L
Journal of Experimental Marine Biology and Ecology, 240 (1999) 179–191
Effect of growth hormone and
g
-aminobutyric acid on
Brachionus plicatilis (Rotifera) reproduction at low food or
high ammonia levels
a,b ,* c c
Wenresti G. Gallardo , Atsushi Hagiwara , Yuichi Tomita ,
d
Terry W. Snell
a
Graduate School of Marine Science and Engineering, Nagasaki University, Bunkyo 1-14,
Nagasaki852-8131, Japan
b
Southeast Asian Fisheries Development Center(SEAFDEC), Aquaculture Department, 5021 Tigbauan,
Iloilo, Philippines
c
Faculty of Fisheries, Nagasaki University, Bunkyo-machi 1-14, Nagasaki 852-8521, Japan
d
School of Biology, Georgia Institute of Technology, Atlanta, GA 30332-0230, USA Received 31 August 1998; received in revised form 1 February 1999; accepted 8 April 1999
Abstract
21
Growth hormone (GH, 0.0025 and 0.025 I.U. ml ) andg-aminobutyric acid (GABA, 50mg
21
ml ) enhance rotifer population growth in batch cultures. In order to further understand the mechanism of their actions, we conducted experiments culturing isolated females at low food and
6
high free ammonia levels. At an optimum food level of 7310 Nannochloropsis oculata cells
21 21
ml or at low free ammonia level of 2.4mg ml , the F offspring of rotifers treated with GH at1
21
0.0025 I.U. ml had significantly higher population growth rate (r) and net reproduction rate
5
(Ro), and shorter generation time than untreated rotifers. At a lower food level of 7310
21 21
cells ml or at high free ammonia level of 3.1mg ml , rotifers treated with GABA at 50mg
21
ml had significantly higher r and Ro, and shorter generation time. These results indicate that GABA is effective in enhancing rotifer reproduction when rotifers are cultured under stress whereas GH enhances rotifer reproduction when culture conditions are optimal. Significant effects
1 2
were also observed in F and F generations which were not treated with hormones. These data may be useful for treating rotifer mass cultures to mitigate the effects of stress caused by high population densities. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Rotifera; Brachionus plicatilis; Growth hormone;g-Aminobutyric acid; Reproduction; Food limitation; Free ammonia
*Corresponding author.
1. Introduction
Environmental stress such as food limitation and high free ammonia concentration is common in rotifer mass cultures when population density increases, resulting in decreased reproduction and survival. To mitigate the stress of high population densities, hormonal treatment may be effective. In an earlier paper (Gallardo et al., 1997), we reported that in batch cultures of the rotifer Brachionus plicatilis, population growth of
21
rotifers treated with growth hormone (GH, 0.0025 and 0.025 I.U. ml ) or g
-amino-21
butyric acid (GABA, 50 mg ml ) was 1.7 and 2 times higher than that of controls, respectively. It was surprising to find that a vertebrate hormone is effective on rotifers, but vertebrate growth hormone has also been found to be effective on other invertebrates such as oysters (Paynter and Chen, 1991) and abalone (Taylor et al., 1996). Also, GABA has been found to be effective for inducing metamorphosis in the red abalone
Haliotis rufescens (Searcy-Bernal et al., 1992).
To confirm our batch culture results (Gallardo et al., 1997) and examine the effect on subsequent generations of hormone-treated rotifers, we conducted individual culture experiments on isolated females carried through the third generation (Parent, F , F ). It1 2
is possible that significant effects would be observed in subsequent generations even if these were not treated with hormones.
Results of our individual culture experiment aimed at confirming the batch culture result showed the absence of a positive effect of GABA in contrast to our batch culture results. This led us to hypothesize that GABA may be effective in enhancing rotifer reproduction when culture conditions are not optimum. Therefore, in our succeeding experiments, the rotifers were exposed to low food and high free ammonia levels to determine whether GABA and possibly GH also could mitigate the effects of these stressors. In the batch cultures where GH and GABA were effective, food became limited and water quality deteriorated as a result of increasing population density. This paper reports the effect of GH and GABA on rotifer reproduction in optimal and limited environments or in the presence of toxic levels of free ammonia.
