L
Journal of Experimental Marine Biology and Ecology, 240 (1999) 213–228
Hunger rapidly overrides the risk of predation in the subtidal
scavenger Nassarius siquijorensis (Gastropoda: Nassariidae):
an energy budget and a comparison with the intertidal
Nassarius festivus in Hong Kong
*
B. Morton , K. Chan
The Swire Institute of Marine Science, The University of Hong Kong, Cape d’Aguilar, Shek O, Hong Kong Received 4 January 1999; received in revised form 23 April 1999; accepted 1 May 1999
Abstract
This study shows that, as with its intertidal counterpart, Nassarius festivus, the rate at which subtidal Nassarius siquijorensis moves towards food bait is similar for starved and well-fed individuals. This study also investigates another facet of nassariid nutrition related to the degree of hunger, i.e. the effect of simulated predation upon a feeding assemblage. Individuals which fed within 7 days, cease feeding and depart palatable food if crushed conspecifics are added. Between 7 and 13 days since its last meal, however, N. siquijorensis will feed when food is available, despite the possibility of predation. For the intertidal N. festivus, the critical time for hunger to override the risk of predation is between 14 and 21 days. The difference between subtidal and intertidal species may be due to a difference, in terms of days, that a meal can provide for their energy expenditure, particularly with regard to respiration. The bigger, subtidal, N. siquijorensis needs to feed more frequently than the smaller, intertidal, N. festivus. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Carrion; Scavenging; Nassarius; Hong Kong; Energy budget; Predation risk; Respira-tion
1. Introduction
Due to the unpredictable supply of carrion in the sea, there are probably no obligate scavengers, only facultative (opportunistic) species which would normally pursue a
*Corresponding author. Tel.:1852-2809-2179; fax:1852-2809-2197.
E-mail address: swireml@hkucc.hku.hk (B. Morton)
predatory life style (Britton and Morton, 1993, 1994a). Taylor (1980) identified 93.3% of the gut contents of the subtidal nassariid gastropod Nassarius siquijorensis (A. Adams, 1852) in Hong Kong as comprising fish bones and scales and unidentifiable tissue. Later, Taylor and Shin (1990) showed gut contents to comprise 26.7% sediment, 9.7% fish bones and scales, 8.6% unidentifiable tissue, 5.4% crustacean fragments, 18.3% ophiuroid ossicles but also identifiable fragments from a variety of polychaetes, identified by their setae. N. siquijorensis may, thus, consume living prey, i.e. possibly polychaetes and ophiuroids but is, largely, a consumer of carrion. Britton and Morton (1994b) pointed out that subtidal Nassarius mendicus from Monterey Bay, California, recognized and consumed carrion, but had limited powers of distance chemoreception, i.e. from a maximum distance of 10 cm. Liu and Morton (1994) demonstrated that N.
siquijorensis recognized carrion, moved purposefully towards it and consumed it. The
distance for the majority of N. siquijorensis to detect food was 35 cm. N. siquijorensis is dominant in soft subtidal habitats in Hong Kong because of an ability to withstand anoxia for periods of up to 8.5 days (Chan and Morton, 1997) and because of the ready availability of trawler-damaged carrion (Morton, 1996).
Intertidal nassariids live on gently sloping beaches of sand and mud experiencing moderate wave action, a moderate to large tidal range and slowly receding tides. Tidal waters, flowing slowly over stranded carrion pick up chemical cues which nassariids are able to detect. Such scavengers normally lie quiescent in the sediment, only the siphon protruding above it, but emerge quickly once a chemical cue from carrion is received. Distance chemoreception takes them unerringly to its source. They can arrive from considerable distances, i.e. between 1.5 to 2.5 m for small intertidal species, e.g.
Nassarius pyrrhus and Nassarius festivus from Western Australian and Hong Kong
shores, respectively (Morton and Britton, 1991; Britton and Morton, 1992). Contact chemoreception completes the detection process and eversion of the proboscis (the ‘proboscis search reaction’; Kohn, 1983) initiates feeding.
Nassariids move towards food rapidly and consume large quantities quickly, i.e. between 50 and 60% of the body weight for the intertidal Nassarius festivus (Morton, 1990; Cheung, 1994) and 61% of the body weight for the subtidal Nassarius
siquijorensis (Liu and Morton, 1994) in feeding bouts lasting for an average of 8 and 12
min, respectively. After feeding, they depart the food and reburrow quickly. Snails clustered around moribund flesh constitute a potentially attractive source of food for larger predators, e.g. portunid (Stenzler and Atema, 1977) and xanthid crabs (Zipser and Vermeij, 1978), the latter attracted to the leaking body fluids of the object of nassariid interest and thus to the snails themselves.
Tryon (1882) made the first brief mention of the escape reaction of the genus
Nassarius when he stated that some ‘Nassas’ spring up and throw themselves over when
disturbed whilst feeding. When the metapodial tentacles of Nassa (5Nassarius)
reticulata are stimulated by contact with the sea star Astropecten bispinosus, it exhibits a
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 215
chemnitzii. A comparison of the literature for N. luteostoma, N. reticulata, and N.
mutabilis shows that the escape response is somewhat similar in all three species. It
differs only in whether the snail either somersaults or leaps diagonally forward.
