www.elsevier.com/locate/ibmb
Substrate-stereoselectivity of a high-affinity glutamate transporter
cloned from the CNS of the cockroach
Diploptera punctata
C. Donly
a,*, J. Jevnikar
a, H. McLean
b, S. Caveney
baSouthern Crop Protection and Food Research Centre, Agriculture and Agri-Food Canada, 1391 Sandford St., London, Ontario, Canada N5V
4T3
bDepartment of Zoology, University of Western Ontario, London, Ontario, Canada N6A 5B7
Received 30 August 1999; received in revised form 17 December 1999; accepted 21 December 1999
Abstract
A cDNA encoding a Na+-dependent glutamate transporter has been cloned from the brain of the cockroachDiploptera punctata. The cDNA encodes a transporter protein of 481 amino acids, designated DipEAAT1, which when expressed in baculovirus infected insect cells, resulted in a 40–50 fold increase in [3H]L-glutamate uptake. DipEAAT1 mRNA is expressed in the brain, as is the RNA
encoding TrnEAAT1, a related transporter recently isolated from the caterpillarTrichoplusia ni. The affinity of these transporters for L-glutamate and several structural analogues was compared. Both have a high affinity for L-glutamate, their presumed primary substrate, but quite different affinities for D-aspartate. TrnEAAT1 was found to be similar to other glutamate transporters in that its ability to transport [3H]L-glutamate into cells was inhibited strongly by D- and L- isomers of aspartate and its analogues.
DipEAAT1, by contrast, was inhibited weakly by all D- isomers tested. The affinity of DipEAAT1 for [3H]D-aspartate was found
to be an order of magnitude lower than that of TrnEAAT1, revealing an unusual stereoselectivity for aspartate substrates by the cockroach transporter. The activity of DipEAAT1 was also unaffected by the presence of Zn++ in the bathing solution, despite the presence of a putative Zn++-binding motif conferring Zn++-sensitivity on some mammalian glutamate transporters.2000 Elsevier Science Ltd. All rights reserved.
Keywords:Glutamate transporter;Diploptera punctata; Cockroach; Cloning; Substrate-stereoselectivity
1. Introduction
D-aspartate is a high-affinity transport substrate used to characterize the Na+-dependent uptake systems for the excitatory amino acid L-glutamate in the mammalian brain (Balcar and Johnston, 1972; Robinson et al., 1993) and non-neuronal tissues (Schneider et al., 1980; Balcar, 1992). D-aspartate competes equally with its naturally occurring L-isomer in blocking L-glutamate uptake in rat brain slices and synaptosomal preparations. D-gluta-mate, on the other hand, does not inhibit L-glutamate uptake by neuronal tissues (Balcar and Johnston, 1972; Robinson et al., 1993). Gazzola et al. (1981) commented on this stereoselective anomaly in a study of Na+ -depen-dent dicarboxylic amino acid transport in human skin
* Corresponding author. Tel.:+1-519-457-1470; fax:+ 1-519-457-3997.
E-mail address:[email protected] (C. Donly).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 0 4 - 7
fibroblasts. As in the mammalian CNS, this transport system was competitively inhibited by both L- and D-isomers of aspartate, but was selectively stereospecific and not blocked by the D-isomers of glutamate and cys-teate. In insect tissues, too, aspartate (but not D-glutamate) competes strongly with L-glutamate for both high-affinity (Bermudez et al., 1988; McLean and Cav-eney, 1993; Caveney et al., 1996) and low-affinity Na+ -dependent uptake (Kingan and Hishinuma, 1987).
In insects, a glutamate transporter cDNA isolated and cloned from the head of the caterpillar Trichoplusia ni, TrnEAAT1, was also shown to accept both L- and D-enantiomers of aspartate as transport substrates (Donly et al., 1997), while a glutamate transporter isolated from
Drosophila embryos, dEAAT1, discriminates only weakly between these two substrates (Seal et al., 1998). The situation, however, is quite different for a trans-porter cDNA isolated from the brain of the cockroach,
Diploptera punctata. We report here that functional
characterization of this cockroach transporter,
DipEAAT1, in a baculovirus-cell expression system revealed that this transporter is strongly stereoselective. [3H]D-aspartate is not a high affinity transport substrate for this transporter, nor are D-aspartate or threo -3-hyd-roxy-D-aspartate potent blockers of L-glutamate uptake.
