Journal of Experimental Marine Biology and Ecology 255 (2000) 51–74
www.elsevier.nl / locate / jembe
Comparative culture and toxicity studies between the toxic
dinoflagellate Pfiesteria piscicida and a morphologically
similar cryptoperidiniopsoid dinoflagellate
a ,* a a a
Harold G. Marshall , Andrew S. Gordon , David W. Seaborn , Brian Dyer ,
b b
William M. Dunstan , A. Michelle Seaborn
a
Department of Biological Sciences, Old Dominion University, Norfolk, VA 23529-0266, USA
b
Department of Ocean, Earth, and Atmospheric Sciences, Old Dominion University, Norfolk, VA 23529-0266, USA
Received 10 July 2000; received in revised form 24 July 2000; accepted 28 August 2000
Abstract
A series of fish bioassays using cultures of the toxic dinoflagellate, Pfiesteria piscicida and a cryptoperidiniopsoid dinoflagellate indicated various degrees of toxicity for Pfiesteria piscicida and no toxicity by the cryptoperidiniopsoid. P. piscicida maintained toxicity in the presence of live fish, and this toxicity was perpetuated following a series of inoculations to other culture vessels. Differences in the onset and magnitude of the fish deaths occurred, requiring 16 days for the initial fish death when using P. piscicida from a culture that had previously been maintained on algal cells, to kills within hours when using a culture that had recently (previous day) killed fish. Autopsies of moribund fish from the test and control fish bioassays indicated a general lack of bacterial infection, which ensued following death of other autopsied fish. Moreover, bacterial comparisons of waters in the fish bioassay and control fish cultures indicated that similar bacterial concentrations were present. Neither oxygen or ammonia levels were determined to be factors in the fish death. Life stages of a cryptoperidiniopsoid dinoflagellate from Virginia estuaries were also identified, including motile zoospore, gametes, planozygote, amoebae, and cyst stages. The cryptoperidiniopsioid did not initiate fish deaths in bioassays conducted over a 14-week period at
21
zoospore concentrations of ca. 700–800 cells ml . Elemental X-ray analysis of the scales from cysts of this dinoflagellate and P. piscicida indicate that they both contain silicon. Overall, the data from this study demonstrate that the cryptoperidiniopsoid possesses several similar life stages and feeding patterns as P. piscicida, but was not toxic to fish. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Pfiesteria piscicida; Cryptoperidiniopsis gen. nov.; Toxicity; Dinoflagellates
*Corresponding author. Tel.: 11-757-683-3595; fax: 11-757-683-5283.
1. Introduction
Estuarine and laboratory studies have identified dinoflagellates of the toxic Pfiesteria complex (TPC; thus far including Pfiesteria piscicida, Steidinger et al., 1996; and P.
shumwayae sp. nov., Glasgow, 2000) with production of toxins associated with fish kill
events and human illness (Burkholder et al., 1992; Glasgow et al., 1995; Burkholder and Glasgow, 1997; Grattan et al., 1998). The most frequent and extensive occurrences of these fish deaths have been in North Carolina’s Albemarle–Pamlico Estuarine System, with a lesser degree of incidence and fish mortality in tributaries of the eastern shore of Chesapeake Bay (Lewitus et al., 1995; Burkholder and Glasgow, 1997; Burkholder et al., 1999; Magnien et al., 1999). TPC species have also been found in other estuaries in benign forms, from New York through the Gulf Coast (Burkholder and Glasgow, 1997; Rublee et al., 1999).
These studies indicate that Pfiesteria spp. exist as nontoxic (i.e., non-toxin producing) heterotrophs feeding on algae and other organisms, and that under certain environmental conditions, the cells become toxic in the presence of fish. Pfiesteria spp. have been further described by Burkholder and Glasgow (1995, 1997), Burkholder et al. (1999), and Burkholder (in press) as existing in several functional types based on their capability of toxin production (Woods Hole Oceanographic Institution (WHOI), 2000). Active toxin-producing cells in the presence of live fish are considered the Toxic A functional type (TOX-A), which become temporarily nontoxic (TOX-B functional type) when removed from access to live fish and fed algae, or other prey. These TOX-B cells have the potential for toxin production and become TOX-A, or actively toxic, when re-introduced to live fish. In addition, there are strains of Pfiesteria that do not have, or apparently have permanently lost, the toxin production response that is triggered by the presence of live fish. This third functional type is called non-inducible (NON-IND), and on the basis of present understanding, cannot be induced to produce bioactive substances that cause fish disease and death (WHOI, 2000; Burkholder, in press).
Toxic strains of TPC species demonstrate the following traits: they show strong attraction toward live fish or their fresh tissues and secreta / excreta (attraction measured using motion analysis techniques as in Kamykowski et al., 1992; Burkholder and Glasgow, 1997); they produce bioactive substances or toxins that cause fish disease and death (Fairey et al., 1999); and they are stimulated to produce these substances in the
presence of live fish or their fresh tissues (separated from the live animal ,2 h;
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 53
´ ´ ´
Pfiester and Popovsky (1979), Pfiester and Lynch (1980), Popovsky (1982), Popovsky and Pfiester (1990), and Buckland-Nicks et al. (1990), among others.
The ecological and habitat relationships that stimulate Pfiesteria development and toxin production have been discussed by Burkholder et al. (1992, 1995a, 1999), Burkholder and Glasgow (1997), Magnien et al. (1999), and others. The laboratory and
field studies that have associated fish mortality with P. piscicida have been reported over
a broad salinity range and at various water temperatures. However, the toxic events have most commonly occurred at low to mid-salinity values (5–15 psu) and temperatures above 268C. Most of these events have been in nutrient-enriched waters of shallow, poorly flushed estuaries (Burkholder and Glasgow, 1997; Burkholder et al., 1997; Magnien et al., 1999). These conditions also favor the development of the algal prey that
Pfiesteria may utilize as a food resource in the absence of abundant live fish (Burkholder
and Glasgow, 1995, 1997).