2. Materials and methods
2.1. Effective hormone concentration
GH (porcine) and GABA were purchased from Sigma. Individual culture experiments were carried out in 24-well microplates (Iwaki, Japan). Concentrations tested were 0.0025, 0.025, 0.25 and 2.5 I.U. of GH and 0.05, 0.5, 5, 50 and 500mg of GABA per ml
6 21
of Nannochloropsis oculata suspension at 7310 cells ml . For each concentration eight replicate wells were prepared.
W.G. Gallardo et al. / J. Exp. Mar. Biol. Ecol. 240 (1999) 179 –191 181
concentration. These isolated rotifers were exposed for 48 h which was the same time as our batch culture experiments (Gallardo et al., 1997). After 24 h the first offspring were removed so as not to confuse it with the mother during transfer to the new well on the 48th hour. Thereafter, the mother was transferred daily to new culture medium without hormone. The number of offspring were counted daily until the mother died. When the offspring reached sexual maturity (egg-bearing), they were classified as to amictic or mictic based on the type of egg they carried (Hagiwara et al., 1988). The experimental condition was 258C, 22 ppt salinity, and darkness.
The intrinsic rate of natural increase (r) was computed based on Birch (1960). From the raw data, fecundity or net reproduction rate (Ro), lifespan and reproductive period were calculated.
2.2. Effect of hormone at different food levels
4 5 6
Rotifers were cultured in three food levels (7310 , 7310 , and 7310 N. oculata
21 21 21
cells ml ) with or without hormone (0.0025 I.U. ml GH or 50mg ml GABA). The same procedure was followed as in the first experiment. From the survival and fecundity schedules, the r values, Ro, lifespan and reproductive period were calculated.
A separate experiment was conducted to determine the effect of GH and GABA on
6
parent rotifers and on the generation time and size of their F and F offspring at 71 2 310
5 21
and 7310 cells ml food levels. As in the previous experiment, egg-bearing amictic females (P) of similar age were used. For each treatment, 12 females each bearing one egg were pipetted individually into culture wells and incubated at 258C in darkness. After 16 h, these rotifers were observed every hour at 1003 magnification, and as soon as neonates (F ) appeared, they were transferred to a new food suspension without1
hormone. The time of neonate appearance was recorded and the mother was removed. After 20 h, observations were made every hour and the time was recorded at the appearance of eggs and neonates (F ). To determine if generation time is related to body2
size, the lorica length and width of the mother were measured using a compound microscope at 1003 magnification with an ocular micrometer. For easy measurement without significant shrinkage, rotifers were fixed with 6.5% HCl (Fu et al., 1991). Body size of maternal rotifer at first offspring was measured to determine if hormone affects both reproductive and somatic growth because normally, high reproduction causes smaller size because more energy is allocated for reproduction over somatic growth (King and Anderson, 1971; Duncan, 1989).
2.3. Effect of hormone at different free ammonia levels
21
Free ammonia concentrations of 2.4 and 3.1mg ml were tested. In each ammonia
21
concentration, rotifers were cultured with or without hormone (0.0025 I.U. ml GH or
21
2.4. Statistical analyses
Two-way ANOVA was performed to determine any interaction between hormone concentration and rotifer generation. One-way ANOVA was conducted to identify significant differences among treatment means within each generation. Multiple com-parison by Tukey’s method was conducted to determine which treatments were significantly different. We usedSYSTAT version 5.0 (Systat) for all statistical analyses.
3. Results
3.1. Effective hormone concentration
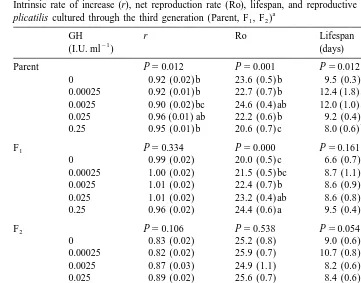
GH effect on Ro, lifespan and reproductive period differed among generations (two-way ANOVA, P,0.05) but effect on r did not differ. One-way ANOVA showed significant differences between treatment means in each generation. Parental rotifers (P)
21
treated with GH at 0.25 I.U. ml had significantly lower net Ro and a shorter reproductive period than the control, but the first generation offspring (F ) of rotifers1
21
treated with GH at 0.0025, 0.025 and 0.25 I.U. ml had significantly higher Ro (Table 1). In F2 rotifers, there was no significant difference in any parameter between treatments. No significant difference was found in the intrinsic rate of natural increase (r) and the lifespan of rotifers between treatments and control in all generations.