Nassarius vibex also utilizes an escape reaction when it is stimulated by either the sea
star Luidia alternata or by the gastropods Fasciolaria hunteria and F. tulipa (Gore, 1966). Other examples of escape responses among gastropods are seen when potential prey comes into close proximity with a predator (Field, 1977; Parsons and Macmillan, 1979; Vermeij et al., 1987; Dix and Hamilton, 1993).
When a Mediterranean trail-following opisthobranch, Haminoea navicula, is either molested or injured, conspecifics following its mucous trail exhibit an alarm response upon encountering the location where the trauma occurred (Cimino et al., 1991). Species of the sand-dwelling gastropod Umbonium perform twisting and leaping movements in the presence of potential predators (Kiruchi and Dol, 1987). The presence of either conspecific tissues or body fluids in water may also initiate either an escape or an alarm response. Within 10 min after introduction of water containing body fluids of crushed conspecifics, a significant number of tide-pool inhabiting Littorina littorea moved to shelter, with some quadrupling their crawling speed immediately after exposure to the
contaminated water (Hadlock, 1980). The mud snail Nassarius obsoletus [5Ilyanassa
obsoleta] moves toward and feeds upon crushed mussels (Modiolus [Geukensia] demissus) and snails (Littorina littorea), but departs rapidly when placed in the vicinity
of crushed conspecifics (Atema and Burd, 1975). Stenzler and Atema (1977) expanded these observations by showing that three species, i.e. N. obsoletus, Nassarius vibex and
Nassarius trivittatus, all responded negatively to crushed conspecifics, but that the
escape response was diminished by hunger.
This study was designed to determine: (a) whether or not Nassarius siquijorensis possesses either an alarm or escape response to the presence of dead conspecifics; (b) if so, how long after introduction of crushed conspecifics to a feeding group do individuals invoke such a response, and (c) to what extent can an alarm response be over-ridden by hunger, as expressed by 28 days starvation post-satiation. A similar study has been carried out with the intertidal species, Nassarius festivus (Morton et al., 1995), so that the last objective (d) was to compare these subtidal and intertidal species and to obtain an explanation for any differences identified in terms of energy expenditure.
2. Materials and methods
2.1. Feeding experiments
For each feeding experiment, six sets of 40 individuals of Nassarius siquijorensis, i.e.
a total of 240 individuals, were placed in 40 cm340 cm experimental trays with
non-flowing seawater. The 40 individuals in each tray were arranged in a 24-cm-diameter circle around three shrimps placed in the centre. For each of the six trays, the numbers of individuals feeding upon the shrimps were recorded each minute for 40 min. When 20 (50%) individuals had arrived at the bait, 5 g of coarse, shell, sand was sprinkled upon two of the feeding groups to simulate the mechanical but not chemical part of the stimulus introduction (Stenzler and Atema, 1977). For the second two feeding groups, the bodies of 10 crushed Monodonta labio (an intertidal gastropod N.
siquijorensis would never have encountered and to simulate non-conspecific predation)
were scattered upon the feeding nassariids. For the third two feeding groups, the bodies of 10 crushed N. siquijorensis (to simulate conspecific predation) were scattered upon them. Regardless of whether sand, crushed Monodonta or crushed conspecifics was added to each feeding cluster, feeding individuals were permitted to feed without other disturbance for the duration of the experiment, i.e. 40 min.
Because few satiated Nassarius siquijorensis were attracted to the bait at time zero, a second experiment was conducted using different individuals in which six groups of 20
N. siquijorensis were placed initially upon the shrimps. After all individuals were so
situated, two control groups received sand, two more groups received 10 crushed
Monodonta while the two final groups received the bodies of 10 crushed N.
siqui-jorensis. In this case, the numbers of individuals remaining at the site of the bait, but not
necessarily feeding, were recorded every minute for 40 min.
From these experiments it has been possible to calculate the times taken for 50% of each group of control and experimental individuals to (a) find the bait and (b) ascertain how long they remained feeding upon it, if at all, when sand or either crushed
Monodonta or crushed conspecifics were added after increasingly long periods of
starvation.
2.2. Activity
Forty individuals of Nassarius siquijorensis were each placed in separate 55-ml pots containing seawater and sand into which they could burrow. One group of 20 individuals was fed daily to satiation and the other group was starved for 8 days. The activity pattern of each individual was recorded from 06:00 to 16:00 h. Observations were made for 10 min each hour and the times spent moving, resting and buried in the sand in each observation period was recorded for each individual. The mean % time spent moving / individual in the two groups in a 10-h cycle was compared by a Mann–Whitney rank sum test using arcsine transformed data (Zar, 1984).
2.3. Respiration rate
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 217
seawater and covered underwater with lids without trapping any air bubbles. All vials
were placed in a large tank filled with seawater at a temperature of 208C (the average
sea-bed temperature in Hong Kong). The N. siquijorensis were forced to move continuously by inverting the vials when they reached the tops. The experiment lasted for 20 min so that dissolved oxygen levels inside the vials would not fall below 30% saturation. Dissolved oxygen levels in each vial were measured by a YSI model 33 S-C-T oxygen meter at the start and at the end of the experiment. Ten more vials were filled with seawater without N. siquijorensis and served as controls.