Fig. 1. Amino acid sequence comparison of glutamate transporters cloned from insects. Residues identical in all 3 sequences are shaded in black, while positions having 2 out of 3 matches are shaded grey. Bars are drawn over regions of sequence hydrophobicity that may correspond to transmembrane structures. Two histidine residues in the DipEAAT1 sequence analogous to a putative Zn++binding site are boxed. The sequence of TrnEAAT1 is from Donly et al. (1997) and the sequence of DrmEAAT1 is from Seal et al. (1998). The sequence of the DipEAAT1 cDNA reported here has been deposited in the GenBank/EMBL database under the accession # AF208521.
2. Methods
2.1. Isolation of a cockroach glutamate transporter cDNA
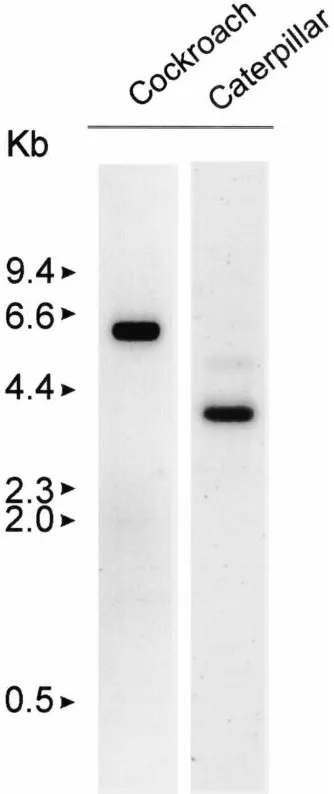
Fig. 2. Expression of glutamate transporter mRNAs. RNAs extracted from the brains of the cockroach (D. punctata-left) and the caterpillar (T. ni-right) were separated by denaturing agarose gel electrophoresis and transferred to nylon membranes. 32P-Labeled fragments of the
DipEAAT1 and TrnEAAT1 cDNAs were hybridized with the corre-sponding RNAs and then the washed blots exposed to X-ray film. RNA products of approximately 5.0 kb (cockroach) and 3.6 kb (caterpillar) were observed to be present in the insect brains.
punctata (Donly et al., 1996). Eleven positive clones were identified and the corresponding phagemids sub-sequently excised (Stratagene). A single clone was selec-ted and subjecselec-ted to complete automaselec-ted dideoxynucleo-tide chain termination sequencing (Mobix).
2.2. RNA isolation and Northern blot analysis
RNA was isolated from brains of mated female cock-roaches using the Pharmacia QuickPrep mRNA purifi-cation system (Donly et al., 1996). The isolated RNA was used for cDNA library construction (Stratagene), cDNA synthesis using the First-Strand cDNA Synthesis Kit (Pharmacia Biotech), or Northern blotting. Total
RNA was isolated from brains of Trichoplusia ni cater-pillars by homogenization in Trizol Reagent (Life Technologies), and used directly for Northern blotting. 4 µg of polyA+-enriched cockroach brain RNA, or 10 µg of total caterpillar brain RNA were separated on denaturing agarose gels and transferred to Hybond N+ nylon (Amersham), where they were fixed in a CL-1000 Ultraviolet Crosslinker (UVP Inc.). Hybridizations were performed at 65°C in QuikHyb solution (Stratagene), using32P-labeled fragments of each transporter. For the cockroach RNA, the 250 bp fragment of theD. punctata
transporter cDNA generated by PCR (above) was used as the probe, while for the caterpillar RNA, an 800 bp
fragment comprising the 59 segment of the TrnEAAT1
cDNA was used. Washes and exposure to X-ray film
were performed according to standard protocols
(Sambrook et al., 1989).