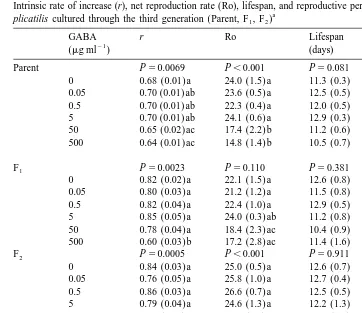
GABA effect on r, Ro, lifespan and reproductive period did not differ significantly among generations (two-way ANOVA, P.0.05) but within each generation, significant differences in r and Ro were observed (one-way ANOVA, P,0.01). Parental rotifers
21
treated with 50 and 500 mg ml GABA had significantly lower Ro compared to the
21
control. Furthermore, the r and Ro of F and F of rotifers exposed to 5001 2 mg ml GABA was significantly lower than the controls (Table 2). There was no significant difference in lifespan and length of reproductive period between treatments in each generation. Mictic female production of rotifers did not differ between rotifers with or without hormone treatment.
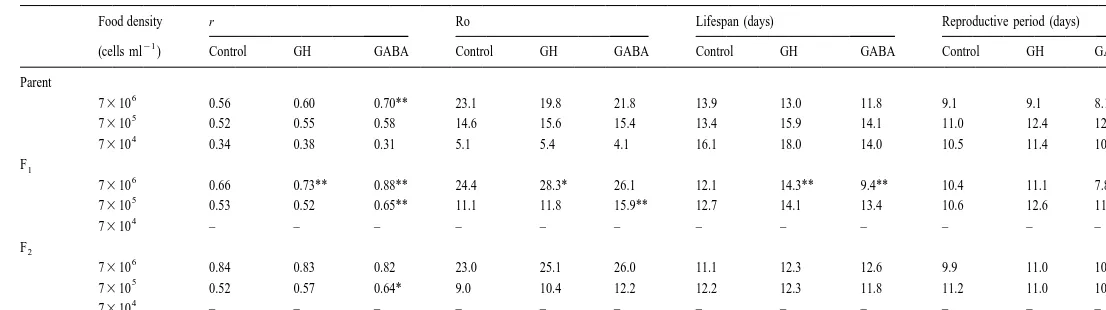
3.2. Effect of hormone at different food levels
21
GH (0.0025 I.U. ml ) resulted in higher r, Ro and lifespan of F rotifers when food1
6 21 5
level is at 7310 N. oculata cells ml , but not at lower food levels of 7310 and
4 21 6 21
7310 cells ml (Table 3). On the other hand, at 7310 cells ml (control), there was no significant difference in Ro between rotifers with or without GABA treatment
21 5 21
(50 mg ml ), but at a lower food density of 7310 cells ml , the Ro of the F of1
GABA-treated rotifers was significantly higher than those without GABA treatment. The
6 21
r values of P and F of GABA-treated rotifers at 71 310 cells ml were significantly
6 21
W.G. Gallardo et al. / J. Exp. Mar. Biol. Ecol. 240 (1999) 179 –191 183 Table 1
Intrinsic rate of increase (r), net reproduction rate (Ro), lifespan, and reproductive period of GH-treated B.
a plicatilis cultured through the third generation (Parent, F , F )1 2
GH r Ro Lifespan Reproductive
21
(I.U. ml ) (days) period (days)
Parent P50.012 P50.001 P50.012 P50.000
Concentration 2.95 0.023 8.47 0.000 3.62 0.008 4.88 0.001
Generation 90.09 0.000 20.70 0.000 6.00 0.003 61.56 0.000
Conc3Gen 1.66 0.116 2.15 0.037 2.49 0.016 7.77 0.000
a
Values are mean and standard error (in parenthesis) of 6–8 replicates per treatment. One-way ANOVA P values are indicated for each generation. In a column, means followed by a common letter are not significantly different at the 5% level. Means lacking letters not significantly different. Two-way ANOVA results for each variable are shown at the bottom of the table.