In Virginia estuaries, intensive investigations over the past 3 years have documented numerous ‘pfiesteria-like organisms’ (PLOs; Marshall, 1999; WHOI, 2000). These organisms superficially resemble Pfiesteria spp. in general appearance under light microscopy (LM), but they mostly have been tested as benign or incapable of producing bioactive substances that cause fish stress, disease or death (this study; Burkholder, in press; J. Burkholder and H. Glasgow, North Carolina State University, Raleigh, NC, unpublished data). PLOs have similar size and morphological features as Pfiesteria spp., and most cannot be distinguished from Pfiesteria spp. using only LM. Therefore, we have used scanning electron microscopy (SEM) of both suture-swollen cells (Glasgow, 2000), and membrane stripping protocols by K. Steidinger (pers. commun.) and Truby (1997) to determine the plate tabulation and plate characteristics of these PLOs. The toxin-producing capability is assessed by fish bioassays as in Burkholder et al. (1995b, 1999); Burkholder and Glasgow (1997); and Burkholder (in press). Pfiesteria-like organisms in Virginia estuaries have included at least two cryptoperidiniopsoids (Cryptoperidiniopsis sp. [gen. nov.; Dr. K. Steidinger, Florida Department of En-vironmental Protection — Florida Marine Research Institute, St. Petersburg, FL, pers. commun.] and Cryptoperidiniopsis brodyi [gen. et sp. nov.; Steidinger, pers. commun.], along with certain Gymnodinium spp. and Gyrodinium spp. (e.g., Gyrodinium
galatheanum Braarud [5Gymnodinium galatheanum Braarud]). These PLOs are widely
distributed in Virginia estuaries and in other areas of the Chesapeake Bay (Marshall et al., 1999; Seaborn et al., 1999). Many of the smaller Virginia estuaries along the Potomac River and several of the smaller inlets along the mid-western shoreline of the
21
Chesapeake Bay also have supported at times high concentrations (.200 cells ml ) of
these cells (Marshall et al., 1999; H. Marshall, unpublished data).
Little is known about the comparative ecology of TPC species versus PLOs.
Comparative growth studies with a TOX-B (temporarily nontoxic) P. piscicida, a
1996). Thus, it can be discerned from Pfiesteria spp. under light microscopy, especially
4 21
with epifluorescence. In high cell densities (.10 zoospores ml ), several isolates of
G. galatheanum have also been reported as toxic to fish (Steidinger, 1993; Nielsen,
1993; Burkholder, 1998). However, isolates from North Carolina, Maryland, and Florida waters thus far have shown neither attraction to live fish nor ichthyotoxicity in repeated bioassays (Glasgow, 2000; Burkholder, in press). The objectives of the present study were to compare P. piscicida and a cryptoperidiniopsoid dinoflagellate in terms of their basic stage morphologies and potential toxicity to fish, toward strengthening insights about distinguishing characteristics of co-occurring Pfiesteria versus pfiesteria-lookalike species.
2. Materials and methods
2.1. Cultures
Cultures of two pfiesteria-like species were developed from water (Gyrodinium
galatheanum) and sediment (Cryptoperidiniopsis sp.) samples taken from Virginia
estuaries between 1997 and 1999 (Seaborn et al., 1999). Unialgal cultures of these species were established after numerous individual cell isolations and subsequent dilutions. Scanning electron microscope examination of the cryptoperidiniopsoid species indicated that it was different from C. brodyi (gen. et sp. nov.; Steidinger, pers.
commun.). This species ([DEQ002) and G. galatheanum were monitored daily and
routinely checked for contaminants and other dinoflagellates through light microscopy. Subsequent DNA sequencing analysis by Dr. D. Oldach (U.MD) indicated the cryptoperidiniopsoid to be a Cryptoperidiniopsis sp. (gen. nov.) that was separate from
C. brodyi (gen. et sp. nov), but closely related to P. piscicida. The identity of G.
galatheanum was confirmed by K. Steidinger (pers. commun.) with SEM and by D.
Oldach through a Heteroduplex mobility assay (Oldach et al., 2000). These cultures were maintained in falcon flasks in f / 2-Si medium at 15 psu, under ambient light and
room temperature, and were given Cryptomonas sp. (CCMP [767, Bigelow
Labora-tory) as a food source. Prior to using the Cryptomonas culture, it was routinely examined for contaminants, with cells routinely isolated to establish a series of sub-cultures.
A toxic Pfiesteria piscicida culture ([271A-1) was provided by Burkholder and
Glasgow (NCSU). This culture had been isolated from the Neuse Estuary, North Carolina, cloned in uni-dinoflagellate culture with algal prey (cryptomonads) as a food source at NCSU. Pfiesteria spp. have not been cultured successfully without a prey source (e.g., Burkholder and Glasgow, 1995; Steidinger et al., 1996), and it has not been possible to induce toxin production unless live fish are added (Burkholder and Glasgow, 1997). Thus, a clonal culture contains an isolate of (a uni-dinoflagellate) P. piscicida or
P. shumayae (sp. nov.), with its associated endosymbiont bacterial consortium
(Steiding-er et al., 1995) and its prey (Burkhold(Steiding-er, in press). The culture was allowed to graze
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 55
Section 2.3, below) to test for ichthyotoxic activity (five fish per replicate culture vessel,
n53; Burkholder et al., 1995a,b; Burkholder and Glasgow, 1997; Glasgow, 2000;
Burkholder, in press). The control fish remained healthy while the Pfiesteria-exposed fish all died, and accompanying tests indicated no difference between control and test culture vessels in presence / abundance of bacteria or other microflora / fauna that could
act as fish pathogens. Culture [271A-1 was thus evaluated as a toxic strain, was
identified to species by H. Glasgow and J. Burkholder (SEM of suture-swollen zoospores; Burkholder and Glasgow, 1995; Glasgow, 2000), and made available for our studies.
The culture ([271A-1) was shipped to our laboratory as TOX-A or actively toxic P.
piscicida, taken from the fish-killing cultures. However, since toxic strains of Pfiesteria
spp. are known to rapidly cease toxin production in the absence of live fish (within hours; Burkholder and Glasgow, 1997), this strain was received in TOX-B (temporarily nontoxic) status. We initially maintained the culture for 4 weeks with Cryptomonas as the food source. We also identified this isolate using SEM analysis (Leo model 435VP SEM) and, as had been done in the Burkholder and Glasgow laboratory, we again cross-confirmed the species identification as P. piscicida by two independent laboratories (Dr. P. Rublee of UNC-Greensboro, and Dr. D. Oldach of U. MD) using gene
sequencing techniques (Rublee et al., 1999; Oldach et al., 2000). This P. piscicida
culture was used in comparative growth studies (Seaborn et al., 1999) and in our initial fish bioassay studies (Fish bioassay I) with this species. A second TOX-A P. piscicida
isolate ([2200, Neuse Estuary) that had been similarly prepared was supplied by the
Burkholder and Glasgow laboratory, and was used for additional fish bioassays and morphological comparisons with the cryptoperidiniopsoid (Cryptoperidiniopsis sp. gen. nov.). Prior to the bioassays, the identity of this species was also cross-confirmed by the two laboratories mentioned above. This isolate was maintained with fish in culture facilities in a TOX-A mode (see Section 2.3 fish bioassays, below), without the addition of algal prey.
2.2. Morphology of life stages
2.3. Fish bioassays
The Pfiesteria piscicida cultures used in these bioassays were previously identified as the toxin producing source of fish deaths in the Burkholder and Glasgow laboratory.