Ten individuals of Nassarius siquijorensis (shell lengths of between 10.9 and 18.4 mm) were put into separate 55-ml plastic vials. Sand from the beach outside the Swire
Institute of Marine Science was dried in an oven at 808C for 2 days to halt microbial
activity and 16.5 g were added to each vial. The experiment started only when all individuals were buried with their siphons protruded. The determination of respiration rate was as above. Differences in respiration rate during resting and moving were compared by a Student t-test. Energy expended on respiration was calculated using a
21
conversion factor of 13.98 J mg O2 (Ivlev, 1934).
3. Results
3.1. Feeding experiments
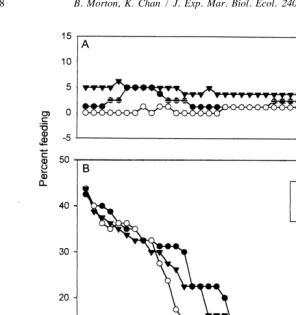
At the start of the feeding experiment, satiated Nassarius siquijorensis were clearly not interested in the food provided (Fig. 1). When afforded the opportunity to seek a further meal, on day zero, typically less than 5% did feed (Fig. 1A). Even when placed upon the shrimps, many moved off them (Fig. 1B). Although the presence of crushed conspecifics at the bait site may have helped drive away members of this group, illustrated in Fig. 1B by downward-pointing triangles, there was no significant difference between these and the control individuals receiving sprinkled sand and crushed
Monodonta (closed and open circles, respectively) with respect to the numbers
remaining in the vicinity of the bait (Wilcoxon matched pairs signed ranks test,
P50.0652).
The speed at which experimental and control groups of Nassarius siquijorensis approached the food is given in Table 1. Analysis of variance (ANOVA) indicated no
significant difference between two or more of the groups at the a 50.05 level with
respect to rate of movement towards the food prior to additions of either sand, crushed
Monodonta or crushed conspecifics (df53, F51.37, P50.321). Despite the variance
between some groups, when movement rates for experimental and control groups are
21 21
combined (Table 1, Grand means), the mean values of 4.60 cm min , 2.06 cm min
21
and 4.50 cm min for all individuals, indicate that there was no difference in the
attractiveness of the bait between experimental and control groups, prior to the addition of crushed conspecifics, crushed Monodonta and sand, respectively.
Fig. 1. Feeding responses of recently (within 2 h) satiated Nassarius siquijorensis. (A) Percentage numbers of individuals reaching the bait from a distance of 24 cm. (B) Percentage numbers of individuals remaining at the bait, but not necessarily feeding, after being placed upon it. Filled triangles represent individuals exposed to crushed conspecifics and food; unfilled circles represent individuals exposed to crushed Monodonta and filled circles represent individuals exposed to sand.
Table 1
21
Mean rates (cm min ) at which 50% of experimental, Monodonta and control groups of Nassarius
siquijorensis moved towards the bait, after 7, 14, 21 and 28-day periods of starvation
Day Control Monodonta Experimental
groups group group
7 2.00 1.71 2.40
14 4.00 2.40 6.00
21 6.00 2.40 6.00
28 6.00 1.71 4.00
B
.
Morton
,
K
.
Chan
/
J.
Exp
.
Mar
.
Biol
.
Ecol
.
240
(1999
)
213
–
228
219
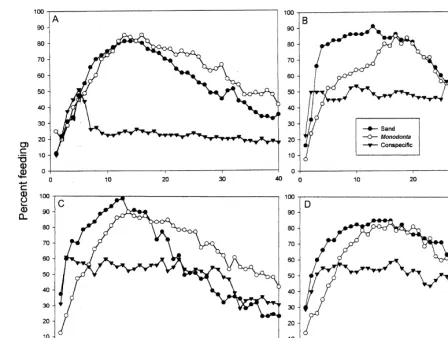
Fig. 2. Feeding responses of Nassarius siquijorensis after: (A) 7 days; (B) 14 days; (C) 21 days and (D) 28 days post initial satiation. (All experimental groups were placed 24 cm from the bait at time 0.) Filled triangles represent individuals exposed to crushed conspecifics after 50% of each group commenced feeding. Closed circles represent individuals exposed to sprinkled sand after 50% of each group commenced feeding and open circles represent individuals exposed to crushed
of 20 experimental animals (50%) arrived at the bait on day 7, with this feeding density lasting less than 1 min. When crushed conspecific tissue was added, individuals began to move off it immediately. Three minutes later, 11 individuals (27%) of the experimental groups remained feeding whereas 5 min later, nine individuals (22.5%) were still at the bait. Animals continued to leave the bait until the end of the experiment. A mean maximum of 32.5 individuals (81.25%) of the sand and 34 (85.0%) of the crushed
Monodonta control groups clustered around the bait. The feeding density in these two
groups remained at .20 individuals for 21 min after addition of sand and 26 min after
addition of crushed Monodonta. An average of 14 (35.0%) feeding individuals remained in the sand control group even at the end of the experiment (40 min), as did 16.5 (41.25%) of the Monodonta control group.
