors containing two differentasubunits, Mol. Pharamacol.
of the hypothalamic-pituitary-adrenocortical axis, Trends Neurosci. 45 (1994) 475–480.
20 (1997) 78–84. [30] W. Wisden, D.J. Laurie, H. Monyer, P.H. Seeburg, The distribution [18] J.P. Herman, W.E. Cullinan, M.I. Morano, H. Akil, S.J. Watson, of 13 GABA receptor subunit mRNAs in the rat brain. I.
A
Contribution of the ventral subiculum to inhibitory regulation of the Telencephalon, diencephalon, mesencephalon, J. Neurosci. 12 hypothalamo-pituitary-adrenocortical axis, J. Neuroendocrinol. 6 (1992) 1040–1062.
(1995) 433–442. [31] S. Ymer, P.R. Schofield, A. Draguhn, P. Werner, M. Kohler, P.H. [19] E.W. Hillhouse, N.G.N. Milton, Effect of noradrenaline and g- Seeburg, GABA receptor b subunit heterogeneity: Functional
A
aminobutyric acid on the secretion of corticotropin-releasing factor- expression of cloned cDNAs, EMBO J. 8 (1989) 1665–1670. 41 and arginine vasopressin from the rat hypothalamus in vitro, J. [32] J.-H. Zhang, M. Sato, M. Tohyama, Region specific expression of Endocrinol. 122 (1989) 719–723. the mRNAs encoding beta subunits (b, b and b ) of GABA
1 2 3 A
[20] L. Jacobson, R.M. Sapolsky, The role of the hippocampus in receptor in rat brain, J. Comp. Neurol. 303 (1991) 637–657. feedback regulation of the hypothalamo-pituitary-adrenocortical