Their bioassay procedure to identify toxin producing P. piscicida has been briefly
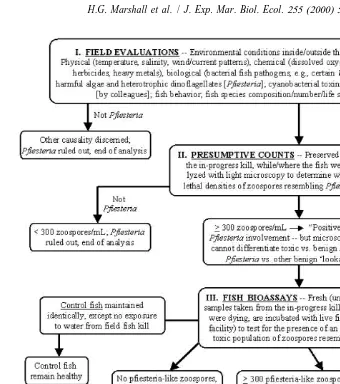
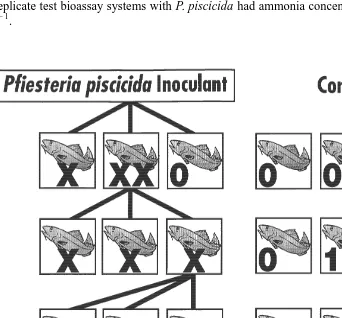
summarized in various publications and used in their bioassay studies (e.g., Burkholder et al., 1995a,b, 1999; Burkholder and Glasgow, 1997; Glasgow, 2000), with a more detailed description provided by Burkholder (in press). This approach evaluates the presence of toxic agents in relation to fish deaths that may occur across a range of environmental conditions (low to high nutrients, salinities, organics, toxic substances of other types, bacterial concentrations, protozoan densities, etc.). The Pfiesteria cultures that were provided for our studies by the Burkholder and Glasgow laboratory were identified as toxic strains based on bioassay results coming from the procedure outlined in Fig. 1. This cause / effect relationship is based on the presence of toxic rather than
infectious agents to determine ichthyotoxic activity of P. piscicida. The bioassay steps
include: (a) confirmation of the presence of Pfiesteria piscicida at potentially lethal densities at an in-progress fish kill event, identified by SEM examination and gene
sequencing analysis; (b) isolation of P. piscicida from a water sample taken from the
in-progress kill, in positive fish bioassays, and development of clonal culture(s) of these cells (uni-dinoflagellate and axenic except for endosymbiont bacteria; containing residual
21
axenic algal or other benign prey, such as ca. five to 10 cryptomonads ml ); (c)
addition of the clonal Pfiesteria isolate to healthy fish cultures, resulting in fish deaths, while fish in control cultures remained healthy; and (d) re-isolation and re-cloning of the organism from the second set of positive fish bioassays where deaths occurred, with subsequent species identification verified by SEM and gene sequencing, plus cross-confirmation of toxicity by an independent laboratory experienced in culturing toxic
Pfiesteria.
Fish bioassays were used to test for ichthyotoxic activity of P. piscicida versus the cryptoperidiniopsoid dinoflagellate and G. galatheanum. The toxins of Pfiesteria spp. are incompletely characterized (Fairey et al., 1999). Thus, at present, properly conducted fish bioassays are the ‘gold standard’ — i.e., the only reliable technique available — for assessing toxin-producing capability of the known toxic Pfiesteria spp. and potentially toxic pfiesteria-like dinoflagellates (Burkholder and Glasgow, 1997; Burkholder et al., 1999, Glasgow, 2000; Burkholder, in press). The technique requires maintaining healthy fish in aerated culture vessels that are amenable to cell production and toxic activity of TPC species, then adding the cloned dinoflagellate population to a subgroup of replicate fish cultures, while maintaining another set as replicate controls. Actively toxic, fish-killing strains of TPC species have been associated with serious human health impacts in laboratory and field exposures, apparently through production of aerosolized neurotoxins (Glasgow et al., 1995; Grattan et al., 1998; Duke University Medical Center records, Durham, NC). Therefore, we constructed a custom-designed biohazard III containment system at ODU (modified from the biohazard III containment system in the Burkholder and Glasgow laboratory at NCSU), and fish bioassays to detect and culture toxic Pfiesteria were conducted within that system.
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 57
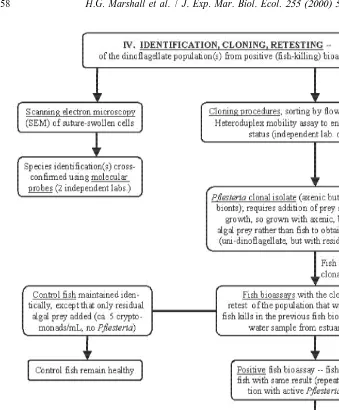
Fig. 1. Standardized steps of the Burkholder / Glasgow fish bioassay procedure used to evaluate the role of Pfiesteria in fish kills, and grow toxic Pfiesteria (Burkholder et al., 1992, 1995a,b, 1999; Burkholder and Glasgow, 1995, 1997; Glasgow, 2000; modified from Burkholder, in press). These represent a modified approach of Koch’s postulates (steps III, IV) regarding toxic rather than infectious organisms. The toxin producing Pfiesteria piscicida zoospores (TOX-A functional type) were identified and isolated through this procedure by the Burkholder and Glasgow laboratory, with cultures of these zoospores provided for the bioassays used in this study.
Fig. 1. (continued )
fish died, they were replaced with live fish. No sediment was introduced into the culture vessels. Triplicate 1-ml water samples were taken from each series (I–IV) of bioassay and control vessels at 2–3-day intervals, preserved in acidic Lugols’s solution and
examined in a Palmer–Maloney counting cell at 3400 magnification for Pfiesteria
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 59
OH). Ammonia was measured colorimetrically using an ammonia test kit (Aquarium Pharmaceuticals, Chalfont, PA).
In our initial bioassay study (I), the TOX-B functional type of P. piscicida isolate
[271-A was inoculated into three bioassay holding systems (initial concentration ca.
21
50–60 zoospores ml ), each containing three tilapia. Three similar control fish systems
(with three tilapia each) were also maintained.
In a second set of fish bioassays (II), we used TOX-A P. piscicida isolate[2200 and
inoculated three replicate bioassay systems ([1, [2, [3) containing 10 tilapia each
2l
(initial concentration ca. 50–60 zoospores ml ). Three similar systems each with 10 tilapia were also maintained as controls without P. piscicida. These bioassays were otherwise conducted similarly as the first set. When fish died, they were replaced to re-establish the original total of 10 fish. Actively toxic status of the P. piscicida isolate was maintained by continual replacement of the dead fish with live fish. When fish
deaths occurred during the fourth day in replicate [2 of this series (II), a third
experimental series (III) of three bioassays ([4,[5,[6), plus three additional controls,
was established, each containing10 tilapia. An inoculant from the surface waters of
replicate [2 was introduced into [4, [5, and [6 (initial concentration ca. 50–75
2l
zoospores ml ). A similar pattern of replacing dead fish with live fish, and recording
Pfiesteria and bacterial concentrations was followed and the practice continued for
another 10 days. After 10 days, a similar inoculant was transferred from [6 to three
additional replicate bioassay systems (series IV) with test fish ([7, [8, [9; initial
2l
concentration ca. 50–60 zoospores ml ). Three additional control fish bioassay systems
without P. piscicida were also maintained.