2.3. Baculovirus expression
The cockroach glutamate transporter cDNA
(DipEAAT1) was ligated into the transfer vector pFastBac1 for expression using the Bac-to-Bac baculo-virus expression system (Life Technologies). Two differ-ent versions of the cDNA were used for expressions. One version included the entire cloned cDNA, while the other included only the open reading frame (ORF). The shortened version was created using oligonucleotide pri-mers designed from the ends of the peptide ORF with restriction endonuclease recognition sequences at their
59 ends. The PCR amplification was performed using
Expand polymerase (Roche) to minimize the occurrence of errors in the amplified products. Both versions pro-duced similar expression levels. The caterpillar cDNA (TrnEAAT1) was previously cloned in the Bac-to-Bac expression system as described in Donly et al. (1997). The constructs were transposed to bacmid as directed by the supplier (Life Technologies) and used to transfect Sf9 cells grown in TC-100 medium supplemented with 10% FBS (Life Technologies). Viral stocks were ampli-fied in BTI-TN-5B1-4 (High Five) cells (Invitrogen). High Five cells were maintained in monolayer culture at
27°C in EX-CELL 405 serum-free medium (JRH
Biosciences). For expression of transporters, cells were infected at a multiplicity of infection of 0.35–0.95 in 12 well microtitre plates using 200,000–300,000 cells per well. Cells were assayed for activity after 48 h.
2.4. Transport assays
in 12 well plates were washed with saline containing 66 mOsm Na+(composition listed in Donly et al., 1997) and then exposed to 500 µl saline containing 10 µl [3 H]L-glutamate (specific activity 46–63 Ci/mmol) or [3 H]D-aspartate (specific activity 20 Ci/mmol) (Amersham) for 5 min at 27°C. Uptake was terminated by washing the cells in Na+-free saline. The cells were air dried and the accumulated radiolabel extracted in 500µl 70% ethanol. A 200 µl aliquot was added to scintillation fluid and its radioactivity measured.
For kinetic studies, the corresponding unlabeled amino acid was added to the saline to give final L-gluta-mate or D-aspartate concentrations between 1 µM and 500µM. Only the Na+-dependent accumulation of radi-olabel by the cells (greater than 95% of total uptake at non-saturating concentrations of amino acid) was used to determine transporter kinetics. [3H]L-glutamate was used for the competitive inhibition studies. In these experiments, unlabeled inhibitor was added to the medium over the range 1 µM to 500µM.
Amino acids were obtained from Sigma (L-glutamate, D-glutamate, L-aspartate, D-aspartate, L-cysteate) or from Tocris Cookson (D-cysteate, threo -3-hydroxy-L-aspartate and threo-3-hydroxy-D-aspartate).
3. Results and discussion
3.1. Molecular cloning of DipEAAT1
By comparing the amino acid sequences of known mammalian glutamate transporters, regions of conserved
Fig. 3. High-affinity Na+-dependent [3H]L-glutamate uptake by cells expressing DipEAAT1 or TrnEAAT1. The uptake data shown are corrected
for Na+-independent uptake, which constitutes typically less than 5% of total uptake. Eadie–Hofstee analysis (inset) of the Na+-dependent transport in this sample experiment indicates that the two transporters have similar affinities for L-glutamate (Kmvalues of 32.8µM and 31.1µM, respectively,
at 66 mM Na+). Transport activity was compared in pairs of multi-well plates run in parallel. The calculated maximum rates of uptake (V
max) for
cells expressing DipEAAT1 and TrnEAAT1 in this experiment were 605 pmol well21min21and 539 pmol well21min21, respectively. Average
values are given in the text.
sequence homology can be detected and utilized for the design of degenerate PCR primers. Such primers may then be used to amplify fragments of genes for homolo-gous proteins from other organisms, ranging from nema-todes (Radice and Lustigman, 1996) to insects (Donly et al., 1997). When such primers were used for PCR with cDNA from brains of the cockroach D. punctataas the target, a 250 bp DNA fragment resulted which showed strong similarity to other glutamate transporter genes. Using this fragment as a probe, eleven clones were iso-lated from a cockroach brain cDNA library, and one clone was selected for complete sequencing.
The resulting sequence revealed an ORF encoding a 481 amino acid protein which we designate DipEAAT1 (D. punctata exitatory amino acid transporter 1). The deduced amino acid sequence of DipEAAT1 was found to be highly similar to other known glutamate trans-porters, showing up to 47% similarity with the human transporter hEAAT3. However, this sequence shows the greatest similarity to other insect transporters, with 53% identity to both caterpillar (TrnEAAT1) and fruit fly (DrmEAAT1) glutamate transporters (Fig. 1).
3.2. Glutamate transporter expression
(Fig. 2). Northern analysis showed the DipEAAT1 gene is expressed as an approximately 5 kb mRNA in cock-roach brain, while the TrnEAAT1 gene is expressed in caterpillar brain as an approximately 3.6 kb mRNA (Fig. 2 and Donly et al., 1997). The Drosophila transporter DrmEAAT1 is expressed in the fruitfly embryonic CNS (Seal et al., 1998).