On day 14, the mean arrival time at the food for 50% of the individuals was 2 min for the sand control group and 2 and 5 min for the conspecific and Monodonta control groups, respectively (Fig. 2B). The addition of crushed conspecifics to the experimental groups did not, however, elicit an escape response, as it had on day 7. A mean maximum of 22.5 conspecific influenced animals (56.25%) clustered about the bait on day 14, with feeding densities fluctuating around 50% of the test animals remaining after 27 min. Similarly, a mean maximum of 36.5 sand control and 34 Monodonta influenced individuals (91.25 and 85.0%) clustered around the bait, with feeding densities of
.50% remaining after 28 and 29 min, respectively.
On day 21, the mean arrival time at the food by 50% of the individuals was 2 min for the sand control group and 2 and 5 min for the conspecific and Monodonta influenced groups, respectively (Fig. 2C). As on day 14, feeding individuals in the experimental groups did not depart the bait when crushed conspecifics were added to it. A maximum of 24 experimental animals (60.0%) clustered about the bait on day 21, with feeding
densities of .50% of the test animals remaining after 27 min. A mean maximum of
39.5 sand control individuals (98.75%) and 36 (90.9%) of the Monodonta influenced
individuals clustered around the bait, with feeding densities of .50% remaining for 23
and 32 min, respectively.
On day 28, the mean arrival time at the food for 50% of the sand control individuals was 2 min and 3 and 7 min, respectively for the conspecific and Monodonta influenced groups (Fig. 2D). As on the previous days, the addition of crushed conspecifics to the experimental groups did not elicit an escape response. A mean maximum of 24 experimental animals (60.0%) clustered around the bait on day 28, with a feeding
density of .50% of the test animals remaining after 18 min. Similarly, a mean
maximum of 34 sand control and 33.5 Monodonta influenced individuals (85.0% and
83.75%) clustered around the bait, with feeding densities of .50% remaining after 28
and 27 min, respectively.
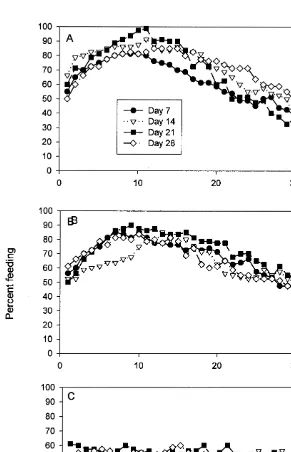
Two different patterns become clear when the data are re-organized and plotted from the times either sand or crushed Monodonta or crushed conspecifics were added to the control and experimental groups, respectively (Fig. 3). First, the responses of the sand-sprinkled group to the presence of food in the absence of crushed conspecifics or
Monodonta tissue appear variable but, in fact, suggest a simple pattern. One week
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 221
control individuals spent time feeding than they did on day 7 (Fig. 3A, downward-pointing triangles). By day 21, the majority of the group had gone 2 weeks without feeding, which is reflected in the highest (almost 100%) percentage number of individuals joining the feeding cluster on this day (Fig. 3A, squares). Finally, by day 28 (Fig. 3A, diamonds), there was a further, slight, decreased interest in feeding.
A similar pattern to the above was seen with the feeding individuals exposed to crushed Monodonta. One week following the ‘fed to satiation’ meal, i.e. day 7, there was a renewed interest in feeding again (Fig. 3B, circles). By day 14, fewer individuals initially showed an interest in feeding again (Fig. 3B, downward pointing triangles) but
did return to the food eventually. By day 21, most individuals (|90%) showed an
interest in feeding (Fig. 3B, squares) and by day 28 (Fig. 3B, diamonds), the same numbers as seen on day 7 were feeding.
The second pattern is even clearer. With the addition of crushed conspecifics to the feeding cluster on day 7 (Fig. 3C, circles), a significant percentage of Nassarius
siquijorensis left it. As this did not happen with the control and Monodonta influenced
groups, we must assume that the addition of damaged nassariid tissue triggered an alarm response in some feeding individuals. By day 14, hunger overrode the risk of predation, suggested by the presence of crushed conspecifics. Even with the addition of conspecific tissue, however, the feeding clusters on day 14 (Fig. 3C, downward pointing triangles) remained on the food. A similar pattern was repeated on days 21 and 28 (Fig. 3C, squares and diamonds, respectively). The percentages of N. siquijorensis remaining on the food, even after 14, 21 and 28 days were, overall, well below the numbers remaining
on the sand control and crushed Monodonta-influenced food on the same days (|50–
60% versus 80–100%).
3.2. Activity
Well-fed individuals of Nassarius siquijorensis were more active than starved individuals. Three out of 20 individuals moved within the 10 h of the study period while only one of the starved individuals was active. For the rest of the time, however, both the well-fed and starved individuals remained resting and buried in the sand. The mean
% time spent moving per individual was 1.563.67 and 0.562.24 for well-fed and
starved individuals, respectively. A Mann–Whitney rank sum test, however, showed no
significant difference between the two groups (T5430, P50.595), that is, regardless of
its level of hunger, N. siquijorensis is an ‘await’ scavenger. It rarely forages.