The cryptoperidiniopsoid (Cryptoperidiniopsis sp. [gen. nov.] [CB002) and G.
galatheanum, were introduced to separate bioassay facilities holding three to six tilapia
each, with control sets with three to six fish also established to determine their toxicity to fish over time. These assays were continued for 10 and 14 weeks, respectively, for G.
galatheanum and the cryptoperidiniopsoid species.
2.4. Fish autopsies
Autopsies were performed on fish in the test bioassay systems to assess the presence and quantity of bacteria within the blood of the fish. These fish appeared stressed after exposure to P. piscicida by their sluggish and spastic pattern of movement. Fish during the bioassays often rested on or near the bottom of the bioassay vessels prior to death. From each of the three replicate bioassays in experimental series II–IV, we randomly selected 10 of these moribund fish and 10 dead fish for autopsy. The surface of each fish was disinfected prior to autopsy with laboratory disinfectant (Conflikt, Fisher Scientific). Disinfectant was removed by rinsing with sterile Instant Ocean and wiping with a sterile swab, and an incision was made below the dorsal fin with a sterile scalpel. Blood was removed from the incision with a sterile swab and applied to bacteriological medium (TCBS, Difco, Detroit, MI) and incubated at room temperature for 24 h. The resulting growth was streak plated onto Trypticase Soy agar (Difco, Detroit, MI) for purification
´
MD). This system consists of 23 standard biochemical tests for the identification of Enterobacteriaceae and other Gram-negative bacteria. Triplicate 1-ml water samples were also taken from each series (I–IV) of bioassay and control vessels at 2–3-day intervals, for determining bacterial abundance in the water by acridine orange direct count (Hobbie et al., 1977).
3. Results
3.1. Morphology of life stages
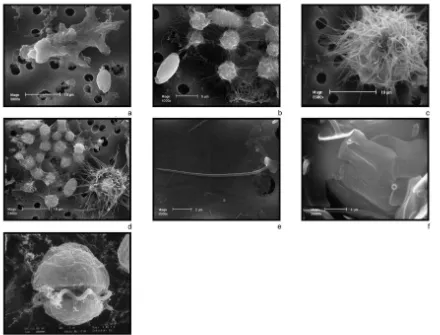
Seven life stage morphologies of Cryptoperidiniopsis sp. (gen. nov.) were identified during this study. These included a cyst, a resting stage, zoospores, gametes, planozygotes, lobose amoebae, and a rhizopodal amoeba with slender, retractable, pointed pseudopodia (Fig. 2a–g). These pseudopodia were non-branching, but when joined by other similar amoebae, would form a network around algal prey (Fig. 2b). In
P. piscicida we noted motile zoospores, rhizopodal amoebae, lobose amoebae, filose
(star) amoebae, and a cyst stage. These P. piscicida life stages are similar to those described by Burkholder et al. (1992) and Burkholder and Glasgow (1995, 1997). In the motile zoospore stage, both P. piscicida and Cryptoperidiniopsis sp. (gen. nov.) used a peduncle to feed on microalgal prey. The peduncle was extended from behind a hinged plate on the ventral surface of these cells, and attached to the prey. A swarming action around the attacked cell by several zoospores was common, and resulted in the transfer of the interior algal cell contents through the peduncle into the food vacuole located in
¨
the zoospore epitheca (process called myzocytosis; Schnepf and Elbrachter, 1992). This feeding process resulted in a swelling of the zoospore and extension of the food vacuole into the hypotheca sometimes occurred following high feeding activity. Examples of this type of feeding behavior in dinoflagellates have been described earlier by Spero (1982),
¨
Gaines and Elbrachter (1987), Glasgow et al. (1998), Lewitus et al. (1999), and others.
Gyrodinium galatheanum also isolated and cultured from Virginia estuaries, exhibited
similar feeding behavior with extended peduncle when fed Cryptomonas
In the original description of the dinoflagellate that came to be formally named P.
piscicida (Burkholder et al., 1992; Steidinger et al., 1995), it was noted that a cyst stage
of this species possesses scales that resemble those found on chrysophytes. Burkholder (1999) also illustrated several types of Pfiesteria cysts that differ in their origin, size, and the type of scales present. The cyst we have identified from this Cryptoperidiniopsis sp. (gen. nov.) has elongated spine-like projections resting upon a surface scale,
somewhat similar to one of the P. pfiesteria cysts described by Burkholder (1999). This
dinoflagellate has two types of scales covering the cyst. There is an inner layer of surface scales (Fig. 2f) external to the cell membrane that are flat, and slightly
panduriform (1.833.4 mm). They have dissimilar surfaces, containing small circular
H
.G
.
Marshall
et
al
.
/
J.
Exp
.
Mar
.
Biol
.
Ecol
.
255
(2000
)
51
–
74
61
these loosely attached to the surface of the other scales. They consist of a flattened bottom portion that rests on these inner scales, with each possessing an elongated and bluntly pointed spine (Fig. 2e). The bottom portion of these scales is cordiform in shape
(0.9–1.930.6–2.0mm). The shaft of the spine is hollow (length of 6.6–10.7mm, mean
diameter 0.25mm; n515 spines measured). SEM X-ray analysis revealed that silicon is
a component of both the cyst (Fig. 3a) and scale types. The examination of cysts derived from the TOX-A P. piscicida using SEM X-ray analysis indicated these cysts also contained silicon (Fig. 3b).
3.2. Fish bioassays
In the first set of fish bioassays (I), a strain of TOX-B Pfiesteria piscicida (potentially
toxic, but previously fed algal prey, [271-A) was added to three replicate test fish
21
bioassay systems (initial Pfiesteria density ca. 50–60 zoospores ml ). There was no
immediate or recognizable reaction from the fish for the first 15 days of exposure to P.
piscicida. The first fish death occurred after 16 days. The dead fish were replaced with
three live tilapia which died within 24 h. Thereafter, fish deaths occurred on an intermittent basis in all three replicate bioassays. There were no fish deaths in the controls.