Based on amino acid sequence similarities, the three cloned insect transporters appear to be most closely related to hEAAT1 and hEAAT3, with DipEAAT1 showing 46% and 47% identity and TrnEAAT1 showing 40% and 42% to the human proteins, respectively. How-ever, TrnEAAT1 is slightly more similar functionally to hEAAT1 than to any of the other cloned human gluta-mate transporters (Donly et al., 1997). Also, TrnEAAT1 appears to be expressed in glial cells in the CNS, but not in neurons (T. Malutan, unpublished observations). This more closely resembles the cellular pattern of distri-bution of EAAT1 in the mammalian brain and not that of EAAT3, which is neuronal in expression (Faruta et al., 1997). The cellular distribution of DipEAAT1 in the cockroach CNS remains to be determined.
3.3. Transporter kinetics and competitive inhibition
The relative affinities of the cockroach and caterpillar transporters for radiolabeled L-glutamate were determ-ined 48 h after virally infecting High Five cells with either DipEAAT1 or TrnEAAT1 cDNA. All experiments were run in parallel in saline containing 66 mM Na+.
The uptake parameters for [3H]L-glutamate were
determined by Eadie–Hofstee analysis of the Na+- and concentration-dependent uptake data (Fig. 3). The affin-ity of DipEAAT1 for L-glutamate (Km=30.5±4.7µM at a maximum velocity of uptake (Vmax) by the cells
rang-ing from 173 to 662 pmol well21 min21 in n=9
experiments), was found to be similar to that in cells virally infected with TrnEAAT1 cDNA (Km=37.7±6.6 µM atVmaxranging from 259 to 800 pmol well21min21 inn=7 experiments) (Fig. 3). The uptake of L-glutamate by DipEAAT1 was absolutely dependent on the presence of Na+ in the saline, as reported also for the caterpillar (Donly et al., 1997) and fruit fly glutamate transporters (Seal et al., 1998). The apparent uptake of [3 H]L-gluta-mate by DipEAAT1-infected cells in nominally Na+-free saline (Na+ replaced by equimolar choline, saline pH adjusted with KOH) was 4.8±0.75% of the uptake in the presence of Na+ and similar to that seen in cells not
infected with DipEAAT1-virus (5.9±0.3% control
uptake) that were tested in parallel.
Many structural analogues of glutamate and L-aspartate, both naturally-occurring (Caveney et al., 1996) and synthetic (McLean and Caveney, 1993) are potent competive inhibitors of L-glutamate uptake in insects. A comparison was made of the ability of several of these
compounds to inhibit [3H]L-glutamate uptake by
DipEAAT1 and TrnEAAT1. Fig. 4 shows that at a saline concentration of 100µM, the D-enantiomers of aspartate and threo-3-hydroxy-aspartate are weak inhibitors of uptake by DipEAAT1 yet strong inhibitors of uptake by TrnEAAT1. The D- analogues of glutamate and cysteate do not inhibit L-glutamate uptake by either transporter. The poor ability of D-aspartate to inhibit L-glutamate uptake by DipEAAT1 implies that this transporter is able to discriminate against (i.e. not accept) 4-carbon D-dicarboxyamino acids as transport substrates. This ster-eoselectivity is not seen in other glutamate transporters cloned from insects (Donly et al., 1997; Seal et al., 1998) nor in mammalian glutamate transporters (Gazzola et al., 1981). To show directly that D-aspartate is indeed a
low-affinity transport substrate for cells expressing
DipEAAT1, the kinetics of [3H]D-aspartate uptake by DipEAAT1 and TrnEAAT1 were compared. Fig. 5 shows a sample experiment. The affinity of DipEAAT1 for D-aspartate (Km=222.4 mM,Vmaxranging from 94 to 140 pmol well21 min21, n=4) was on average 10-fold lower than that of TrnEAAT1 (Km=20.4 µM,Vmax rang-ing from 55–85 pmol well21 min21,n=4).
