L
Journal of Experimental Marine Biology and Ecology, 240 (1999) 303–321
Predation by xanthid crabs on early post-settlement
gastropods: the role of prey size, prey density, and habitat
complexity
1 2 *,3
Melody Ray-Culp , Megan Davis , Allan W. Stoner
Caribbean Marine Research Center, 805 E. 46th Place, Vero Beach, FL 32963, USA Received 17 March 1998; received in revised form 27 April 1999; accepted 4 May 1999
Abstract
Small predators in marine benthic communities create a hazardous environment for newly settled invertebrates, especially for the smallest individuals. To explore the effects of predation on a newly settled gastropod, queen conch (Strombus gigas Linnaeus), by a xanthid crab (Mi-cropanope sp.), prey size, prey density, and habitat complexity were manipulated in five laboratory experiments. All crabs .3.1 mm CW killed all conch ,2 mm SL when individual crabs (,14 mm carapace width (CW)) were offered individual conch that were 2–35 days old after metamorphosis (1.2–8.8 mm shell length (SL)). Only 10% of the crabs .5.0 mm CW, however, killed conch that were .5.0 mm SL, suggesting that conch may reach a size refuge from xanthid crabs at 5 mm SL. Furthermore, when given a choice, crabs (4.8 mm CW) preferred smaller conch (2.0 mm SL) to larger (3.7 mm SL), suggesting that 1 week of additional growth in shell length is advantageous to survivorship. Proportional mortality decreased as conch density increased when crabs were offered conch at seven different densities (two to 96 individuals). Crabs proved to be effective predators regardless of the amount of seagrass structure provided in a microcosm experiment, and could consume two conch in 10 s. The high densities of xanthid crabs that occur in the wild, their effectiveness as predators, and their large appetites point to the important role that small predators may potentially play in structuring the population dynamics of their small prey immediately after settlement. 1999 Elsevier Science B.V. All rights reserved.
Keywords: Conch; Predation; Seagrass; Strombus gigas; Xanthid crab
*Corresponding author. Tel.:11-732-872-3129; fax:11-732-872-3128. E-mail address: al.stoner@noaa.gov (A.W. Stoner)
1
Present address: 3033 Sixth Street SW, Vero Beach, FL 32968, USA. 2
Present address: Harbor Branch Oceanographic Institution, Inc., 5600 US 1 North, Ft. Pierce, FL 34946, USA. 3
Present address: Northeast Fisheries Science Center, National Marine Fisheries Service, 74 Magruder Road, Highlands, NJ 07732, USA.
1. Introduction
Predation is an important regulator of the population dynamics of marine invertebrate communities. From the moment a pelagic larva approaches the benthos to settle, it is vulnerable to a host of benthic predators, and the first day of post-settlement life may be exceptionally hazardous for benthic invertebrates (Stoner, 1990; Gosselin and Qian, 1996). Despite the severity of mortality immediately following settlement (often 98– 100%), little is known about this critical period, especially for motile species (Osman and Whitlatch, 1995; Gosselin and Qian, 1997).
Studying early post-settlement processes in the soft-bottom benthic community is problematic because settlers are difficult to detect, extract, or observe non-destructively. Often the magnitude of recruitment is estimated from the abundance of older individuals large enough to be observed, with studies usually reporting findings for juveniles that have already been settled on the benthos for weeks or months (Keough and Downes, 1982; Hunt and Scheibling, 1997). As a result, settlement magnitude and mortality may be underestimated, and observable survivorship patterns may obscure the important patterns that resulted from the earliest post-settlement events (Stoner, 1990; Hunt and Scheibling, 1997). Further compounding these problems is the likelihood that different data collection methods and sampling frequencies lead to different conclusions about the same system (Minchton and Scheibling, 1993).
Despite the difficulty of determining the effects of predation on a species immediately after settlement, doing so is critical to the interpretation of observed distribution patterns. The vulnerability of an individual to predation at any particular time in its life depends, among other things, on its size, how many other conspecifics it lives with, and the amount and quality of protection its habitat provides. Crustacean predators tend to choose relatively small molluscan prey (Juanes, 1992), and some molluscs survive by outgrowing their predators, eventually reaching a size refuge at which mortality due to predation declines significantly (Paine, 1976; Whetstone and Eversole, 1981; Jensen and Jensen, 1985). For some invertebrates, such as abalone (McShane, 1991) and littleneck clams (Boulding and Hay, 1984), the probability of individual mortality increases as density increases. But for others, including bryozoans (Keough, 1986), barnacles (McGuinness and Davis, 1989), and queen conch (Ray and Stoner, 1994), there is safety in numbers, and the probability of being killed is negatively dependent on density. There is also refuge in the structure provided by aquatic vegetation, and seagrass meadows are well known providers of food and shelter for many fishes and invertebrates (Kikuchi, 1980; Orth, 1992). Animals occupying vegetated areas tend to survive at higher rates than those in unvegetated areas (Heck and Wilson, 1987; Ray and Stoner, 1995).
Such conclusions about the effects of size, conspecific density, and habitat structure on mortality in the field have been reached by investigating animals that are large enough to be observed. These three variables must also affect life stages that are too small to observe in the field. This laboratory study was conducted to explore the effects of prey size, prey density, and habitat complexity on predation by a small (,14 mm carapace width) xanthid crab (Micropanope sp.) on queen conch (Strombus gigas Linnaeus) immediately after metamorphosis.