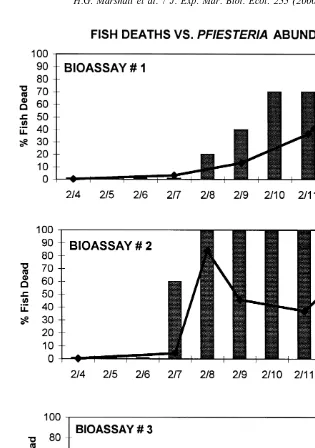
In the second set of fish bioassays (II), a strain of TOX-A P. piscicida (previously
given live fish, rather than algal prey, [2200) was added to the initial three test fish
21
bioassay systems (initial Pfiesteria density ca. 50–60 zoospores ml ). After 72 h of
exposure to P. piscicida, six of 10 fish had died in replicate bioassay system [2, and
21
zoospores had increased ca. 10-fold to ca. 600 cells ml (Fig. 4). The six dead fish and
four remaining live fish were removed and replaced with 10 live fish. Within 24 h, all 10 21
fish had died, and the zoospore density had increased to ca. 1180 cells ml . Further
daily replacement of dead with live fish over the next 4 days resulted in death of all fish, 21
with zoospore concentrations reaching ca. 5000 cells ml . Fish deaths in replicate
bioassay system[1 did not occur until day 5 of exposure to P. piscicida (zoospores at
21 21
ca. 460 cells ml ), with all fish dead by day 9 (zoospores at 10 000 cells ml ).
In contrast to these results in the first two replicates, fish did not die in replicate 21
bioassay[3 even when Pfiesteria concentrations were .30 000 cells ml (day 9). At
day 14, the still-live fish in replicate [3 were transferred to replicate [2, and all fish
were dead within 24 h. When 10 replacement fish were added to replicate[3, no fish
deaths occurred over an additional 14-day period. In replicates[1 and[2, toxicity was
maintained by replacing dead with live fish, with the shortest time to fish death occurring within 1.5 h of exposure to TOX-A P. piscicida. Throughout the experimental period, none of the 30 fish in the three replicate control bioassay systems (without exposure to P.
piscicida) died, and all appeared healthy. These toxic P. piscicida have since been continuously maintained in our laboratory by providing them with live fish.
On the fifth day of the above bioassays, another series of inoculations (bioassay III)
from replicate [2 with P. piscicida were transferred to three additional replicates,[4,
21
[5, and[6 (initial P. piscicida density at 50–75 zoospores ml ). Fish deaths began to
occur on the fourth day of exposure to P. piscicida in replicate[4, on the seventh day in
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 63
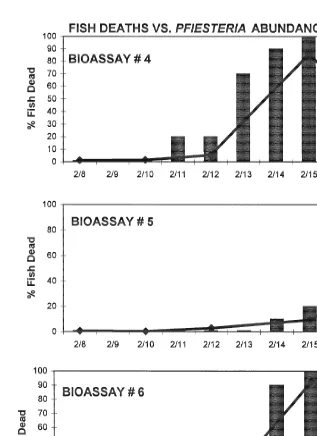
Fig. 4. Results of fish bioassay II. Bars indicate percent of fish (10) deaths over time in reference to 21
concentrations of Pfiesteria piscicida zoospores ml in culture vessels[1–3.
21
piscicida during the initial fish deaths were ca. 500–1200 zoospores ml . By the eighth
day, death of all 10 fish had occurred in replicates [4 and [6, with P. piscicida at
21
8000–11 000 zoospores ml . Replicate[5 required a longer time interval for total fish
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 65
Fig. 5. Results of fish bioassay III. Bars indicate percent of fish (10) deaths over time in reference to 21
concentrations of Pfiesteria piscicida zoospores ml in culture vessels[4–6.
piscicida by replacing dead with live fish throughout an 18-day period. Over the test
period, one of the 30 fish in the three replicate controls died, and examination revealed no dinoflagellates present in the water of that control vessel.
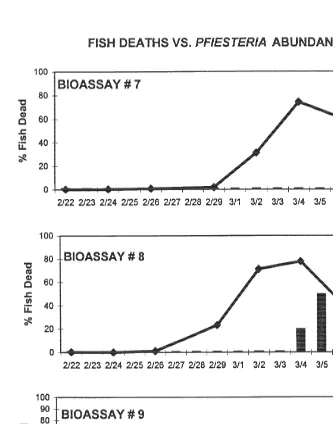
On the 18th day, fish bioassays[7, [8, [9 (10 fish per replicate) were inoculated
21
bioassay systems of fish without exposure to P. piscicida were maintained in triplicate as well, with no deaths occurring in the controls over the 15-day experimental period. In
contrast, fish deaths occurred in the replicate bioassay systems containing P. piscicida at
11 days ([9), 12 days ([8), and 15 days ([7) (Fig. 6), corresponding to zoospore
Fig. 6. Results of fish bioassay IV. Bars indicate percent of fish (10) deaths over time in reference to 21
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 67
21
concentrations of 1000–16 000 cells ml . The results of the entire series (II, III, IV) are
illustrated in Fig. 7.
In each of the test replicates with P. piscicida within all three of the experimental series, the fish exhibited noticeable stress prior to death. They moved with irregular, spastic motion, and had little forward advancement in their attempts to swim to the surface, after which they slowly settled to the bottom of the bioassay systems. This pattern was repeated until the fish died. All oxygen measurements taken during fish deaths indicated there were comparable oxygen levels in both the bioassay and the
21
controls (.4 mg dissolved oxygen l ), above levels generally considered to stress fish
(Meyer and Barclay, 1990). Ammonia levels also were similar within the test bioassay 21
systems and the controls. Ammonia was generally ,0.25 mg l . In one control
21
replicate, ammonia was 8 mg l on one date, but no fish died in this replicate; and all
replicate test bioassay systems with P. piscicida had ammonia concentrations ,0.05 mg
21
l .
All fish bioassays conducted with the Cryptoperidiniopsoid sp. (gen. nov.) were negative. This set of bioassays was conducted over a 14-week period, with cell
21
abundance reaching ca. 600–750 zoospores ml . None of the fish died in either the
bioassay, or the control vessels, and all appeared healthy. Similar results were found for the fish bioassays using G. galatheanum. Over a 10-week period of exposure with cell
21
concentrations reaching ca. 700–800 zoospores ml , no fish deaths occurred and all
fish appeared healthy.
3.3. Fish autopsies and bacteria analysis
In nine of the 10 moribund fish taken from the fish bioassays (II–IV), the blood contained no bacteria-forming colonies on TCBS medium. Autopsy of the 10th previously moribund fish revealed the presence of oxidase-positive, non-fermentative, gram-negative rods in the blood. In contrast, the 10 fish that were randomly sampled for autopsy after death all contained bacteria in their blood that grew on TCBS medium, including several Vibrio and Aeromonas spp.