The stereoselective behaviour of DipEAAT1 was further confirmed by determining the concentration of the D- and L-enantiomers of aspartate andthreo -3-hyd-roxy-aspartate that inhibited 50% of [3H]L-glutamate uptake by DipEAAT1, in comparison to TrnEAAT1 (Fig. 6 and Fig. 7). The inhibition curves fitted to the data sets in Fig. 6 gave IC50values and stoichiometries of inhibitor binding (expressed as Hill coefficients) for the inhibition of DipEAAT1 activity by D-aspartate as 150.1 µM and 1.14, and by L-aspartate as 31.1µM and 1.26, respectively. The comparable inhibition and
stoi-Fig. 4. Inhibition of [3H]L-glutamate uptake by structural analogues
of L-glutamate and L-aspartate. Cells were exposed to saline contain-ing 0.44µM [3H]L-glutamate and 100µM inhibitor. glutamate,
Fig. 5. Transporters DipEAAT1 and TrnEAAT1 differ considerably in their affinity for [3H]D-aspartate as a transport substrate. Eadie–Hofstee
analysis of the uptake data (inset) revealed that in this representative experiment the affinity of DipEAAT1 for D-aspartate (Km=207.6µM) was about one-tenth that of TrnEAAT1 (Km=19.1µM). The maximum rate of [3H]D-aspartate uptake by cells expressing DipEAAT1 (Vmax=93.7 pmol
well21min21) was slightly greater than that of cells expressing TrnEAAT1 (64.3 pmol well21min21). Average values are given in the text.
Fig. 6. Stereoselective inhibition of DipEAAT1 by aspartate. The inhibition of 0.44 µM [3H]L-glutamate uptake by cells expressing
DipEAAT1 by different concentrations of D-aspartate (solid squares) was compared with the inhibition of [3H]L-glutamate uptake by cells
expressing TrnEAAT1 (solid circles). D-aspartate is a weak inhibitor of L-glutamate uptake by DipEAAT1 in comparison to its ability to block uptake by TrnEAAT1. L-aspartate strongly inhibits glutamate uptake by both transporters (open symbols). Data shown are the means
±SD of three or more replicate experiments for both inhibitors.
chiometry values for L-aspartate inhibition of
TrnEAAT1 were 13.52 µM and 1.21; and for
D-aspart-ate, 17.4 µM and 1.02, respectively. A similar pattern was seen when threo-3-hydroxy-aspartate was used to inhibit L-glutamate uptake (Fig. 7). The curves fitted through the data sets give IC50values for the inhibition of DipEAAT1 activity and stoichiometries of inhibitor
binding by threo-3-hydroxy-D-aspartate as 224.6 µM and 0.93, and by threo-3-hydroxy-L-aspartate as 24.9 µM and 1.50, respectively. The comparable inhibition and stoichiometry values for TrnEAAT1 inhibition by
threo-3-hydroxy-D-aspartate were 22.2 µM and 1.15; and by threo-3-hydroxy-L-aspartate, 6.7 µM and 1.23, respectively. These inhibition data underscore the stereo-selective behaviour of DipEAAT1 indicated by the data in Fig. 4.
3.4. Effects of Zn++ on transport
Fig. 7. Stereoselective inhibition of DipEAAT1 bythreo-3-hydroxy-aspartate. Inhibition of 0.44µM [3H]L-glutamate uptake bythreo
-3-hydroxy-D-aspartate (solid symbols) in cells expressing either DipEAAT1 or TrnEAAT1, compared to the inhibition of the two transporters bythreo -3-hydroxy-L-aspartate (open circles).Threo-3-hydroxy-D-aspartate is a weak inhibitor of L-glutamate uptake by cells expressing DipEAAT1 (solid squares) but a strong inhibitor of uptake by cells expressing TrnEAAT1 (solid circles). Data shown are the means±SD of three or more replicate experiments for both inhibitors.
G to H in this position resulted in zinc sensitivity (Vandenberg et al., 1998).
Analysis of the protein structure within the second extracellular domain of DipEAAT1 (Fig. 1) revealed the presence of 2 similarly positioned histidine residues ana-lagous to H146 and H156, which are absent from
TrnEAAT1. However, the addition of Zn++ to our
physiological saline failed to suppress glutamate trans-port by cells expressing DipEAAT1. Glutamate uptake in saline containing 1 µM, 10 µM or 100 µM ZnSO4 was similar to that seen by cells incubated in Zn++-free saline (98±7%, 102±7% and 110±7% of control uptake, respectively, n=3). As expected, glutamate uptake by cells expressing TrnEAAT1 was also unaffected by incu-bation in these Zn++-containing salines (98±7%; 102±7% and 110±7% of control uptake in 1µM, 10 µM or 100 µM ZnSO4, respectively).
