SEA/ HLM/ 324
Dist ribut ion: General
Guidelines on
St andard Operat ing Procedures
for Microbiology
Sudarshan Kum ari
Sudarshan Kum ari
Sudarshan Kum ari
Sudarshan Kum ari
Regional Advisor, Blood Safety & Clinical Technology
WHO, SEARO
R.L. Ichhpujani
R.L. Ichhpujani
R.L. Ichhpujani
R.L. Ichhpujani
World Health Organization
Regional Office for South- East Asia
New Delhi
Page Page Page Page 3333
© World Health Organization 2000
This document is not a formal publication of the World Health Organization (WHO),
Page Page Page Page iiii
Acknowledgem ents
WHO grat efully acknowledges the valuable contributions of Dr Rajesh Bhatia,
Consultant and Head, Departm ent of Microbiology, National Institute of
Communicable Diseases, Delhi; Dr C.S. Bhaskaran (former Director, Instit ute
of Preventive Medicine, Hyderabad); Dr K.B. Sharm a (form er Regional Adviser,
Health Laboratory Services, WHO Regional Office for South- East Asia) and Mr
K.K. Khanna (form er Joint Director, National Institute of Com municable
Page Page Page Page iiiiiiiii iii
Preface
Laborat ory services have becom e an integral and inseparable com ponent of
m odern m edicine and public health. Laborat ories play a decisive role in the
diagnosis, treatm ent, prognosis and monitoring of both communicable and
noncommunicable diseases. Intermediate and peripheral health facilities in
developing countries are crucial to prim ary health care. Therefore, reliable,
reproducible and rapid laboratory services, organized in a cost- effective
manner, will go a long way in providing quality health services at the dist rict
hospitals and health cent res.
Quality assurance in laboratory services, aimed at im proving reliabilit y,
efficiency and facilitating inter- laboratory com parabilit y in t esting, is the
backbone of quality health care delivery. The use of standard operating
procedures in laborat ory testing is one of the m ost crucial factor in achieving
quality. This helps both in proper pat ient m anagem ent and generates reliable
disease surveillance dat a. This publication provides guidelines on standard
operating procedures for diagnosing diseases of public health importance at
interm ediat e and peripheral levels. Guidelines on early warning signals about
epidem ic- prone diseases based on laboratory data and on collecting and
effectively transport ing the appropriate clinical specim ens to the
referral/ central laboratories for diseases for which diagnostic services are not
as yet developed at the interm ediat e laboratories are also provided.
The publication contains guidelines on the use of conventional
procedures which m ay be adapted as per local needs. The em phasis is on
providing feasible, pract ical, easy- to- reproduce, specific, sim ple and
cost-effective techniques for the diagnosis of com municable diseases. Necessary
biosafet y guidelines have been provided and situations where referrals to
higher- level laboratories are indicated have also been identified.
It is hoped that this publication will be useful in achieving its objective of
im proving the qualit y of laborat ory services at interm ediat e and peripheral
SECTION A: GENERAL LABORATORY PRACTICES SECTION A: GENERAL LABORATORY PRACTICESSECTION A: GENERAL LABORATORY PRACTICES
SECTION A: GENERAL LABORATORY PRACTICES
1 11
1 Organization and Functions of LaboratoriesOrganization and Functions of LaboratoriesOrganization and Functions of LaboratoriesOrganization and Functions of Laboratories 1
Peripheral laboratory services ... 1
Interm ediat e laboratory services ... 3
2 22 2 Collection and Transportation of ClinCollection and Transportation of ClinCollection and Transportation of ClinCollection and Transportation of Clinical Specimensical Specimensical Specimensical Specimens 5
Criteria for rejection of specim ens... 5
Collection of specimens... 6
Transportation of specim ens ... 9
3 4 Staining Staining TechniquesStaining TechniquesStaining Techniques 17Techniques Methylene blue staining ... 17