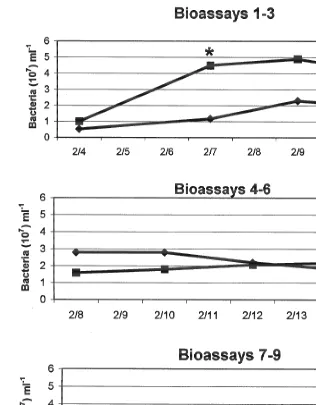
In experimental series II of the fish bioassays, the mean bacterial concentrations in the
water column of both the test replicates (fish bioassays [1, [2, [3, with P. piscicida)
and the controls increased over the first 5 days before slightly decreasing by the seventh day (Fig. 8). Mean bacterial abundance in test fish bioassays were significantly different from that of controls on only one of four dates analyzed (7 Feb., Student’s t-test,
P50.023). In analyses from experimental series II ([4, [5, [6 with P. piscicida),
there were no significant differences in mean bacterial abundance from test fish bioassay
versus control water. Of eight dates for series III ([7, [8, [9 with P. piscicida), the
bacterial abundance was similar in the test bioassays and the controls, except for one
date (24 Feb., P50.011). The bacterial cell concentrations in this last group were the
lowest of the three sets, but these results were also the most comparable and extended over a 16-day period. Overall, there were no significant differences in the bacterial abundance between the fish bioassays and the control vessels for 14 of the 16 comparison dates.
4. Discussion
Dinoflagellates resembling Pfiesteria spp. are widely distributed in Virginia estuaries and in the lower Chesapeake Bay (Marshall et al., 1999). Among the more common forms in these waters are the cryptoperidiniopsoid species that have morphological features, life cycle stages, and some behavioral traits similar to those of Pfiesteria
piscicida. There are at least two cryptoperidiniopsoid species in Virginia estuaries and
the Chesapeake Bay. One is Cryptoperidiniopsis brodyi (gen. et sp. nov.), the other is presently considered Cryptoperidiniopsis sp. (gen. nov.) that is, as yet, incompletely described. A characteristic behavioral pattern shared by both of these
Cryptoperidiniop-sis species and Pfiesteria can be observed with light microscopy. This behavior conCryptoperidiniop-sists
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 69
Fig. 8. Mean bacterial concentrations in each set of the experimental fish bioassays and the corresponding control vessels (e.g., bioassays 1–3, series II; bioassays 4–6, series III; bioassays 7–8, series IV). *Dates when cell concentrations were significantly different.
these dinoflagellates (Burkholder and Glasgow, 1995; Glasgow et al., 1998; Seaborn et al., 1999).
This research corroborates reports of a complex life cycle with amoeboid stages in P.
piscicida (Burkholder et al., 1992; Burkholder and Glasgow, 1995, 1997; Steidinger et
cryptoperidiniopsoid, Cryptoperidiniopsis sp. (gen. nov.). These include the presence of several amoeboid and cyst stages. Gradual transformation changes may be noted using long-term and / or intensive light microscopy. For example, we have observed dramatic transformations in overnight observations of unialgal cultures that initially consisted of
high zoospore densities of either P. piscicida or Cryptoperidiniopsis sp. (gen. nov.) that
were food-depleted (without algal prey for 5–7 days). The next day, these cultures were observed to be devoid of most zoospores, comprised instead of high concentrations of either amoeboid or cyst stages. When cryptomonad algal prey were subsequently added to these cultures, high densities of zoospores were re-established and the amoeboid and cyst stages were significantly reduced in abundance. In another approach, positive linkages among these life stages can be confirmed from genetic sequencing of the various stages. For example, we have sent blind samples consisting of zoospores versus amoebae, taken from our cultures of Cryptoperidiniopsis brodyi (gen. et sp. nov.), to Dr. D. Oldach (UMD; Oldach et al., 2000). The gene sequencing results for both the zoospores and the amoebae were evaluated as positive for this species, and represent stages of its life cycle. The Burkholder and Glasgow laboratory has confirmed various stages in the life cycle of P. piscicida and P. shumwayae (sp. nov.) from molecular analysis in independent laboratories (Dr. P. Rublee of UNC-Greensboro, and Dr. D. Oldach of UMD; e.g., Rublee et al., 1999). Similar action to cross-confirm life stage relationships for other dinoflagellates with complex life cycles would be appropriate, and are recommended.
Various life stages are also identified in this study of Cryptoperidiniopsis (nov. gen.). These include a distinct cyst stage that is characterized by two types of scales that are different than those noted for P. piscicida (Burkholder et al., 1992, 1995b; Burkholder and Glasgow, 1995, 1997). Burkholder and Glasgow (1995, 1997) and Burkholder (1999) have recognized multiple cyst forms in Pfiesteria that are produced from different life stages of the species, so it is likely that multiple cyst stages will also be found in the life cycle of this and other cryptoperidiniopsoid spp. When fully characterized, these species-specific morphological differences in the cyst scales may prove to be of value in discerning the identification among pfiesteria-like species verses
Pfiesteria spp. The presence of silicon in the cysts of both Pfiesteria piscicida and the Cryptoperidiniopsis sp. (gen. nov.) is of additional interest, since silicon is not
commonly found in dinoflagellates. Its presence here may indicate an early evolutionary infusion of this trait (scale formation) into the genome of these dinoflagellates by a protozoan, or chrysophyte algal endosymbiont. Similarly, the ‘chrysophyte-like’ cyst of
P. piscicida zoospores that was reported by Burkholder et al. (1992), has fostered
speculation that Pfiesteria may be an evolutionary ‘old’ dinoflagellate genus that encompasses both chrysophyte and dinoflagellate characteristics.
Although the onset and initial strength of toxicity varied among the bioassay experiments, Pfiesteria piscicida was directly associated with a series of fish deaths that were produced in this study. The presence of P. piscicida was linked to fish deaths in the
initial bioassay, and in eight of the nine subsequent bioassays. The P. piscicida culture
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 71
zoospores, there was also an increase in the duration before fish were affected. This was clearly demonstrated in the third set of bioassays in which 11–15 days elapsed before fish death occurred. However, once an actively toxic P. piscicida culture was established, the culture continued to be toxic as long as dead fish were replaced with live fish. The
most rapid time to fish death was 1.5 h of transfer into the actively toxic P. piscicida
culture. These results support those reported in previous studies by Burkholder et al. (1992, 1995a,b, 1999), Noga et al. (1993), Lewitus et al. (1995), Burkholder and Glasgow (1997), and Glasgow (2000) describing toxicity of Pfiesteria spp. to fish, and the variability in the onset of toxicity by P. piscicida using TOX-A and TOX-B functional types of Pfiesteria (Burkholder and Glasgow, 1997; Burkholder et al., 1999; WHOI, 2000; Burkholder, in press).
The serial transfer of P. piscicida zoospores from one set of bioassays through two additional bioassays perpetuated the toxicity in these dinoflagellate populations over time in the laboratory, using different and multiple compliments of fish. Of note, during the bioassay experiments only one fish (out of 90) in the control experiments died, with none of the deaths occurring within the toxic bioassays associated with depressed oxygen or high ammonia levels. Autopsies of live fish that exhibited symptoms of
Pfiesteria toxicity showed absence of bacterial infection in nine out of 10 fish examined.