3.3. Respiration rate
The mean respiration rate of resting individuals of Nassarius siquijorensis
21 21
(0.12560.0407 mg O h individual ) was significantly lower (T51211.0, P,
2
21 21
0.0001) than moving individuals (0.18760.0479 mg O h individual ). When dry
2
tissue weights were used in calculating respiration rate, the mean values for resting and 21 21
moving individuals were 0.07060.022 mg O h g dry weight and 0.09960.023 mg
2 21 21
O h2 g dry weight, respectively. There was, again, a significant difference between
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 223
3.4. Energy budget
As no data on absorption efficiency and energy expenditure on mucus production and excretion have been obtained, it is not possible to calculate a complete energy budget for
Nassarius siquijorensis. Notwithstanding, the great majority, |80%, of nassariid energy
is thought to be spent on respiration, hence their typically quiescent life style in the absence of food (Cheung, 1994). The calculation used to determine energy expended on
21
respiration (J day ) was as follows:
21 21
Energy expended on respiration day individual when moving
5Respiration rate3conversion factor3% time moving324 h
21 21 21
Energy expended on respiration day individual when buried
5Respiration rate3conversion factor3% time buried324 h
21 21 21
50.125 mg O h individual 313.98 J mg O 30.985324 h
2 2
21 21
541.31 J day individual
The total energy expended on respiration by Nassarius siquijorensis was, thus, 42.25 J
21 21
individual day of which 97.78% was expended when buried, the remainder whilst
moving (Table 2).
Liu and Morton (1994) have calculated for Nassarius siquijorensis the mean time 21
spent feeding on a single meal (19.14 min individual ) and the mean amount of food
21
eaten during a single meal individual (0.090 g dry weight). The mean dry tissue
weight of N. siquijorensis of the same size range as used in this experiment was calculated to be 0.335 g and using the above information the mean consumption rate
21 21 21
min can, therefore, be calculated as 0.014 g dry weight g dry weight min .
21
The energy intake (J meal ) for Nassarius siquijorensis was calculated as follows:
21
mean calorific value of shrimp (Penaeus setiferus; 80 kcal 100 g (Spotte, 1992), i.e. 1
21
cal54.1868 J)3mean consumption rate3feeding time meal (min)5(80 000 / 100)3
21 21 21
4.1868 J g 30.014 g dry weight g dry weight min 30.335 g dry weight319.14
21
min5300.67 J meal .
Table 2
The energy budget of Nassarius siquijorensis
Buried Moving
Percentage of time spent (%) 98.50 1.50
21 21
Respiration rate (mg O h2 individual ) 0.125 0.187
21
Energy expended on respiration (J day ) 41.31 0.94 42.25 (Total)
21 a
Mean feeding time meal (min) 19.14
21
Energy intake (J meal ) 300.67
a
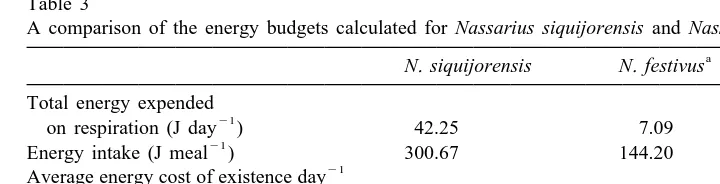
Table 3
A comparison of the energy budgets calculated for Nassarius siquijorensis and Nassarius festivus a
N. siquijorensis N. festivus Total energy expended
21
on respiration (J day ) 42.25 7.09
21
Energy intake (J meal ) 300.67 144.20
21 Average energy cost of existence day
21
(% of the energy ingested meal ) 14 5
Ingested food containing the energy
needed for 24 h of existence (times) 7 20
a
After Cheung (1994).
21
The average energy cost of existence day is thus: (41.3110.94) / 300.6731005
21
14.05% of the energy ingested meal .
21 21
Energy intake for Nassarius siquijorensis was, thus, 300.67 J individual meal .
The energy intake during a single meal can, therefore, provide seven times the energy required for survival. Table 3 compares the calculated energy budgets for Nassarius
siquijorensis and Nassarius festivus (the latter after Cheung, 1994). N. siquijorensis
expended more energy on respiration than N. festivus. Although the energy intake for N.
siquijorensis is higher than that for N. festivus, the average energy cost of existence
21
day is higher for the former than the latter. At a single meal, N. siquijorensis can
ingest food containing seven times the energy needed for 24 h of existence while N.
festivus can ingest food containing 20 times.
4. Discussion
Like its intertidal counterpart, Nassarius festivus, this study suggests that, for
Nassarius siquijorensis, hunger is not reflected in speed to food, that is, individuals
always move at a relatively constant rate. The time taken for 50% of the experimental animals to reach the food, however, differed with the state of hunger. This observation is, we believe, a measure of an individual’s decision about whether or not to seek the sensed food. It thus seems that each individual responds to the scent of food and makes a decision about whether or not to find it. This is probably related to the strength of the stimulus, as a reflection of its proximity, and the degree of individual hunger.