Gram staining ... 18
Albert’ s staining ... 19
India ink staining ... 20
Ziehl Neelsen staining ... 20
Iodine staining for ova and cyst s ... 21
Quality control of stains ... 22
5 55 5 Bacteriological Bacteriological MediaBacteriological MediaBacteriological Media 23Media Types of m edia ... 23
Preparation of m edia and checking of pH ... 24
Nutrient broth... 24
Nutrient agar ... 25
Glucose broth ... 25
Blood agar ... 25
Page Page Page Page vivivivi
Page Page Page Page
XLD agar ... 25
Buffered glycerol saline ... 26
Loeffler serum m edium ... 26
Blood t ellurit e agar ... 27
Salt broth (10%) ... 27
Peptone wat er ... 28
Alkaline peptone water ... 28
MacConkey agar ... 28
Bile salt agar ... 28
Selenite F broth ... 30
Media for carbohydrate ferm entation ... 30
Lowenstein Jensen m edium ... 31
Cary and Blair transport m edium ... 32
Stuart transport m edium ... 32
Venkataram an- Ram akrishnan (V.R.) holding m edium ... 33
MacConkey broth for bacteriological ex amination of water ... 33
Sabouraud Dex trose Agar (SDA) ... 34
Perform ance of plated m edia ... 36
6 66 6 Cultivat ion of Bact eria on Laboratory MediaCultivat ion of Bact eria on Laboratory MediaCultivat ion of Bact eria on Laboratory MediaCultivat ion of Bact eria on Laboratory Media 39
Instrum ent for seeding m edia... 39
Aseptic t echniques ... 41
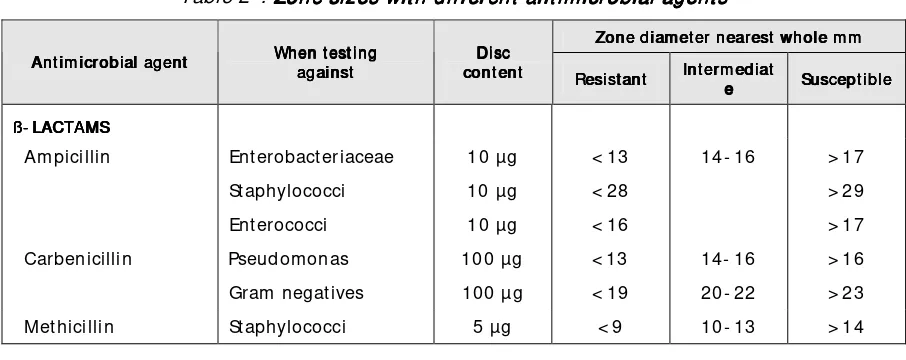
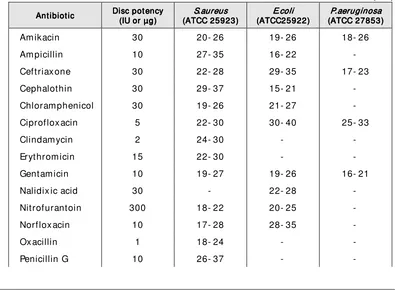
7 77 7 Antim icrobial Antim icrobial Susceptibilit y TestingAntim icrobial Susceptibilit y TestingAntim icrobial Susceptibilit y Testing 43Susceptibilit y Testing Modified Kirby- Bauer m ethod ... 44
Quality assurance in susceptibilit y t est ... 47
Biosafet y ... 48
Referral ... 4 8 When intermediate laboratories need not undertake suscpetibility test s?... 48
Salient features of qualit y assurance in antibiotic suscept ibilit y testing ... 48
8 88 8 Safet y in Laborat oriesSafet y in Laborat oriesSafet y in Laborat oriesSafet y in Laborat ories 53
Laborat ory biosafet y levels ... 53
Preventive m easures against laboratory infections... 54
Pipetting ... 54
Hypoderm ic syringes and needles ... 54
Opening containers ... 55
Laborat ory access ... 56
Clothing... 56
Accidents in laboratory ... 56
Accidents and spills ... 57
Guidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for MicrobiologyGuidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for Microbiology
Page Page Page Page viiviiviivii
Page Page Page Page
General laborat ory direct ions for safety ... 58
9 99 9 Qualit y Qualit y AssuranceQualit y AssuranceQualit y Assurance Assurance 59 What is the objective of QA? ... 59
Factors affecting the quality ... 59
Maintenance of equipm ent ... 61
Perform ance tests on culture m edia ... 62
Quality control for com m only- used t est s ... 63
Quality control of im m unological tests ... 63
QA of antibiotic susceptibility testing ... 63
In service training of staf f ... 65
Participation in ex t ernal quality assessment ... 65
SECTION B: STANDARD SECTION B: STANDARD SECTION B: STANDARD SECTION B: STANDARD PROCEDURES FOR SPECIPROCEDURES FOR SPECIPROCEDURES FOR SPECIPROCEDURES FOR SPECIFIC DISEASESFIC DISEASESFIC DISEASESFIC DISEASES 10 1010 10 CholeraCholeraCholeraCholera 67
Causative organism ... 67
Specim ens ... 67
Collection, storage and t ransportat ion of specimens ... 67
Materials... 68
Procedure ... 69
Quality assurance ... 72
Antim icrobial susceptibility t est ing ... 72
Reporting of result s ... 72
Referral ... 7 2 Biosafet y ... 72
11 1111 11 DiphtheriaDiphtheriaDiphtheriaDiphtheria 73
Collection and t ransport ation of specimens... 73
Storage ... 74
Processing of swabs ... 74
Staining of smears ... 74
Reporting of result s ... 76
Quality assurance ... 76
Other significant organisms isolated from throat swabs ... 76
Im portant points about diagnosis of diphtheria... 76
Susceptibility to antim icrobial agents ... 77
Biosafet y ... 77
Page Page Page Page viiiviiiviiiviii
Page Page Page Page
Isolation of the organism ... 79