Caribbean, and southeastern Florida (Abbott, 1974). Despite extensive experimental work with juveniles .55 mm in shell length (SL), which are regularly observed in clearly established aggregations that persist over many years, animals ,10 mm SL are cryptic, rarely found in the wild, and little is known about their ecology (Sandt and Stoner, 1993; Stoner and Ray, 1993). Veligers hatch from benthic egg masses in the summer and are pelagic for up to 1 month (Davis et al., 1993). Upon encountering appropriate trophic cues (Davis and Stoner, 1994), they undergo metamorphosis at |1.2
mm shell length (SL) and recruit into benthic habitat. During the first 1.5 years of life, when juveniles are ,75 mm SL, they suffer severe mortality from predation, though survivorship steadily increases with size (Ray et al., 1994).
To quantify small predators that were capable of feeding on newly settled conch, a dredge survey was conducted in 1992 at Shark Rock, a well-studied conch nursery area near Lee Stocking Island, Bahamas (Stoner et al., 1998). The most abundant small
2
predators collected were xanthid crabs (at 286 / m ), and the single-most abundant
4
xanthid crab was identified to the genus Micropanope Stimpson . As a group, shell-crushing crabs are highly mobile predators, equipped with strong and effective master claws (Bertness and Cunningham, 1981). They sometimes out-consume other important invertebrate predators (Menge, 1983) and can influence intertidal zonation patterns of gastropods (Behrens Yamada and Boulding, 1996). Xanthid crabs are important and abundant predators on hard clams (Whetstone and Eversole, 1981), ribbed mussels (Lin, 1990), and scallops (Streib et al., 1995) in temperate waters. Given their great densities in juvenile conch habitat, they probably also have a strong influence on the early survival of conch and other small prey.
2. Methods
2.1. Predator and prey handling
In a series of predator–prey experiments with xanthid crabs (Micropanope sp.) and the smallest post-metamorphic queen conch obtainable, we examined size-specific predation, consumption, prey size choice, functional response, and habitat complexity. As is the case for many invertebrate species (Hunt and Scheibling, 1997), finding sufficient quantities of small conch in the wild, immediately after they had undergone metamorphosis, was not possible. Therefore, all experimental conch were hatchery-reared and purchased from the Caicos Conch Farm, a commercial hatchery in the Turks and Caicos Islands, British West Indies. These animals were flown to our laboratory on Lee Stocking Island, Bahamas, a few days after metamorphosis, at 1–2 mm shell length (SL). Stock availability at the hatchery played a primary role in the timing of experiments, which were conducted from May through August 1993. Transit time to our laboratory was less than 15 h, and our intent was to have animals available for experimentation as soon after metamorphosis as possible.
4
Experimental animals were maintained in an outdoor flow-through seawater system. They were fed a diet of benthic diatoms that had been rinsed from seagrass detritus collected nearby from the Shark Rock conch nursery, where conch aggregations have been mapped since 1989, and newly settled conch and their predators were dredged in 1992 (Stoner and Ray, 1993; Stoner et al., 1998). Given the cryptic nature of small conch, predation experiments could not be monitored in the field. Therefore, all experiments were conducted in the laboratory.
Live xanthid crabs were sorted from the Shark Rock detritus. Only those identified to the genus Micropanope and whose chelae and other appendages were intact were used in our experiments. Data collected for crabs that molted during an experiment were not analyzed because molting crabs did not feed. The carapace width (CW) of each crab was measured using either the ocular micrometer on a dissecting microscope or dial calipers, depending on the crab’s size. Crabs were starved for at least 24 h before conch were offered to them.
Each conch was carefully examined to ascertain good health, as evidenced by two criteria: (1) the conch was actively moving, i.e. crawling along the substrate or, if dislodged, actively using its operculum to right itself; (2) its shell was unbroken, and the color was not faded. At the end of each experiment, each conch was again carefully examined. When all or most of the tissue was gone from a dead conch’s shell, the cause of death was attributed to crab predation. In this study, a total of 4480 conch were offered to crabs; of those conch that were found dead, only 10 were not killed by crabs. The dead conch had no shell damage, and the tissue, while perhaps still intact, had begun to decay.
Experiments were conducted within a temperature range of 26–318C and at a salinity of 37–39‰, conditions typical of the ambient environment.
2.2. Size-specific predation
The purpose of the first experiment was to determine the upper size limits of conch that could be killed by xanthid crabs in the size range previously observed in the Shark Rock nursery area, 1.0–14.5 mm CW (Stoner et al., 1998). A total of 339 paired trials were conducted in which one conch was offered to one crab. In order to avoid giving crabs an opportunity to learn better prey handling techniques, they were used only once. Conch were measured for shell length, apex to siphonal canal, and were tested at less than 35 days after metamorphosis in the size range available, 1.2–8.8 mm SL.
Experiments with crabs ,3 mm CW were conducted in small, covered petri dishes (diameter 37 mm, height 11 mm), and those with crabs .3 mm CW were conducted in round, white, flat-bottom polyethylene containers (diameter 10.5 cm, height 5.5 cm). All containers were filled with seawater, which was changed daily.