In contrast, blood from dead fish contained bacteria. These results indicate that bacterial septicemia was not the cause of fish mortality in these bioassays, although bacteria rapidly spread into the circulatory system after death. Further results of the bacterial concentrations in this study show no significant differences in bacterial abundance in the water from test fish bioassays with Pfiesteria versus the water from control fish cultures, in 14 of the 16 comparisons over the testing period. These findings indicate that bacterial abundance was not a factor related to the fish deaths. In addition, the systematic examination of the water in all the bioassay series and the control facilities throughout these experiments indicated no perceptible differences in the presence of any microflora, or microfauna in the control or test fish bioassays throughout the study.
In contrast to these data supporting ichthyotoxicity of Pfiesteria piscicida at lower cell 21
densities (e.g., 250–300 zoospores ml ; Burkholder et al., 1995a), the experiments with
the Cryptoperidiniopsis sp. (gen. nov.) and G. galatheanum, indicate that neither of 21
these species is toxic to fish at the cell concentrations attained (ca. 700–800 cells ml )
over periods up to several months. These dinoflagellates did not increase cell production to higher densities when grown with fish, with comparable cell densities present in control vessels without fish. Moreover, the fish in test bioassays with both species remained comparable in health to the control fish, showed no signs of stress or disease. As mentioned, other research has demonstrated no ichthyotoxicity of various strains and species of Cryptoperidiniopsis (gen. nov.) from the mid-Atlantic and southeastern US (Delaware to Florida: Burkholder et al., 1995a; Glasgow, 2000; Burkholder, in press). At
3 21
extremely high cell densities (115310 zoospores ml ), G. galatheanum has been
reported as a toxin producer associated with deaths of juvenile cod (Gadus morhua) by Nielsen (1993), and with reduced growth rate in mussels (Mytilus edulis) by Nielsen and
3 21
Stroemgren (1991) at concentrations above ca. 120310 zoospores ml . If our strain
indicated that the two ‘pfiesteria-lookalike’ species examined are strikingly distinct from
P. piscicida. Although they appear physically similar to Pfiesteria, they differ in the
important trait of a toxic response toward fish. Further research will strengthen insights about the comparative ecology of toxic Pfiesteria complex species in comparison to these and other dinoflagellates.
Acknowledgements
Financial support for various components of this study was provided by the Virginia Department of Health, the Virginia Department of Environmental Quality, the Center for Disease Control and Prevention, and the University of Miami (grant ES05705). Appreciation is given to personnel from the Virginia Department of Environmental Quality and Department of Health for providing the samples analyzed in this program. Special thanks and appreciation is given to JoAnn Burkholder, Howard Glasgow, Matthew Lynn, David Oldach, Parke Rublee, Karen Steidinger, and Ernest Truby for their contributions during various phases of this work. [SS]
References
Buckland-Nicks, J.A., Reimechen, T.E., Taylor, F.J.R., 1990. A novel associations between an endemic stickleback and a parasitic dinoflagellate. 2. Morphology and life cycle. J. Phycol. 26, 539–548. Burkholder, J.M., 1998. Implications of harmful microalgae and heterotrophic dinoflagellates in management
of sustainable marine fisheries. Ecol. Appl. 8 (Suppl. 1), S37–S62. Burkholder, J.M., 1999. The lurking perils of Pfiesteria. Sci. Am. 281, 42–49.
Burkholder, J.M., in press. The toxic Pfiesteria complex. In: Hallegraeff, G., Blackburn, S., Bolch, C., Lewis, R. (Eds.), Harmful Algal Blooms. IOC UNESCO, Paris.
Burkholder, J.M., Glasgow, Jr. H.B., 1995. Interactions of a toxic estuarine dinoflagellate with microbial predators and prey. Arch. Protistenk. 145, 177–188.
Burkholder, J.M., Glasgow, Jr. H.B., 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: Behavior, impacts, and environmental controls. Limnol. Oceanogr. 42, 1052–1075.
Burkholder, J.M., Noga, E.J., Hobbs, C.W., Glasgow, H.B. Jr., Smith, S.A., 1992. New ‘phantom’ dinoflagellate is the causative agent of major estuarine fish kills. Nature 358 / 360, 407–410, 768. Burkholder, J.M., Glasgow, Jr. H.B., Hobbs, C.W., 1995a. Fish kills linked to a toxic ambush–predator
dinoflagellate: distribution and environmental conditions. Mar. Ecol. Prog. Ser. 124, 43–61.
Burkholder, J.M., Glasgow, Jr. H.B., Steidinger, K.A., 1995b. Stage transformation in the complex life cycle of an ichthyotoxic ‘ambush–predator’ dinoflagellate. In: Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C. (Eds.), Harmful Marine Algal Blooms, Technique et Documentation, Lavoisier. Intercept, Paris, pp. 567–572.
Burkholder, J.M., Mallin, M.A., Glasgow, H.B., Larsen, L.M., McIver, M.R., Shank, G.C., Deamer-Melia, N., Briley, D.S., Springer, J., Touchette, B.W., Hannon, E.K., 1997. Impacts to a coastal river and estuary from rupture of a large swine waste holding lagoon. J. Environ. Qual. 26, 1451–1466.
Burkholder, J.M., Mallin, M.A., Glasgow, Jr. H.B., 1999. Fish kills, bottom-water hypoxia, and the toxic Pfiesteria complex in the Neuse river and estuary. Mar. Ecol. Prog. Ser. 179, 301–310.
Bursa, A., 1970a. Dinamoebidinium coloradense spec. nov. and Katodinium auratum spec. nov. in Como Creek, Boulder County, Colorado. Arct. Alp. Res. 2, 145–151.
H.G. Marshall et al. / J. Exp. Mar. Biol. Ecol. 255 (2000) 51 –74 73 Fairey, E., Edmunds, S., Deamer-Melia, N.J., Glasgow, Jr. H.B., Johnson, F., Moeller, P., Burkholder, J.M., Ramsdell, J., 1999. Reporter gene assay for fish killing activity produced by Pfiesteria piscicida. Environ. Health Perspect. 107, 711–714.
¨
Gaines, G., Elbrachter, M., 1987. Heterotrophic nutrition. In: The Biology of Dinoflagellates. Botanical Monographs, Vol. 31. Blackwell Scientific, Oxford, pp. 224–268.