5
significantly shorter than the untreated rotifers. At a lower food density of 7310 cells
21
ml the r values of F and F of GABA-treated rotifers were significantly higher than1 2
untreated rotifers, whereas the lifespan and reproductive period did not differ sig-nificantly between treated and untreated rotifers.
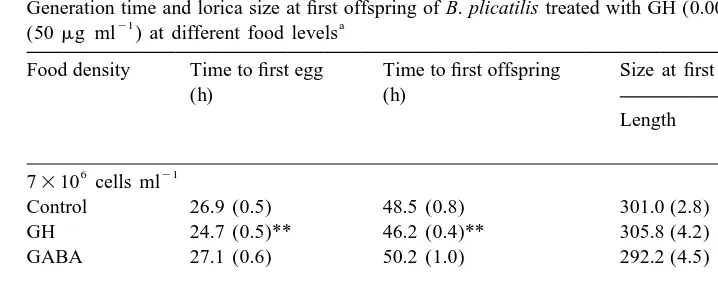
6 21
At 7310 cells ml food level, the time to first egg and first offspring of GH-treated
5
rotifers was significantly shorter than the untreated rotifers. However, at 7310 cells
21
ml food level, there was no significant difference between treatments (Table 4). On the other hand, with GABA treatment, the time to first egg and first offspring of
5 21
GABA-treated rotifers at 7310 cells ml food level was significantly shorter than the untreated rotifers. There was no significant difference in time to first egg and first
6 21
Table 2
Intrinsic rate of increase (r), net reproduction rate (Ro), lifespan, and reproductive period of GABA-treated B.
a plicatilis cultured through the third generation (Parent, F , F )1 2
GABA r Ro Lifespan Reproductive
Concentration 10.26 0.000 10.64 0.000 1.13 0.351 2.28 0.052
Generation 23.02 0.000 11.80 0.000 1.62 0.203 1.14 0.323
Conc3Gen 1.92 0.051 1.33 0.225 0.90 0.536 0.82 0.613
a
Values are mean and standard error (in parenthesis) of 6–8 replicates per treatment. One-way ANOVA p values are indicated for each generation. In a column, means followed by a common letter are not significantly different at the 5% level. Means lacking letters are not significantly different. Two-way ANOVA results for each variable are shown at the bottom of the table.
3.3. Effect of hormone at different free ammonia levels
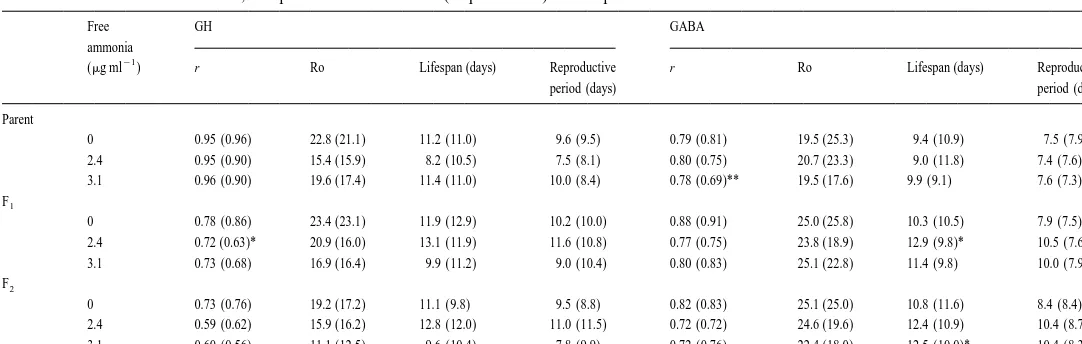
In controls lacking hormone, r, Ro, lifespan and reproductive period decreased with increasing free ammonia concentration particularly among parent rotifers, but with the addition of GABA, the r of parent rotifers at 3.1 ppm free ammonia was significantly higher than the untreated rotifers (Table 5). Higher lifespan and reproductive period
21
were observed in the F of GABA-treated rotifers at 2.41 mg ml and in the F at 3.12 mg
21 21
ml . On the other hand, at 2.4mg ml free ammonia, the F of GH-treated rotifers had1
W
Intrinsic rate of increase (r), net reproduction rate (Ro), lifespan, and reproductive period of B. plicatilis treated with GH (0.0025 I.U. ml ) or GABA (50mg ml )
a
at different food levels, compared with the controls. N58 for each treatment
Food density r Ro Lifespan (days) Reproductive period (days)
21
(cells ml ) Control GH GABA Control GH GABA Control GH GABA Control GH GABA
Table 4
21
Generation time and lorica size at first offspring of B. plicatilis treated with GH (0.0025 I.U. ml ) or GABA
21 a
(50mg ml ) at different food levels
Food density Time to first egg Time to first offspring Size at first offspring
(h) (h)
Length Width
(mm)
6 21
7310 cells ml
Control 26.9 (0.5) 48.5 (0.8) 301.0 (2.8) 199.0 (1.8)
GH 24.7 (0.5)** 46.2 (0.4)** 305.8 (4.2) 200.8 (2.9)
GABA 27.1 (0.6) 50.2 (1.0) 292.2 (4.5) 196.2 (1.8)
5 21
7310 cells ml
Control 33.7 (0.7) 56.3 (0.9) 287.3 (3.5) 190.0 (2.0)
GH 33.3 (0.7) 55.1 (0.9) 288.3 (2.2) 186.7 (2.0)
GABA 30.3 (1.4)* 52.0 (1.1)** 284.7 (4.2) 185.0 (3.3)
a