In the first 111 trials, observations of conch status were made after each of three consecutive 24 h periods. Kills were always made within the first 48 h. Therefore, all subsequent trials were terminated after 48 h.
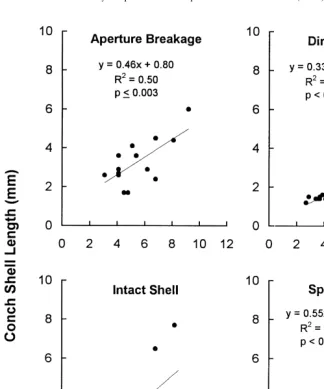
Shell damage was recorded for each killed conch using four categories: (1) shells were classified as ‘crushed’ when they were broken into small fragments; (2) shells with their ‘spire removed’ had the apex cut off; (3) shells with ‘aperture breakage’ had damage to their aperture or outer body whorl; sometimes this damage resulted when the crab peeled the shell back along the spire line to expose the tissue; (4) shells with no damage were classified as ‘intact.’ Analysis of covariance was used to test the relationship between conch size and predation method, with crab size as the covariate. Pairwise comparisons were tested using the Tukey HSD multiple comparison test. 2.3. Consumption
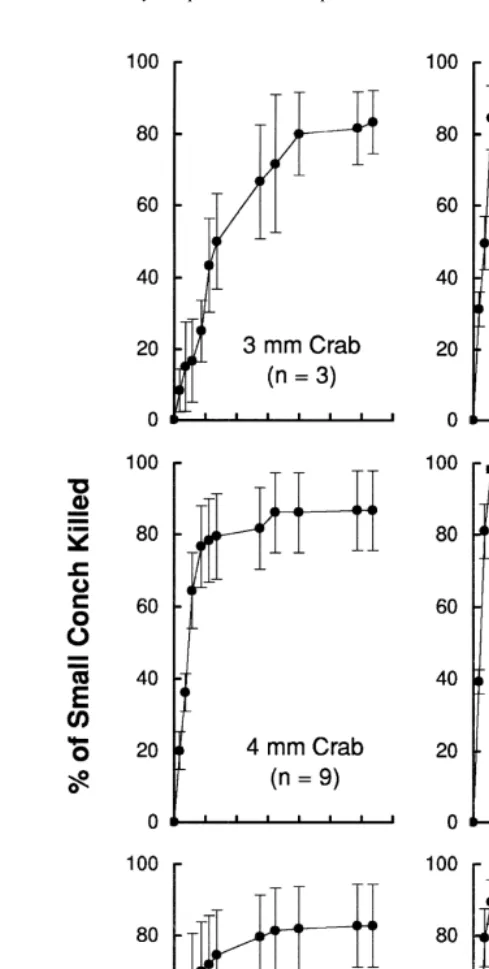
The purpose of the next experiment was to determine the number of small conch (smallest available, mean of subsample 1.6 mm SL, SD50.2, n5100) that individual xanthid crabs (3.1–10.4 mm CW, n554) could consume. As in the first experiment, an attempt was made to collect crabs that covered the size range dredged at the Shark Rock nursery in 1992 (Stoner et al., 1998). Each crab was placed in a polyethylene container (described above) filled with seawater at 10:00 h and offered 10 conch. When the crab had consumed more than five conch, it was offered another 10. If a crab had not consumed five conch by 21:00 h on the first day of observation, it was still given another 10 conch in anticipation of evening feeding. The intent was to permit ad libitum feeding. The number of conch consumed and the type of damage to their shells were recorded during 11 observation periods spaced over 51 h.
All but four of the crabs $6 mm CW killed all of the small (1.6 mm SL) conch offered within 7 h (see Results). Therefore, a second run was conducted with crabs that were.6 mm CW and conch that were more than twice the size (mean of subsample 4.1 mm, SD50.4, n520) of those conch used in the first run. Fifteen of these larger conch were offered to each of four medium-sized crabs (range 7.5–7.9 mm CW), and 20 were offered to each of four large-sized crabs (range 8.5–11.5 mm CW) during a 24 h period. Four crabs were given additional conch (see Table 1 for totals) in our continued effort to permit ad libitum feeding. The number of larger crabs that could be tested in the second run of the experiment was limited by the number of larger conch available.
2.4. Microcosm experiments 2.4.1. General approach
Experiments on prey size choice, functional response, and habitat complexity were conducted in microcosms that simulated the natural seagrass (Thalassia testudinum
¨
Table 1
The number of large conch (4.1 mm shell length) killed in 24 h by eight xanthid crabs that varied in carapace width (CW)
Microcosms were made from white plastic cylinders (diameter 15 cm, height 18 cm) with no tops. Polypropylene screen (mesh size 0.230.2 mm) was attached to the bottom of each microcosm and covered with a layer (2–3 cm deep) of fine, azoic sand (0.2–0.5 mm). The microcosms were placed in a shallow wet table (1.2 m32.4 m30.3 m), and each was provided with a continuous flow of sand-filtered seawater.