The reason for the insensitivity of DipEAAT1 to extracellular Zn++ is unclear. Possibly the Zn++-binding histidine and cysteine residues in the second extracellu-lar loop of Zn++-sensitive transporters need to act in con-cert with other exposed Zn++-coordinating amino acid residues which are lacking in DipEAAT1. Further examples of Zn++sensitive transporters need to be found to more completely delineate the Zn++binding site.
3.5. Glutamate transporters in insects
Comparisons of the protein structures of DipEAAT1, TrnEAAT1 and DrmEAAT1 with known mammalian transporters have not shown a specific correlation to any
one of the at least five mammalian subtypes known. DipEAAT1 and TrnEAAT1 are closest in amino acid sequence identity to the human transporters hEAAT1 and hEAAT3. However, as mentioned earlier, from pharmacological and physiological information it has been possible to establish some finer level correlations, which suggest a slightly closer relationship to hEAAT1 for TrnEAAT1. The presence of at least part of a Zn++ -binding motif in the second extracellular loop of DipEAAT1, also aligns this protein more closely with hEAAT1 than with hEAAT3 since it resembles that con-ferring Zn++-sensitivity on hEAAT1 (Vandenberg et al., 1998). This motif has a limited distribution among gluta-mate transporters, being present in hEAAT1 and hEAAT4, but missing from hEAAT2, hEAAT3 and hEAAT5, and the lepidopteran transporter TrnEAAT1.
Unfortunately, the substrate-stereoselectivity of
DipEAAT1 characterized here does not reveal any further correlations with known mammalian transporters as this property appears to be unique.
hints that the insect CNS may yet express more than one structurally and pharmacologically distinct glutamate transporter. This remains to be confirmed.
References
Arriza, J.L., Fairman, W.A., Wadiche, J.I., Murdoch, G.H., Kav-anaugh, M.P., Amara, S.G., 1994. Functional comparisons of three glutamate transporters cloned from human motor cortex. J. Neuro-sci. 14, 5559–5569.
Balcar, V.J., 1992. Na+-dependent high-affinity uptake of L-glutamate in cultured fibroblasts. FEBS Lett. 300, 203–207.
Balcar, V.J., Johnston, G.A.R., 1972. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J. Neurochem. 19, 2657–2666.
Bermudez, I., Botham, R.P., Beadle, D.J., 1988. High- and low-affinity uptake of amino acid transmitters in cultured neurons and muscle cells of the cockroach,Periplaneta americana. Insect Biochem. 18, 249–262.
Besson, M.T., Soustelle, S., Birmann, S., 1999. Identification and structural characterization of two genes encoding glutamate trans-porter homologues differently expressed in the nervous system of
Drosophila melanogaster. FEBS Lett. 443, 97–104.
Caveney, S., McLean, H.M., Watson, I., Starratt, A.N., 1996. Affinity of an insect Na+-dependent transporter for plant-derived cyclic
sub-strates. Insect Biochem. Molec. Biol. 26, 1027–1036.
Donly, B.C., Fuse, M., Orchard, I., Tobe, S.S., Bendena, W.G., 1996. Characterization of the gene for leucomyosuppressin and its expression in the brain of the cockroach Diploptera punctata. Insect Biochem. Molec. Biol. 26, 627–637.
Donly, B.C., Richman, A., Hawkins, E., McLean, H., Caveney, S., 1997. Molecular cloning and functional expression of an insect high-affinity Na+-dependent glutamate transporter. Eur. J. Biochem. 248, 535–542.
Faruta, A., Martin, L.J., Lin, C.-L.G., Dykes-Hoberg, M., Rothstein, J.D., 1997. Cellular and synaptic localization of the neuronal gluta-mate transporters excitatory amino acid transporters 3 and 4. Neu-roscience 81, 1031–1042.
Gabrielsson, B., Robson, T., Morris, D., Chung, S.H., 1986. Effects of divalent metal ions on the uptake of glutamate and GABA from synaptosomal fractions. Brain Res. 884, 218–223.