Values are mean and standard error (in parenthesis) of 6-8 replicates females per treatment. Level of significant difference with the control: *P,0.05, **P,0.01.
4. Discussion
4.1. Effective hormone concentration
Consistent with the batch culture experiment results (Gallardo et al., 1997), GH at
21
0.0025 and 0.025 I.U. ml resulted in significantly higher fecundity of F rotifers from1
isolated females. However, since there was no significant difference in r values, GH did not have an effect on developmental time. The lower fecundity and reproductive period
21
of parent rotifers treated with 0.25 I.U. ml , indicate that this concentration is too high for rotifers in individual cultures. In the succeeding food and ammonia experiments, GH
21
at 0.0025 I.U. ml was used to determine if a lower concentration of GH could still produce significant effects in the presence of stress.
In the case of GABA, it was surprising not to obtain a significant result with 50
21
W
.G
.
Gallardo
et
al
.
/
J.
Exp
.
Mar
.
Biol
.
Ecol
.
240
(1999
)
179
–
191
187
Table 5
21 21
Intrinsic rate of increase (r), net reproduction rate (Ro), lifespan, and reproductive period of B. plicatilis treated with GH (0.0025 I.U. ml ) or GABA (50mg ml )
a
at different levels of free ammonia, compared with the controls (in parenthesis). N58 per treatment
Free GH GABA
ammonia
21
(mg ml ) r Ro Lifespan (days) Reproductive r Ro Lifespan (days) Reproductive
period (days) period (days)
Parent
0 0.95 (0.96) 22.8 (21.1) 11.2 (11.0) 9.6 (9.5) 0.79 (0.81) 19.5 (25.3) 9.4 (10.9) 7.5 (7.9) 2.4 0.95 (0.90) 15.4 (15.9) 8.2 (10.5) 7.5 (8.1) 0.80 (0.75) 20.7 (23.3) 9.0 (11.8) 7.4 (7.6) 3.1 0.96 (0.90) 19.6 (17.4) 11.4 (11.0) 10.0 (8.4) 0.78 (0.69)** 19.5 (17.6) 9.9 (9.1) 7.6 (7.3) F1
0 0.78 (0.86) 23.4 (23.1) 11.9 (12.9) 10.2 (10.0) 0.88 (0.91) 25.0 (25.8) 10.3 (10.5) 7.9 (7.5) 2.4 0.72 (0.63)* 20.9 (16.0) 13.1 (11.9) 11.6 (10.8) 0.77 (0.75) 23.8 (18.9) 12.9 (9.8)* 10.5 (7.6)** 3.1 0.73 (0.68) 16.9 (16.4) 9.9 (11.2) 9.0 (10.4) 0.80 (0.83) 25.1 (22.8) 11.4 (9.8) 10.0 (7.9) F2
0 0.73 (0.76) 19.2 (17.2) 11.1 (9.8) 9.5 (8.8) 0.82 (0.83) 25.1 (25.0) 10.8 (11.6) 8.4 (8.4) 2.4 0.59 (0.62) 15.9 (16.2) 12.8 (12.0) 11.0 (11.5) 0.72 (0.72) 24.6 (19.6) 12.4 (10.9) 10.4 (8.7) 3.1 0.60 (0.56) 11.1 (12.5) 9.6 (10.4) 7.8 (9.9) 0.72 (0.76) 22.4 (18.0) 12.5 (10.0)* 10.4 (8.3)
a
4.2. Effect of hormone at different food levels
21
Growth hormone at 0.0025 I.U. ml was effective when food level was at its optimum, but not when it was low. On the other hand, GABA is effective under limited food condition as shown by the higher Ro and r values of the F of GABA-treated1
5 21 6 21
rotifers at 7310 cells ml . The higher r values of P and F at 71 310 cells ml imply that GABA may also be effective in optimum food conditions, but this result is not consistent with the results of our other experiments on GABA, (i.e. effective hormone concentration, generation time, and free ammonia experiments) wherein the r
6 21
values or generation of rotifers at 7310 cells ml (control) were not significantly different from other treatments. In the food experiment, even if production of offspring
6 21