Living shoots of seagrass and seagrass detritus were collected from the Shark Rock nursery area and transplanted into the microcosms at natural densities commonly
2
occupied by conch (250–700 shoots / m ; Stoner and Waite, 1990). Three seagrass densities were created for use in the experiments described in Sections 2.4.2–2.4.4:
2
moderate density seagrass with 10 shoots of seagrass (565 shoots / m ) and 13 g (wet
2
weight) of detritus; low density seagrass with five shoots of seagrass (282 shoots / m ) and 1–3 g (wet weight) of detritus; and a bare sand microcosm with no vegetation. Although small conch are rarely found in the wild, Sandt and Stoner (1993) discovered a population of early juveniles (35–54 mm SL) in bare sand habitat where the conch burrowed into the substrata during the day. In a seagrass meadow nearby, however, they observed smaller individuals (12–25 mm SL) on the surface and speculated that rhizome mats and anoxic sediments discouraged such burrowing behavior. Small conch living in seagrass apparently derive their protection from the above-surface structure provided by living and detrital seagrass (Sandt and Stoner, 1993; Ray and Stoner, 1995). In this experiment, therefore, only enough rhizome material was left attached to a shoot bundle as to preserve the blades intact for transplantation.
2.4.2. Prey size choice
During the experiment testing for size-specific predation, five crabs, all 4.8 mm CW, had killed two conch that were 1.6 and 1.7 mm SL, but did not kill three conch that were 3.6–4.2 mm SL. Therefore, a prey size choice experiment was conducted to determine if 1 week of additional growth in shell length (1.7 mm) would increase conch survivorship. Forty conch from each of two size classes were offered to each of five crabs, all 4.8 mm CW, placed individually into five replicate microcosms simulating moderate density seagrass. The mean shell length for small conch was 2.0 mm (SD50.14, n550, range 1.7–2.2 mm), and for large conch it was 3.7 mm (SD50.23, n550, range 3.4–4.1 mm). The number of killed conch was counted after 24 h. Data were analyzed in a contingency table using the G statistic. Data for one of the five crabs were discarded from the analysis because the crab molted. The numbers of conch killed by the remaining four crabs were pooled, as were the numbers of living conch, and these pooled values were used in the contingency table.
2.4.3. Functional response
To determine the functional response of xanthid crabs, 42 individual crabs (mean 4.8 mm CW, SD50.27, range 4.4–5.1 mm) were offered newly settled conch at seven different densities in microcosms simulating low density seagrass. Density treatments were 2, 6, 12, 18, 24, 48, and 96 conch (mean of subsample 1.7 mm SL, SD50.15, range 1.3–2.0 mm, n550) per microcosm. In addition, a control treatment without a crab was also run with 18 conch per microcosm. There were six replicates for each treatment, including the control treatment, although one of the replicates with 96 conch was later excluded from analysis because the crab molted. The number of conch killed was recorded after 48 h. Conch mortality was examined as a function of conch density using linear regression.
2.4.4. Habitat complexity
To determine the effects of habitat structure on conch predation by xanthid crabs, a microcosm experiment was conducted using three degrees of habitat complexity: moderate density seagrass, low density seagrass, and bare sand. The experiment was conducted twice. In each run, 100 conch were added to each of six replicates for each habitat type. As a control, 100 conch, but no crab, were also added to one additional microcosm for each habitat type in each run.
Subsets of conch were measured prior to experimental setup (see Table 2 for sizes of conch and crabs). Because the conch in the sand treatment received no whole detritus, and, therefore, possibly less food than conch in the treatments with detritus, conch were also measured after the experiment for comparison. The number of killed conch was recorded 48 h after introduction to the crabs in the first run and after 24 h in the second run.
Table 2
Mean percentage of conch killed by xanthid crabs in three microcosm habitat treatments (bare sand, low a
density seagrass, moderate density seagrass) after 48 h (Run 1) and after 24 h (Run 2)
Run 1 Run 2
% of conch killed in each habitat (mean6SE(n))
Sand 1363 (6) 3564 (6)
One hundred conch were offered to each crab, and initial sizes for crabs and a subset of conch are noted. F and P values are given for one-way analysis of variance; data were homogeneous (Cochran’s test, P.0.05).
3. Results
3.1. Size-specific predation
Xanthid crabs killed the conch offered to them in 134 of the 339 paired tests, and 110 (82%) of these kills were made within the first 24 h. In 313 of the tests, crabs were offered conch with shell lengths that were smaller than or equal to the crab’s carapace width. Crabs killed conch in 133 (42%) of these tests. Killed conch were 14–96% (mean 45%, SD519) as large as the crabs. In the 180 tests in which kills were not made, conch were 29–100% (mean 75%, SD516) as large as the crabs. In 26 of the 339 tests, the conch was larger in shell length (101–132%) than the crab was in carapace width. Only one of these conch was killed, a 2.4 mm SL conch killed by a 2.2 mm CW crab.
Also, relatively few of the largest conch tested were killed (Fig. 1). In 104 trials where conch were .5.0 mm SL and crabs were .5.0 mm CW, only 10 conch were killed. However, all crabs that were .3.1 mm CW killed all conch ,2 mm SL (n551 tests).
Crabs used different methods of killing conch depending on their relative sizes (analysis of covariance: F( 3,129 )513.5, P ,0.001; slopes were homogeneous: F( 3,126 )5
Fig. 1. Shell length of conch killed and not killed plotted as a function of xanthid crab carapace width. The line representing x5y is also shown.