Glasgow, H.B. Jr., 2000. The Biology and Impacts of Toxic Pfiesteria Complex Species. Ph.D. Dissertation, Department of Marine, Earth and Atmospheric Sciences, North Carolina State University, Raleigh, NC. Glasgow, Jr. H.B., Burkholder, J.M., Schmeche, D.E., Fester, P., Rublee, P.A., 1995. Insidious effects of a
toxic estuarine dinoflagellate on fish survival and human health. J. Toxicol. Environ. Health 46, 501–522. Glasgow, Jr. H.B., Burkholder, J.M., Lewitus, A.J., 1998. Feeding behavior of the ichthyotoxic estuarine dinoflagellate, Pfiesteria piscicida, on amino acids, algal prey, and fish vs. mammalian erythrocytes. In: Reguera, B., Fernandez, M.L., Wyatt, T. (Eds.), Harmful Microalgae. UNESCO, Paris, pp. 394–397. Grattan, L., Oldach, D., Perl, T., Lowitt, M., Matuszak, D., Dickson, C., Parrott, C., Shoemacher, R.,
Wasserman, M., Hebel, J., Charache, P., Morris, J., 1998. Problems in learning and memory occur in persons with environmental exposure to waterways containing toxin-producing Pfiesteria or Pfiestera-like dinoflagellates. Lancet 352, 532–539.
Hobbie, ley J., Daley, R., Jasper, S., 1977. Use of Nucleopore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33, 1255–1258.
Kamykowski, D., Reed, R., Kirkpatrick, G., 1992. Comparison of sinking velocity, swimming, rotation and path characteristics among six marine dinoflagellate species. Mar. Biol. 113, 319–328.
Lewitus, A.J., Jesien, R., Kana, T., Burkholder, J.M., Glasgow, Jr. H.B., May, E., 1995. Discovery of the ‘phantom’ dinoflagellate in Chesapeake Bay. Estuaries 18, 373–378.
Lewitus, A.J., Glasgow, Jr. H.B., Burkholder, J.M., 1999. Kleptoplastidy in the toxic dinoflagellate, Pfiesteria piscicida. J. Phycol. 35, 303–312.
Magnien, R., Goshorn, D., Michael, B., Tango, P., Karrh, R., Nelson, R., 1999. Findings for water quality, habitat and Pfiesteria related comprehensive assessment and response efforts in 1998. Special Report prepared by the Maryland Pfiesteria Study Team, Annapolis, MD, February 1999, pp. 13–15.
Marshall, H.G., 1999. Pfiesteria piscicida and dinoflagellates similar to Pfiesteria. Virginia J. Sci. 50, 281–286. Marshall, H.G., Seaborn, D., Wolny, J., 1999. Monitoring results for Pfiesteria piscicida and Pfiesteria-like
organisms from Virginia waters in 1998. Virginia J. Sci. 50 (4), 287–298.
Meyer, F., Barclay, L., 1990. Field Manual for the Investigation of Fish Kills. US Fish & Wildlife Service Resource Publication 1777. US Department of the Interior, Washington, DC.
Nielsen, M., 1993. Toxic effect of the marine dinoflagellate Gymnodinium galatheanum on juvenile cod Gadus morhua. Mar. Ecol. Prog. Ser. 95, 273–277.
Nielsen, M., Stroemgren, T., 1991. Shell growth response of mussels (Mytilus edulis) exposed to toxic microalgae. Mar. Biol. 108, 263–267.
Noga, E.J., Smith, S.A., Burkholder, J.M., Hobbs, C.W., Bullis, R.A., 1993. A new ichthyotoxic dinoflagellate: cause of acute mortality in aquarium fishes. Vet. Rec. 133, 48–49.
Oldach, D., Delwiche, C., Jakiobsen, K., Tengs, T., Brown, E., Kempton, J., Schaefer, E., Bowers, H., Glasgow, Jr. H.B., Burkholder, J.M., Steidinger, K.A., Rublee, P.A., 2000. Heteroduplex mobility assay guided sequence discovery: elucidation of the small subunit (18S) rDNA sequence of Pfiesteria piscicida from complex algal culture and environmental sample DNA pools. Proc. Natl. Acad. Sci. USA 97, 4304–4308.
¨ ¨ ¨
Pascher, A., 1916. Uber eine neu Amoeba (Dinamoebe varians) mit dinoflagellatenartigen Schwarmen. Arch. Protistenk. 36, 118–136.
Pfiester, L., Lynch, R., 1980. Amoeboid stages and sexual reproduction of Cystodinium bataviense and its similarity to Dinococcus (Dinophyceae). Phycologia 19 (3), 178–183.
´
Pfiester, L., Popovsky, J., 1979. Parasitic, amoeboid dinoflagellates. Nature 279, 421–424. ´
Popovsky, J., 1982. Another case of phagotrophy by Gymnodinium helveticum Penard f. achroum Skuja. Arch. Protistenk. 125, 73–78.
´
Popovsky, J., Pfiester, L., 1990. In: Dinophyceae (Dinoflagellida). Gustav Fischer, Jena, Stuttgart, p. 272. Rublee, P.A., Kempton, J., Schaefer, E., Burkholder, J.M., Glasgow, Jr. H.B., Oldach, D., 1999. PCR and FISH
¨
Schnepf, E., Elbrachter, M., 1992. Nutritional strategies in dinoflagellates. A review with emphasis on cell biological aspects. Eur. J. Protistol. 28, 3–24.
Seaborn, D., Seaborn, A., Dunstan, W., Marshall, H., 1999. Growth and feeding studies on the algal feeding stage of a Pfiesteria-like dinoflagellate. Virginia J. Sci. 50, 337–344.
Spero, H., 1982. Phagotrophy in Gymnodinium fungiforme (Pyrrhophyta): the peduncle as an organelle of ingestion. J. Phycol. 18, 356–360.
Steidinger, K.A., 1993. Some taxonomic and biologic aspects of toxic dinoflagellates. In: Falconer, I. (Ed.), Algal Toxins in Seafood and Drinking Water. Academic Press, London, pp. 1–28.
Steidinger, K.A., Truby, E., Garrett, J., Burkholder, J.M., 1995. The morphology and cytology of a newly discovered toxic dinoflagellate. In: Lassus, P., Arzul, G., Erad, E., Gentien, P., Marcaillou, C. (Eds.), Harmful Marine Algal Blooms, Technique et Documentation, Lavoisier. Intercept, Paris, pp. 83–88. Steidinger, K.A., Burkholder, J.M., Glasgow, Jr. H.B., Hobbs, C.W., Garrett, J., Truby, E., Noga, E.J., Smith,
S.A., 1996. Pfiesteria piscicida gen. et sp. nov. (Pfiesteriaceae Fam. nov.), a new toxic dinoflagellate with a complex life cycle and behaviour. J. Phycol. 32, 157–164.
Tomas, C.R., 1996. In: Identifying Marine Diatoms and Dinoflagellates. Academic Press, New York, p. 598. Truby, E.W., 1997. Preparation of single-celled marine dinoflagellates for electron microscopy. Microsc. Res.
Tech. 36, 337–340.