When nassariids feed, they do so to satiation and voraciously. Morton (1990) and Cheung (1994) estimated that Nassarius festivus eats between 50 and 60% of its body weight in a single meal, as does the subtidal Nassarius siquijorensis (Liu and Morton, 1994). Once fed to satiation, however, subsequent meals are smaller (Liu and Morton, 1994; Cheung, 1994). Nassariids thus have degrees of hunger. Cheung (1994) has calculated that for N. festivus fed to satiation, the energy obtained would provide it with
|20 days of expenditure. The energy obtained for N. siquijorensis, however, would
provide it with only |7 days of expenditure. It thus seems that the subtidal N.
siquijorensis needs to feed more frequently than the intertidal N. festivus. Since
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 225
beaches (Brown, 1971, 1982), most metabolic energy is required for respiration while quiescent. Cheung (1997), however, showed that the oxygen consumption rate of active
N. siquijorensis was 74% higher than for resting animals and an equivalent figure of
50% has been obtained in this study. Nassariids can survive periods of starvation much greater than 20 days, i.e. at least 100 days for N. festivus (Morton, 1990). Nassarius
obsoletus similarly can survive up to 120 days without food (Curtis and Hurd, 1979)
although this species is an obligate omnivore, requiring both plant and animal material in its diet if growth and reproduction are to be maintained (Brown, 1982). There is no estimate of the maximum number of days that N. siquijorensis can survive without food, but a recent study of the tolerance of this species to anoxia showed that it can survive
periods of starvation for .30 days (Chan and Morton, 1997).
The proximity of dead and dying conspecifics surely signals the likely presence of a predator at a point food source, as was demonstrated for the tide-pool-inhabiting
Littorina littorea (Hadlock, 1980). Experimental re-creation of this scenario by Atema
and Burd (1975) and Stenzler and Atema (1977) for Nassarius obsoletus [5Ilyanassa
obsoleta] also suggested this was true. The latter authors also showed that when
nassariids were denied food for several days and then fed carrion, they were markedly less-inclined to leave the meal in the presence of water exposed to crushed conspecifics than well-fed individuals presented with the same choice. The study by Morton et al. (1995) extended the scope of the above scenario and showed that for starvation periods of up to 14 days there was a marked propensity for Nassarius festivus to cease feeding. Following 21 and 28 days starvation, however, individuals continued to feed, despite the presence of crushed conspecifics. The critical time for hunger to override the perceived risk of predation for this species was thus deemed to lie between 14 and 21 days. In this study, it has been shown that for periods of starvation of up to 7 days, Nassarius
siquijorensis would cease feeding if crushed conspecifics were added to the food, i.e.
they would leave the meal in the likelihood of the presence of a predator suggested by the proximity of dead and dying conspecifics. After 14 days of starvation, however, hunger overrode the perceived risk of predation and individuals continued to feed despite the presence of crushed conspecifics. The critical time for hunger to override the perceived risk of predation for this species thus seems to lie between 7 and 14 days.
The above laboratory studies have been confirmed in the field for seven species of Australian nassariid and buccinid intertidal scavengers (McKillup and McKillup, 1995) with supporting evidence from laboratory studies (McKillup and McKillup, 1994, 1995). These authors argue that food availability influences not only scavenger growth and reproduction but also population size. Assuming that the availability of carrion is naturally limiting (Britton and Morton, 1994b), it is likely that hunger will typically override the risk of predation, at least intertidally.
The difference discerned here between the subtidal Nassarius siquijorensis and the intertidal Nassarius festivus and their reactions to the presence of dead conspecifics is probably due to a difference in the days that a meal can provide for their respective energy expenditures. Table 3 shows that the total amounts of energy expended on
21
respiration by N. siquijorensis and N. festivus are 42.56 and 7.09 J day , respectively.
difference only being detected when, in the former situation, the mantle cavity is drained of water (Houlihan, 1979). N. festivus is a middle to low shore species that, by burrowing, is always immersed (Britton and Morton, 1992). This suggests that the difference between the two species is a real one and that N. siquijorensis has a six times higher respiration rate than N. festivus. Although the average energy intake during a
21 21
meal is 300.67 J meal , the average energy cost of existence day is |14% of the
21
energy ingested meal , which implies that the animal can, at a single meal, ingest food
containing only seven times the energy needed for 24 h survival. Conversely, N. festivus can ingest food containing 20 times the energy needed for 24 h survival (Cheung, 1994). A similar value (18 times) was obtained for the similarly intertidal Bullia digitalis (Brown, 1981). It thus seems that the subtidal N. siquijorensis needs to feed more frequently than the intertidal N. festivus. The subtidal Nassarius mendicus, from California, fed more frequently than the intertidal Bullia digitalis from South Africa, the former averaging 3.14 days (Britton and Morton, 1994b) between meals, whereas the latter fed every 7–10 days (Stenton-Dozey and Brown, 1988). Liu and Morton (1994) showed that starvation caused N. siquijorensis to feed for longer and to find food more quickly. This was also shown on days 14 and 21 of this study. Fig. 2B and C have steeper initial slopes (black triangles) than Fig. 2A, i.e. the food was found more quickly by more individuals and a higher percentage of them remained feeding with time.