3.2. Consumption
Within 22 h all xanthid crabs $6 mm CW (n531) had killed 100% of the 20 small (1.6 mm SL) newly settled conch offered, and all but four of them had killed all conch within 7 h (Fig. 3). One of the crabs in the 8–10 mm CW size class consumed two conch in 10 s. Smaller crabs showed wider variation in their consumption rates. Only the largest (3.9 mm CW) crab in the 3 mm size class killed all conch, while six of the nine 4 mm crabs had killed all conch within 11 h. Crabs $6 mm CW killed conch by crushing the entire shell, while those ,6 mm CW usually removed the spire and left the body whorl intact. Given several conch to choose from at a time, individual crabs sometimes handled a few before selecting one to consume. And before consuming a particular conch, the crab often turned the shell to examine or position it.
Fig. 2. Shell length of killed conch plotted as a function of xanthid crab carapace width for four different types of conch shell remains. Regression statistics are noted on each graph.
The four large crabs (8.5–11.5 mm CW) ate as many as 44 larger conch during the 24 h. Both medium- and large-sized crabs, including the two that did not kill any conch, left the shells of some live conch with aperture damage, indicating that the crabs had handled the animals but not killed them.
3.3. Prey size choice
choice in four tests, xanthid crabs (all were 4.8 mm CW) killed a total of 42 small (2.0 mm SL) conch and only three large (3.7 mm SL) conch.
3.4. Functional response
The number of conch killed increased with density (Fig. 4A), but the proportion killed decreased with density (Fig. 4B). For an individual conch, the probability of being killed was lowest at the highest density (96 conch per microcosm). All 18 conch in each microcosm of the control treatments survived.
3.5. Habitat complexity
Conch in this experiment crawled around and under the detritus, climbed up the seagrass blades, and burrowed just below the sand surface. The structure provided by seagrass shoots and detritus in the microcosms afforded no apparent protection from xanthid crab predation. In fact, the highest mortality in both runs occurred in the most complex habitat tested, though differences among the three treatments were not significant (Table 2). None of the conch in the controls died in the second run. In the first run, there was one dead conch in each of the two seagrass treatments.
In the first run, in which conch were 2.0 mm SL and results were recorded after 48 h, the mean mortality was 13% in sand, and 14% in low seagrass. In the second run, in which conch were 0.6 mm smaller and results were recorded after only 24 h, mortality was more than twice as high, although differences between the runs were not tested statistically. Conch size apparently afforded more protection than habitat complexity.
Final conch SL measurements for each run were not significantly different among the three treatments (one-way analysis of variance on homogeneous data (Cochran’s test,
P.0.05): F( 2,57 )51.34, P.0.25 (Run 1); F( 2,147 )52.30, P.0.10 (Run 2)), sug-gesting that conch in the bare sand treatment were not nutritionally deprived during the experiment.
4. Discussion
The survivorship of sessile invertebrates, such as barnacles and ascidians, can be monitored in the field immediately after settlement using sequential maps of distribution, but individual tracking of survival in motile species is inherently more difficult and has yet to be done (Gosselin and Qian, 1997). This difficulty has resulted in emphasis in the literature on age groups that are readily observed or collected. In this study we manipulated prey size, prey density, and habitat complexity using one motile prey species soon after metamorphosis. Our results provide a foundation for speculation about the potential impact of small predators in the field.
Fig. 4. Mean number (A) and mean percentage (B) of conch killed by xanthid crabs plotted as a function of conch density.
until well after the first few months. There is no practical way to collect large numbers of live newly settled conch in the wild. In any case, small conch, regardless of origin, have little defense against motile crabs except their shells. Despite these two limitations, the results of this study have two important implications: small xanthid crabs have enormous potential for predatory impact on newly settled conch, but a few millimeters of growth in shell length can improve survivorship considerably.
Over the long term, conch escape predation by out-growing their predators. Although small conch can leap away from some threats of immediate danger using their opercula, they cannot outrun highly mobile surface predators such as crabs. And, even though conch are cryptically colored, and they burrow in the substrata, small crabs have access to the same hiding places. Predator–prey encounter rates play an important role in the size and number of prey a crab can consume in the wild (Barbeau and Scheibling, 1994). In this study, crabs proved to be very efficient, fast, and agile predators despite the vegetative structure provided in the two seagrass habitat treatments. They regularly worked through the detritus, turning it over in their search for food. A hungry crab was likely to consume any conch of an edible size that it found.
Brachyuran crabs are important predators of gastropods, and they are highly effective shell crushers, especially of relatively light weight, thin shells (Vermeij, 1977; Bertness and Cunningham, 1981). As conch shell length increases, so does shell thickness. Examination of the shell breakage patterns in our size-specific experiment showed crushing to be the preferred method of killing the smallest, thinnest-shelled conch, and the largest crabs tested were especially efficient at doing so. Xanthid crabs that were 7.0–7.9 mm CW could kill 20 newly settled (1.6 mm SL) conch in 7 h, but they killed only as many as 65% of 20 larger conch (4.1 mm SL) in 24 h. And two of the crabs in this size class did not kill any of the larger conch. Laboratory consumption rates are frequently over-estimates compared to those found naturally in the field because prey in experimental containers cannot escape their predators (Ambrose, 1991) and because there are no alternative prey present. Nevertheless, our results suggest that a single xanthid crab is capable of consuming many small conch per day when conch are readily available. Growing from 1.6 to 4.1 mm SL, however, which conch can accomplish in approximately 2 weeks, resulted in increased conch survivorship.