by GABA-treated rotifers at 7310 cells ml was earlier, as shown by the higher r value, there was no significant effect on fecundity. Thus it can be concluded that GABA is effective in enhancing rotifer reproduction only when food level is below the optimum. Furthermore, the seemingly inconsistent result may be explained by the possible differences in qualitative and quantitative composition of the microbial flora which differ in their GABA utilization patterns, pathogenic activity or other possible interference mechanisms (Searcy-Bernal et al., 1992).
It can be noted that effects on r become more significant at lower food densities in later generations. Without GABA treatment, rotifer reproduction rate is significantly lower at low food level and this agrees with the fact that at limited food condition rotifers invest limited food resources into body growth rather than egg production (Duncan, 1989). However, with GABA treatment, reproduction is significantly increased although not to the level of fecundity observed at optimal food levels.
The results of the experiment to determine the effect of hormone on generation time further confirm our findings that GH is effective at optimum food level whereas GABA is effective at lower food levels. The shorter generation time of GH-treated rotifers at
6 21
optimum food level (7310 cells ml ) and GABA-treated rotifers at low food level
5 21
(7310 cells ml ) confirms and explains the high r values obtained in the previous food experiment. In mass cultures, 2–3 h shorter generation time even under food limitation would have a considerable effect on population growth rate.
Snell and King (1977) demonstrated that higher fecundity results in shorter lifespan because resources directed towards reproduction reduce those available for growth and maintenance. However, in our experiment, GH-treated rotifers had higher fecundity, but lifespan was similar to or sometimes longer than that of the untreated rotifers, implying that GH increases rotifer reproduction and survival. This result would be beneficial to culturists since higher population growth and longer lifespan result in a longer culture period with larger population size. On the other hand, GABA-treated rotifers had higher
6 21
fecundity but shorter lifespan at 7310 cells ml , agreeing with the generalization that
5 21
higher fecundity causes shorter mean lifespan. At lower food levels (7310 cells ml ) which is realistic in mass cultures, GABA resulted in higher fecundity.
W.G. Gallardo et al. / J. Exp. Mar. Biol. Ecol. 240 (1999) 179 –191 189
4.3. Effect of hormone at different free ammonia levels
With the addition of free ammonia into the culture medium, GH was effective at 2.4
21 21
mg ml free ammonia but not at 3.1mg ml . Yu and Hirayama (1986) reported that
21
2.2 mg ml is the no-observed-effect concentration (NOEC) and the highest free
21
ammonia concentration observed in rotifer culture tanks was 3.6 mg ml . Our results imply that GH does not mitigate the toxicity of higher free ammonia concentrations in
21
the culture medium. The effect of GABA at 3.1mg ml free ammonia nitrogen further supports our hypothesis that GABA is effective in the presence of stressors. Snell et al.
21
(1987) reported that at 3.2 mg ml free ammonia, rotifer swimming activity is significantly reduced. In the food experiment, GABA had an effect on r and Ro even in the F generation, whereas GH had an effect only through the F , implying that GABA2 1
may be longer lasting than GH.