Gazzola, G.C., Dall’Asta, V., Bussolati, O., Makowske, M.,
Chris-tensen, H.N., 1981. A stereoselective anomaly in dicarboxylic amino acid transport. J. Biol. Chem. 256, 6054–6059.
Gegelashvili, G., Schousboe, A., 1998. Cellular distribution and kinetic properties of high-affinity glutamate transporters. Brain Res. Bull. 45, 233–238.
Kanai, Y., Hediger, M., 1992. Primary structure and functional charac-terization of a high-affinity glutamate transporter. Nature 360, 467–471.
Kingan, T.G., Hishinuma, A., 1987. Transport and metabolism of L-glutamic acid by abdominal ganglia of the hawkmoth, Manduca sexta. Comp. Biochem. Physiol. 87C, 9–14.
Klockner, U., Storck, T., Conradt, M., Stoffel, W., 1994. Functional properties and substrate specificity of the cloned L-glutamate/L-aspartate transporter GLAST-1 from rat brain expressed inXenopus
oocytes. J. Neurosci. 14, 5759–5765.
McLean, H., Caveney, S., 1993. Na+-dependent medium-affinity uptake of L-glutamate in the insect epidermis. J. Comp. Physiol. B. 163, 297–306.
Radice, A.D., Lustigman, S., 1996. Cloning and characterization of cDNAs encoding putative glutamate transporters from Caenorhab-ditis elegans andOnchocerca volvulus. Mol. Biochem. Parasitol. 80, 41–53.
Robinson, M.B., Sinor, J.D., Dowd, L.A., Kerwin, J.F., 1993. Subtypes of sodium-dependent high-affinity L-[3H]glutamate transport
activity: Pharmacologic specificity and regulation by sodium and potassium. J. Neurochem. 60, 167–179.
Sambrook, J., Fritsch, E.F., Maniatis, T., 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
Schneider, E.G., Hammerman, M.R., Sacktor, B., 1980. Sodium gradi-ent-dependent L-glutamate transport in renal brush-border vesicles. J. Biol. Chem. 255, 7650–7656.
Seal, R.P., Daniels, G.M., Wolfgang, W.J., Forte, M., Amara, S., 1998. Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Receptors and Channels 6, 51–64.
Spirodon, M., Kamm, D., Billups, B., Mobbs, P., Attwell, D., 1998. Modulation by zinc of glutamate transporters in glial cells and cones isolated from the tiger salamander retina. J. Physiol. 506, 363–376.
Vandenberg, R.J., 1998. Molecular pharmacology and physiology of glutamate transporters in the central nervous system. Clin. Exp. Pharm. Physiol. 25, 393–400.

![Fig. 3.High-affinity Na+-dependent [3H]L-glutamate uptake by cells expressing DipEAAT1 or TrnEAAT1](https://thumb-ap.123doks.com/thumbv2/123dok/3120991.1379337/4.598.146.448.448.672/high-afnity-dependent-glutamate-uptake-expressing-dipeaat-trneaat.webp)
![Fig. 4.Inhibition of [3H]L-glutamate uptake by structural analoguesof L-glutamate and L-aspartate](https://thumb-ap.123doks.com/thumbv2/123dok/3120991.1379337/5.598.312.547.485.660/fig-inhibition-glutamate-uptake-structural-analoguesof-glutamate-aspartate.webp)
![Fig. 6.Stereoselective inhibition of DipEAAT1 by aspartate. Theinhibition of 0.44 µM [3H]L-glutamate uptake by cells expressingDipEAAT1 by different concentrations of D-aspartate (solid squares)was compared with the inhibition of [3H]L-glutamate uptake by](https://thumb-ap.123doks.com/thumbv2/123dok/3120991.1379337/6.598.147.447.69.294/stereoselective-inhibition-theinhibition-expressingdipeaat-different-concentrations-aspartate-inhibition.webp)
![Fig. 7.Stereoselective inhibition of DipEAAT1 by threo-3-hydroxy-aspartate. Inhibition of 0.44 µM [3H]L-glutamate uptake by threo-3-hydroxy-D-aspartate (solid symbols) in cells expressing either DipEAAT1 or TrnEAAT1, compared to the inhibition of the two t](https://thumb-ap.123doks.com/thumbv2/123dok/3120991.1379337/7.598.147.447.69.293/stereoselective-inhibition-aspartate-inhibition-glutamate-aspartate-expressing-inhibition.webp)