Conch are highly vulnerable to shell-crushing predators for a significant part of their lives (Davis, 1992; Ray et al., 1994). However, survivorship increases sharply for conch 55–75 mm SL (about 1 year old) when the amount of energy required to crush the shell increases sharply (Jory and Iversen, 1988; Ray et al., 1994). Crabs change their attack strategies depending on the size of their molluscan prey (Juanes, 1992), and our xanthids often examined shells before positioning them for attack. As in this study, crabs tend to crush shells that are relatively small, sometimes severing the spire, and to attack larger shells from the aperture, either by peeling the shell back along the body whorl like a ‘can-opener’ or by chipping away at the leading edge until the meat is reached (Zipser and Vermeij, 1978; Lawton and Hughes, 1985).
stronger shells. They outgrew the size range that smaller crabs could consume. Conch were only vulnerable to predation by crabs with carapace widths that were as large as their shell length or larger, except in one case (2.2 mm CW crab offered 2.4 mm SL conch). Given that the modal carapace width for all xanthid crabs dredged at the Shark Rock nursery was only 1.5 mm (range 1.0–14.5 mm) (Stoner et al., 1998) and that conch settle at |1.2 mm SL (Davis et al., 1993), conch need only increase in size by a small
amount to outgrow the most abundant xanthid size class. Nevertheless, the high densities
2
of xanthid crabs that occur in the wild (to 286 / m ; Stoner et al., 1998), their effectiveness as predators, and their large appetites, undoubtedly make them a formid-able obstacle to conch survivorship.
For a particular prey species, the critical size, which is the largest size of prey vulnerable to predation during a particular time period, is determined by the morphology and behavior of both predator and prey (Zipser and Vermeij, 1978). As in some other predator–prey interactions with crabs and molluscs (Hughes and Seed, 1981; Boulding, 1984), xanthid crabs may prefer small conch because they are more quickly handled, and energy intake is maximized. During the dredge survey conducted at Shark Rock (Stoner et al., 1998), the size class of dead conch most frequently collected was 1–5 mm SL. Spight (1976) concluded that 2 mm SL snails (Thais lamellosa) were more likely to survive predation than 1 mm SL snails, and that larger size was always advantageous. Interestingly, newly settled conch grow fastest on substrata that also induce high percentages of metamorphosis (Stoner et al., 1996), suggesting selective settlement to areas that will enable them to grow quickly.
Rapid declines in density are commonly observed amongst newly settled molluscs. For example, Thais lamellosa suffered up to 99% mortality during their first 2 months after hatching (Spight, 1975); cockle mortality was as high as 82% after their first month after settlement (Jensen and Jensen, 1985); and abalone mortality ranged from 90 to 100% at the end of 5 months after settlement (McShane, 1991). Such declines are most frequently attributed to predation, though a host of abiotic factors (including desiccation, physical disturbance, water conditions) and other biotic factors (including competition, disease, larval condition) are also significant causes of death (for complete reviews, see Gosselin and Qian, 1997; Hunt and Scheibling, 1997). High fecundity among gastropods may be a selective response to offset high early juvenile mortality (Fretter and Manly, 1979). The queen conch may be a case in point, as a single female queen conch may lay several million eggs each summer over a reproductive life span of 15–20 years (Robertson, 1959; Davis et al., 1984).
In our functional response experiment, the probability that an individual conch would be killed decreased with increasing conch density, suggesting that it may be advantage-ous for conch to occur in aggregations. Conch do, in fact, have a contagiadvantage-ous distribution,
2
often occurring in aggregations with 0.1–1 conch / m (Stoner and Ray, 1993). And, larger juveniles do gain protection from conspecifics (Marshall, 1992; Ray and Stoner, 1994). Although queen conch larvae do not settle in response to chemical cues derived from conspecifics, they do settle in response to cues from substrata collected from specific areas that are consistently occupied by juveniles (Davis and Stoner, 1994).
if xanthids are attracted to higher small conch densities in the field, conch mortality may increase with conch density (e.g., Boulding and Hay, 1984), or xanthids may exhibit a numerical response through increased reproduction as a result of abundant food supply (Krebs, 1994). Mortality of newly settled conch will decrease with density only if no such aggregative or numerical responses occur. Although conch settle in response to cues from substrata that also produce high early juvenile growth, these same substrata also support high densities of predators. Xanthid densities showed a high positive correlation with both seagrass shoot density and newly settled conch density (Stoner et al., 1998), and this may represent a population response to the food and shelter that seagrass habitat provides for small invertebrates.
Second, the conch densities in our microcosms were high compared to the only
2
densities of post-settlement conch known in the wild (,16 / m ) (Stoner et al., 1998), and would only occur naturally if large numbers of larvae settle in small patches. If patches do indeed occur, and there is no aggregative or numerical response by xanthids, our functional response experiment suggests that chance of survivorship for a newly settled juvenile may be improved. Predation processes on small conch warrant further investigation in the field.