Significant effects were observed in F and in some cases in F , even though these1 2
animals were not exposed to hormones. GH and GABA hormones could have affected the eggs (F ) carried by maternal females treated with hormone. In most cases, however,1
the effect was not observed in F , implying that the GH and GABA effect diminishes2
after a few generations. Further experimentation is required to answer the question of whether GH- or GABA-treated rotifers would have effects on fish larvae.
As stated in our earlier paper (Gallardo et al., 1997), the GH effect may be direct or indirect. There is a possibility that before absorption by the rotifer, GH which is a large protein (19.5–22 kDa), may be cleaved by proteolytic enzymes from bacteria in the culture or enzymes in the rotifer digestive tract. However, if B. plicatilis has a receptor for GH, it could have a direct effect. The presence of GH or GH-like substance in rotifers will be determined in future research using immunoblots. Similarly, GABA’s effect may either be direct or indirect. As a direct effect, GABA which is an amino acid derivative, may serve as a nutrient for rotifers, or as suggested by Morse (1984), GABA may increase the efficiency of assimilation and utilization of nutrients, thus enhancing reproduction. Acting indirectly, GABA may be utilized or accumulated by the alga N.
oculata on which rotifers feed. It is also possible that GABA induces the secretion of
GH in B. plicatilis as it has been found in rats (Abe et al., 1977; Acs et al., 1984; Murakami, 1985; Mergl et al., 1995), sheep (Spencer et al., 1994) and man (Cavagnini et al., 1980a,b; Koulu et al., 1980; Steardo et al., 1986). Although rotifers are phylogenetically far from mammals, this mechanism is worth examining especially if endogenously-produced GH is present in B. plicatilis. It is plausible that GH is present in rotifers because it has been found in the snail Lymnea stagnalis (Dogterom and Jentjens, 1980; Dogterom and Robles, 1980; Dogterom et al., 1979) and abalone
Haliotis discus hannai (Moriyama et al., 1989, cited by Taylor et al., 1996). Likewise
GABA may be widely present in invertebrates. GABA receptors have been found in crayfish (Pearstein et al., 1997).
Acknowledgements
9-383M) from The Japan Science Society to W.G. Gallardo. The Japanese Government is also gratefully acknowledged for the Monbusho scholarship to W.G. Gallardo for his Ph.D. studies.
References
Abe, H., Kato, Y., Chihara, K., Ohgo, S., Iwasaki, Y., 1977. Growth hormone release by gamma-aminobutyric acid (GABA) and gamma-amino-beta-hydroxybutyric acid (GABOB) in the rat. Endocrinology (Japan) 24, 229–231.
Acs, Z., Makara, G.B., Stark, E., 1984. Growth hormone secretion of the neonatal rat pituitaries is stimulated by gamma-aminobutyric acid in vitro. Life Sci. 34, 1505–1511.
Balompapueng, M.D., Hagiwara, A., Nishi, A., Imaizumi, K., Hirayama, K., 1997. Resting egg formation of the rotifer Brachionus plicatilis using a semi-continuous culture method. Fisheries Sci. 63, 236–241. Birch, L.C., 1960. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 17, 15–26. Cavagnini, F., Benetti, G., Invitti, C. et al., 1980. Effect of gamma-aminobutyric acid on growth hormone and
prolactin secretion in man: influence of pimozide and domperidone. J. Clin. Endocrinol. Metab. 51, 789–792.
Cavagnini, F., Invitti, C., Pinto, M. et al., 1980. Effect of acute and repeated administration of gamma aminobutyric acid (GABA) on growth hormone and prolaction secretion in man. Acta Endocrinol. (Copenh) 93, 149–154.
Dogterom, A.A., Jentjens, T., 1980. The effect of the growth hormone of the pond snail Lymnaea stagnalis on periostracum formation. Comp. Biochem. Physiol. 66A, 687–690.
Dogterom, A.A., Robles, B.R., 1980. Stimulation of ornithine decarboxylase activity in Lymnaea stagnalis after a single injection with molluscan growth hormone. Gen. Comp. Endocrinol. 40, 238–240. Dogterom, A.A., VanLoenhout, H., VanDerSchors, R.C., 1979. The effect of growth hormone of Lymnaea
stagnalis on shell calcification. Gen Comp. Endocrinol. 39, 63–68.