Although the length of time nassariids feed is a reflection of the time since the last meal and the degree of achieved satiation at it, it may also be a reflection of the individual’s hunger-driven need to consume, opportunistically, what it can in order to survive in the face of potential predation. For a nassariid, therefore, there are both advantages and risks involved in pursuing a scavenging mode of life. The benefit of being able to exploit carrion, albeit an ephemeral food resource, is tempered by two costs: (a) being exposed to possible predation while feeding and (b) the possibility of greater time intervals between meals. Both costs affect survival. The perceived risk of predation overrides hunger when starvation is not an important consequence of abandoning food in the presence of potential predation. Hunger overrides the risk of predation when an important consequence of abandoning a meal is death by starvation, even if continued presence at the food may result in death by predation. In the latter case, the probability of being consumed by a predator is no greater and, likely, less than the probability of starvation, if the meal is abandoned. For scavenging nassariids, hunger overrides the risk of predation when the likely alternative is starvation (Morton et al., 1995). This general conclusion seems to be true for both subtidal, as shown in this study, and intertidal species (Morton et al., 1995). In the former, i.e. N. siquijorensis, however, hunger overrides risk more quickly because it has to feed more often to balance the demands of an energy budget that spends more on respiration than the latter, i.e. N.
festivus.
References
Atema, J., Burd, G.D., 1975. A field study of chemotactic responses of the marine mud snail Nassarius
B. Morton, K. Chan / J. Exp. Mar. Biol. Ecol. 240 (1999) 213 –228 227 Britton, J.C., Morton, B., 1992. The ecology and feeding behaviour of Nassarius festivus (Prosobranchia: Nassariidae) from two Hong Kong bays. In: Morton, B. (Ed.), Proceedings of the IV International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong 1989, Hong Kong University Press, Hong Kong, pp. 394–416.
Britton, J.C., Morton, B., 1993. Are there obligate marine invertebrate scavengers. In: Morton, B. (Ed.), Proceedings of the I International Conference On the Marine Biology of the South China Sea, Hong Kong 1990, Hong Kong University Press, Hong Kong, pp. 357–391.
Britton, J.C., Morton, B., 1994a. Marine carrion and scavengers. Oceanogr. Mar. Biol. Annu. Rev. 32, 369–434.
Britton, J.C., Morton, B., 1994b. Food choice, detection, time spent feeding, and consumption by two species of subtidal Nassariidae from Monterey Bay, California. Veliger 37, 81–92.
Brown, A.C., 1971. The ecology of the sandy beaches of the Cape Peninsula, South Africa. Part 2. The mode of life of Bullia (Gastropoda). Trans. R. Soc. South Africa 39, 281–333.
Brown, A.C., 1981. An estimate of the cost of free existence in the sandy-beach whelk Bullia digitalis (Dillwyn) on the west coast of South Africa. J. Exp. Mar. Biol. Ecol. 49, 51–56.
Brown, A.C., 1982. The biology of sandy-beach whelks of the genus Bullia (Nassariidae). Oceanogr. Mar. Biol. Annu. Rev. 20, 309–361.
Carthy, J.D., 1958. An Introduction to the Behaviour of Invertebrates, George Allen and Unwin, London. Chan, K., Morton, B., 1997. The tolerance of Hong Kong species of Nassariidae (Mollusca: Gastropoda) to
anoxia. In: Morton, B. (Ed.), Proceedings of the V International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong 1995, Hong Kong University Press, Hong Kong, pp. 489–502.
Cheung, S.G., 1994. Feeding behaviour and activity of the scavenging gastropod Nassarius festivus. In: Morton, B. (Ed.), Proceedings of the III International Workshop on the Malacofauna of Hong Kong and Southern China, Hong Kong 1992, Hong Kong University Press, Hong Kong, pp. 327–338.
Cheung, S.G., 1997. Respiration studies of the subtidal Nassarius siquijorensis (Gastropoda: Nassariidae) from Hong Kong. In: Morton, B. (Ed.), Proceedings of the V International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong 1995, Hong Kong University Press, Hong Kong, pp. 391–399.
Cimino, G., Passeggio, A., Sodano, G., Spinella, A., Villani, G., 1991. Alarm pheromones from the Mediterranean opisthobranch Haminoea navicula. Experientia 47, 61–63.
Curtis, L.A., Hurd, L.E., 1979. On the broad nutritional requirements of the mud snail, Ilyanassa (Nassarius)
obsoleta (Say), and its polytrophic role in the food web. J. Exp. Mar. Biol. Ecol. 41, 289–297.
Dix, T.L., Hamilton, P.V., 1993. Chemically mediated escape behavior in the marsh periwinkle Littoraria
irrorata Say. J. Exp. Mar. Biol. Ecol. 166, 135–149.