Xanthid crabs are, of course, not the only predators on newly settled conch. A predator screening experiment with newly settled conch revealed a large variety of other animals that were capable of killing them (Ray-Culp et al., 1997). These animals, all collected near Lee Stocking Island, included calappid, portunid, majid, and hermit crabs; alpheid and palaemonid shrimps; panulirid lobsters; file fish; and five families of polychaete worms. Certain species of polychaetes were also capable of feeding on conch larvae that were in the process of settling. The combined impact from all of the small predators that consume conch soon after they settle is likely to be a significant influence that shapes the population dynamics of queen conch. Survivorship may well be close to zero at densities likely to be found in the wild. Nevertheless, individual conch can effectively escape predation from small predators by staying alive long enough to outgrow them.
In conclusion, a single small predator can have an enormous impact in shaping the population dynamics of a prey species by preying on the youngest settlers. The smallest predators on motile species have generally been understudied and warrant further investigation, both in the field and laboratory. Although vegetative shelter may not provide much protection for small molluscs from highly motile and efficient crab predators, higher conspecific densities may increase the probability of individual survivorship. Outgrowing the suite of very small predators, however, appears to be the most important mechanism for survivorship for queen conch.
Acknowledgements
and two anonymous reviewers provided thoughtful comment on the manuscript, and A. Williams kindly identified the xanthid crab.
References
Abbott, R.T., 1974. American Seashells, 2nd ed., Van Nostrand Reinhold, New York, pp. 663.
Ambrose, W.G., 1991. Are infaunal predators important in structuring marine soft-bottom communities? Am. Zool. 31, 849–860.
Barbeau, M.A., Scheibling, R.E., 1994. Behavioral mechanisms of prey size selection by sea stars (Asterias vulgaris Verrill) and crabs (Cancer irroratus Say) preying on juvenile sea scallops (Placopecten magellanicus (Gmelin)). J. Exp. Mar. Biol. Ecol. 180, 103–136.
BehrensYamada, S., Boulding, E.G., 1996. The role of highly mobile crab predators in the intertidal zonation of their gastropod prey. J. Exp. Mar. Biol. Ecol. 204, 59–83.
Bertness, M.D., Cunningham, C., 1981. Crab shell-crushing predation and gastropod architectural defense. J. Exp. Mar. Biol. Ecol. 50, 213–230.
Boulding, E.G., 1984. Crab-resistant features of shells of burrowing bivalves: decreasing vulnerability by increasing handling time. J. Exp. Mar. Biol. Ecol. 76, 201–223.
Boulding, E.G., Hay, T.K., 1984. Crab response to prey density can result in density-dependent mortality of clams. Can. J. Fish. Aquat. Sci. 41, 521–525.
Davis, M., 1992. Predation of hatchery-reared juvenile queen conch, Strombus gigas (L.) by juvenile spiny lobsters, Panulirus argus (L.). M.S. thesis, Florida Institute of Technology, Melbourne, Florida, pp. 37. Davis, M., Bolton, C.A., Stoner, A.W., 1993. A comparison of larval development, growth, and shell
morphology in three Caribbean Strombus species. Veliger 36 (3), 236–244.
Davis, M., Mitchell, B.A., Brown, J.L., 1984. Breeding behavior of the queen conch Strombus gigas Linne held in a natural enclosed habitat. J. Shellfish Res. 4 (1), 17–21.
Davis, M., Stoner, A.W., 1994. Trophic cues induce metamorphosis of queen conch larvae (Strombus gigas Linnaeus). J. Exp. Mar. Biol. Ecol. 180, 83–102.
Fretter, V., Manly, R., 1979. Obervations on the biology of some sublittoral prosobranchs. J. Moll. Stud. 45, 209–218.
Gosselin, L.A., Qian, P.Y., 1996. Early post-settlement mortality of an intertidal barnacle: a critical period for survival. Mar. Ecol. Prog. Ser. 135, 69–75.
Gosselin, L.A., Qian, P.Y., 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282.
Heck, Jr. K.L., Wilson, K.A., 1987. Predation rates on decapod crustaceans in latitudinally separated seagrass communities: a study of spatial and temporal variation using tethering techniques. J. Exp. Mar. Biol. Ecol. 107, 87–100.
Hughes, R.N., Seed, R., 1981. Size selection of mussels by the blue crab Callinectes sapidus: energy maximizer or time minimizer? Mar. Ecol. Prog. Ser. 6, 83–89.
Hunt, H.L., Scheibling, R.E., 1997. Role of early post-settlement mortality in recruitment of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 155, 269–301.
Jensen, K.T., Jensen, J.N., 1985. The importance of some epibenthic predators on the density of juvenile benthic macrofauna in the Danish Wadden Sea. J. Exp. Mar. Biol. Ecol. 89, 157–174.
Jory, D.E., Iversen, E.S., 1988. Shell strength of queen conch, Strombus gigas L.: aquaculture implications. Aquaculture Fish. Manage. 19, 45–51.
Juanes, F., 1992. Why do decapod crustaceans prefer small-sized molluscan prey? Mar. Ecol. Prog. Ser. 87, 239–249.
Keough, M.J., 1986. The distribution of a bryozoan on seagrass blades: settlement, growth and mortality. Ecology 67 (4), 846–857.