Duncan, A., 1989. Food limitation and body size in the life cycle of planktonic rotifers and cladocerans. Hydrobiologia 186 / 187, 11–28.
Fu, Y., Hirayama, K., Natsukari, Y., 1991. Morphological differences between two types of the rotifer
Brachionus plicatilis O.F. Muller. J. Exp. Mar. Biol. Ecol. 151, 29–41.
Galindo, M.D., Guisande, C., Toja, J., 1993. Reproductive investment of several species. Hydrobiologia 255 / 256, 317–324.
Gallardo, W.G., Hagiwara, A., Tomita, Y., Soyano, K., Snell, T.W., 1997. Effect of some vertebrate and invertebrate hormones on the population growth, mictic female production, and body size of the marine rotifer Brachionus plicatilis Muller. Hydrobiologia 358, 113–120.
Hagiwara, A., Hino, A., Hirano, R., 1988. Effects of temperature and chlorinity on resting egg formation in the rotifer Brachionus plicatilis. Nippon Suisan Gakkaishi 54, 569–575.
King, C.E., Anderson, W.W., 1971. Age-specific selection. II. The interaction between r and K during population growth. Am. Natural. 105, 137–156.
Koulu, M., Lamintausta, R., Dahlstrom, S., 1980. Effect of some gamma-aminobutyric acid (GABA)-ergic drugs on the dopaminergic control of human growth hormone secretion. J. Clin. Endocrinol. Metab. 51, 124–129.
Mergl, Z., Acs, Z., Makara, G.B., 1995. Growth hormone secretion and activation of cyclic AMP by growth hormone releasing hormone and gamma-aminobutyric acid in the neonatal rat pituitary. Life Sci. 56, 579–585.
Moriyama, S., Atsuta, S., Kobayashi, M., Kawauchi, H., 1989. Growth hormone-like substance of abalone
Haliotis discus hannai. In: Epple, A., Scanes, C.G., Stetson, M.H. (Eds.), XIth Int. Symp. Comparative
Endocrinology. Malaga, 14–20 May 1989, (as cited by Taylor et al., 1996).
W.G. Gallardo et al. / J. Exp. Mar. Biol. Ecol. 240 (1999) 179 –191 191 Murakami, Y., 1985. Involvement of growth hormone-releasing factor in growth hormone secretion induced by
gamma-aminobutyric acid in conscious rats. Endocrinology 117, 787–789.
Paynter, K.T., Chen, T.T., 1991. Biological activity of biosynthetic rainbow trout growth hormone in the eastern oyster, Crassostrea virginica. Biol. Bull. 181, 459–462.
Pearstein, E., Cattaert, D., Clarac, F., 1997. Crayfish sensory terminals and motor neurons exhibit two distinct types of GABA receptors. J. Comp. Physiol. 180, 71–79.
Searcy-Bernal, R., Salas-Garza, A.E., Flores-Aguilar, R.A., Hinojosa-Rivera, P.R., 1992. Simulatneous comparison of methods for settlement and metamorphosis induction in the red abalone (Haliotis rufescens). Aquaculture 106, 241–250.
Snell, T.W., King, C.E., 1977. Lifespan and fecundity patterns in rotifers: The cost of reproduction. Evolution 31, 882–890.
Snell, T.W., Childress, M.J., Boyer, E.M., 1987. Assessing the status of rotifer mass cultures. J. World Aquacult. Soc. 18, 270–277.
Spencer, G.S., Berry, C.J., Bass, J.J., 1994. Neuroendocrine regulation of growth hormone in sheep. VII. Effects of GABA. Regul. Pept. 52, 181–186.
Steardo, L., Iovino, M., Monteleone, P., Agrusta, M., Orio, F., 1986. Pharmacological evidence for a dual GABAergic regulation of growth hormone release in humans. Life Sci. 39, 979–985.
Taylor, B.E., Donovan, D.A., McLean, E., Donaldson, E.M., Carefoot, T.H., 1996. Effect of recombinant vertebrate growth hormones on growth of abalone, Haliotis kamtschatkana. Aquaculture 140, 153–158. Walz, N., Sarma, S.S.S., Benker, U., 1995. Egg size in relation to body size in rotifers: an indication of
reproductive strategy? Hydrobiologia 313 / 314, 165–170.