Field, L.H., 1977. An experimental analysis of the escape response of the gastropod Strombus maculatus. Pac. Sci. 31, 1–11.
Gonor, J.J., 1965. Predator-prey reactions between two marine prosobranch gastropods. Veliger 7, 228–232. Gore, R.H., 1966. Observations on the escape response in Nassarius vibex (Say), (Mollusca: Gastropoda).
Bull. Mar. Sci. 16, 423–434.
Hadlock, R.P., 1980. Alarm response of the intertidal snail Littorina littorea (L.) to predation by the crab
Carcinus maenas (L.). Biol. Bull. 159, 269–279.
Houlihan, D.I., 1979. Respiration in air and water of three mangrove snails. J. Exp. Mar. Biol. Ecol. 41, 143–161.
Innes, I.J., 1981. A review of the effects of temperature on the oxygen consumption of intertidal gastropods. J. Thermal Biol. 6, 249–256.
Ivlev, V.S., 1934. Eine Micromethode zur Bestimming des Kaloriengenalts von Nabrstoffen. Biochem. Zeitsch. 275, 49–55.
Kiruchi, T., Dol, T., 1987. Defensive escape response of two trochid sand snail species of the genus
Umbonium: the effect of species-specific escape response to asteroid predators. Publ. Amakusa Mar. Biol.
Lab. 9, 47–65.
Kohn, A.J., 1961. Chemoreception in gastropod molluscs. Am. Zool. 1, 291–308.
Liu, J.H., Morton, B., 1994. Food choice, detection, time spent feeding, consumption and the effects of starvation on a subtidal scavenger, Nassarius siquijorensis (Gastropoda: Nassariidae), from Hong Kong. In: Morton, B. (Ed.), Proceedings of the III International Workshop on the Malacofauna of Hong Kong and Southern China, Hong Kong 1992, Hong Kong University Press, Hong Kong, pp. 357–375.
McKillup, S.C., McKillup, R.V., 1994. The decision to feed by a scavenger in relation to the risks of predation and starvation. Oecologia 97, 41–48.
McKillup, S.C., McKillup, R.V., 1995. The responses of intertidal scavengers to damaged conspecifics in the field. Mar. Freshwater Behav. Physiol. 27, 49–57.
Morton, B., 1990. The physiology and feeding behaviour of two marine scavenging gastropods in Hong Kong: the subtidal Babylonia lutosa (Lamarck) and the intertidal Nassarius festivus (Powys). J. Moll. Stud. 56, 275–288.
Morton, B., 1996. The subsidiary effects of dredging (and trawling) upon an epibenthic molluscan community in Hong Kong. Mar. Pollut. Bull. 32, 701–710.
Morton, B., Britton, J.C., 1991. Resource partitioning strategies of two sympatric scavenging snails on a sandy beach in Western Australia. In: Wells, F.E., Walker, D.I., Kirkman, H., Lethbridge, R. (Eds.), Proceedings of the III International Marine Biological Workshop: the Marine Flora and Fauna of Albany, Western Australia, Albany 1988, Western Australian Museum, Perth, pp. 579–595.
Morton, B., Chan, K., Britton, J.C., 1995. Hunger overcomes fear in Nassarius festivus, a scavenging gastropod on Hong Kong shores. J. Moll. Stud. 61, 55–63.
Parsons, D.W., Macmillan, D.I., 1979. The escape response of abalone (Mollusca, Prosobranchia, Haliotidae) to predatory gastropods. Mar. Behav. Physiol. 6, 65–82.
Spotte, S., 1992. Captive Seawater Fishes: Science and Technology, John Wiley, New York.
Stenton-Dozey, J.M.E., Brown, A.C., 1988. Feeding, assimilation and scope for growth in the sandy-beach neogastropod Bullia digitalis (Dillwyn). J. Exp. Mar. Biol. Ecol. 119, 253–268.
Stenzler, D., Atema, J., 1977. Alarm response of the marine mud snail Nassarius obsoletus: specificity and behavioral priority. J. Chem. Ecol. 3, 159–172.
Taylor, J.D., 1980. Diets and habitats of shallow water predatory gastropods around Tolo Channel, Hong Kong. In: Morton, B. (Ed.), Proceedings of the I International Workshop on the Malacofauna of Hong Kong and Southern China, Hong Kong 1977, Hong Kong University Press, Hong Kong, pp. 163–180. Taylor, J.D., Shin, P.K.S., 1990. Trawl surveys of sublittoral gastropods in Tolo Channel and Mirs Bay; a
record of change from 1976–1986. In: Morton, B. (Ed.), Proceedings of the II International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong 1986, Hong Kong University Press, Hong Kong, pp. 857–881.
Tryon, G.W., 1882. Manual of Conchology. IV. Nassidae, etc., G.W. Tryon Publisher, Philadelphia. Vermeij, G.J., Lowell, R.B., Walters, L.J., Marks, J.A., 1987. Good hosts and their guests: relations between
trochid gastropods and the epizoic limpet Crepidula adunca. Nautilus 101, 69–74. Zar, J.H., 1984. Biostatistical Analysis, Prentice-Hall, New Jersey.