Kikuchi, T., 1980. Faunal relationships in temperate seagrass beds. In: Phillips, R.C., McRoy, C.P. (Eds.), Handbook of Seagrass Biology: an Ecosystem Perspective, Garland STPM Press, New York, pp. 153–172. Krebs, C.J., 1994. Ecology: the Experimental Analysis of Distribution and Abundance, 4th ed., Harper and
Row, New York.
Lawton, P., Hughes, R.N., 1985. Foraging behaviour of the crab Cancer pagurus feeding on the gastropods Nucella lapillus and Littorina littorea: comparisons with optimal foraging theory. Mar. Ecol. Prog. Ser. 27, 143–154.
Lin, J., 1990. Mud crab predation on ribbed mussels in salt marshes. Mar. Biol. 107, 103–109.
Marshall, L.S., 1992. Survival of juvenile queen conch, Strombus gigas, in natural habitats: impact of prey, predator and habitat features. Ph.D. thesis, College of William and Mary, Williamsburg, Virginia, pp. 144. McGuinness, K.A., Davis, A.R., 1989. Analysis and interpretation of the recruit–settler relationship. J. Exp.
Mar. Biol. Ecol. 134, 197–202.
McShane, P.E., 1991. Density-dependent mortality of recruits of the abalone Haliotis rubra (Mollusca: Gastropoda). Mar. Biol. 110, 385–389.
Menge, B.A., 1983. Components of predation intensity in the low zone of the New England rocky intertidal region. Oecologia (Berlin) 58, 141–155.
Minchton, T.E., Scheibling, R.E., 1993. Variations in sampling procedure and frequency affect estimates of recruitment of barnacles. Mar. Ecol. Prog. Ser. 99, 83–88.
Orth, R.J., 1992. A perspective on plant–animal interactions in seagrasses: physical and biological deter-minants influencing plant and animal abundance. In: John, D.M., Hawkins, S.J., Price, J.H. (Eds.), Plant–Animal Interactions in the Marine Benthos, Clarendon Press, Oxford, pp. 147–164.
Osman, R.W., Whitlatch, R.B., 1995. Predation on early ontogenetic life stages and its effect on recruitment into a marine epifaunal community. Mar. Ecol. Prog. Ser. 117, 111–126.
Paine, R.T., 1976. Size-limited predation: an observational and experimental approach with the Mytilus – Pisaster interaction. Ecology 57, 858–873.
Ray, M., Stoner, A.W., 1994. Experimental analysis of growth and survivorship in a marine gastropod aggregation: balancing growth with safety in numbers. Mar. Ecol. Prog. Ser. 105, 47–59.
Ray, M., Stoner, A.W., 1995. Growth, survivorship, and habitat choice in a newly settled seagrass gastropod, Strombus gigas. Mar. Ecol. Prog. Ser. 123, 83–94.
Ray, M., Stoner, A.W., O’Connell, S.M., 1994. Size-specific predation of juvenile queen conch Strombus gigas: implications for stock enhancement. Aquaculture 128, 79–88.
Ray-Culp, M., Davis, M., Stoner, A.W., 1997. The micropredators of settling and newly settled queen conch (Strombus gigas Linnaeus). J. Shellfish Res. 16 (2), 423–428.
Robertson, R., 1959. Observations on the spawn and veligers of conchs (Strombus) in the Bahamas. Proc. Malacol. Soc. 33, 164–171.
Sandt, V.J., Stoner, A.W., 1993. Ontogenetic shift in habitat by early juvenile queen conch, Strombus gigas: patterns and potential mechanisms. Fish. Bull. 91, 516–525.
Spight, T.M., 1975. On a snail’s chances of becoming a year old. Oikos 26 (1), 9–14. Spight, T.M., 1976. Ecology of hatching size for marine snails. Oecologia 24, 283–294.
Stoner, A.W., Davis, M., 1994. Experimental outplanting of juvenile queen conch, Strombus gigas: comparison of wild and hatchery-reared stocks. Fish. Bull. 92, 390–411.
Stoner, A.W., Ray, M., 1993. Aggregation dynamics in juvenile queen conch (Strombus gigas): population structure, mortality, growth, and migration. Mar. Biol. 116, 571–582.
Stoner, A.W., Ray, M., Glazer, R.A., McCarthy, K.J., 1996. Metamorphic responses to natural substrata in a gastropod larva: decisions related to postlarval growth and habitat preference. J. Exp. Mar. Biol. Ecol. 205, 229–243.
Stoner, A.W., Ray-Culp, M., O’Connell, S.M., 1998. Settlement and recruitment of queen conch, Strombus gigas, in seagrass meadows: associations with habitat and micropredators. Fish. Bull. 96 (4), 885–899. Stoner, A.W., Waite, J.M., 1990. Distribution and behavior of queen conch Strombus gigas relative to seagrass
standing crop. Fish. Bull. U.S. 88, 573–585.
Stoner, D.S., 1990. Recruitment of a tropical colonial ascidian: relative importance of pre-settlement vs. post-settlement processes. Ecology 71 (5), 1682–1690.
Vermeij, G.J., 1977. Patterns in crab claw size: the geography of crushing. Syst. Zool. 26, 138–151. Whetstone, J.M., Eversole, A.G., 1981. Effects of size and temperature on mud crab, Panopeus herbstii,
predation on hard clams, Mercenaria mercenaria. Estuaries 4 (2), 153–156.