Antim icrobial susceptibility t est ing ... 81
Serodiagnosis ... 81
Quality assurance ... 83
Diagnosis of chronic typhoid carriers ... 84
Biosafet y ... 84
Referral ... 8 4 13 1313 13 Pyogenic Pyogenic MeningitisPyogenic MeningitisPyogenic Meningitis 87Meningitis Causative organism s... 87
Collection, storage and t ransportat ion of specimens ... 88
Abnormalities associated with pyogenic m eningitis... 89
Im m unological test s ... 91
Antim icrobial susceptibility t est ing ... 92
Quality assurance ... 93
Biosafet y ... 93
Referral ... 9 3 14 1414 14 DysenteryDysenteryDysenteryDysentery 95
Collection of faeces ... 95
Macroscopic ex amination ... 96
Microscopic ex amination ... 96
Isolation of organism ... 96
Cautions ... 97
Biosafet y ... 97
Referral ... 9 7 Antim icrobial sensitivit y testing ... 97
Reporting of result s ... 97
Quality assurance ... 98
15 1515 15 GonorrhoeaGonorrhoeaGonorrhoeaGonorrhoea 99
Causative organism ... 99
Specim en collection ... 99
Staining the smear and ex amination ... 10
0 Specim en transportat ion for culture ... 10
1 Culture ... 10
1 Presumptive identification ... 10
Guidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for MicrobiologyGuidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for Microbiology
Page Page Page Page ixixixix
Page Page Page Page
3 Quality assurance ... 10 3 Reporting of result s ... 10
3 Antim icrobial susceptibility t est ing ... 10 3 Referral ... 1 0 4
16 1616
16 SyphilisSyphilisSyphilis Syphilis 10
5 Direct dem onstration ... 10 5 Reporting of result s ... 10 7 Susceptibility testing ... 10 7 Serological evidence ... 10 7 Biological false positives in VDRL... 10 9 Quality assurance in VDRL testing ... 11 0 Referral ... 1 1
0 Biosafet y ... 11 0
17 1717
Page Page Page Page xxxx
Page Page Page Page
Culture for M. tuberculosis ... 11 5 Biosafet y ... 11 6
Quality assurance ... 11 7 Susceptibility to antituberculosis drugs ... 11 7 Reporting of result s ... 11 7 Referral ... 1 1 7
18 1818
18 MalariaMalariaMalariaMalaria 11
9 Preparation of blood smear ... 11 9 Recognition of the m alaria parasite ... 12 1 Com m on defects in m aking blood film s ... 12 3 Reporting of result s ... 12 3 Referral ... 1 2 3
Quality assurance ... 12 3 Biosafet y ... 12 4
19 1919
Guidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for MicrobiologyGuidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for Microbiology
Page Page Page Page x ix ix ix i
Page Page Page Page
0 Susceptibility to antim icrobial agents ... 13 0 Reporting of result s ... 13
0 Referral ... 1 3 1 Biosafet y ... 13 1
20 2020
20 ParasitologiParasit ologiParasitological Ex amination of FaecesParasit ological Ex amination of Faecescal Ex amination of Faecescal Ex amination of Faeces 13 3 Collection of faecal sam ple ... 13 3 Transportation of sam ples... 13 4 Macroscopic ex amination ... 13 4 Microscopic ex amination (temporary wet m ounts)... 13 4 Concentration t echniques ... 13 5 Formal ether sedimentation technique ... 13 6 Biosafet y ... 13
8 Disposal of m orbid m at erial ... 13 8 Quality assurance ... 13 8 Reporting of result s ... 13 8 Referral ... 1 3 8
21 2121
Page Page Page Page x iix iix iix ii
Page Page Page Page
Biosafet y ... 14 5 Quality assurance ... 14 5
Reporting... 14 6 Referral ... 1 4 6
22 2222
22 Bacteriological Ex aminat ion of Wat erBacteriological Ex aminat ion of Wat erBacteriological Ex aminat ion of Wat erBacteriological Ex aminat ion of Wat er 14 7 Microbiological ex am ination of water ... 14 7 Frequency of ex amination ... 15 1 Standards ... 15 1 Reporting... 15 2 Referral ... 1 5 2 Quality assurance ... 15 2
SECTION C: SECTION C: SECTION C:
SECTION C: COLLECTION COLLECTION AND TRANSCOLLECTION AND TRANSPORTATION OF CLINICACOLLECTION AND TRANSAND TRANSPORTATION OF CLINICAPORTATION OF CLINICAL MATERIAL TO PORTATION OF CLINICAL MATERIAL TO L MATERIAL TO L MATERIAL TO REFERRAL
REFERRAL REFERRAL REFERRAL
LABORATORIESLABORATORIESLABORATORIESLABORATORIES
23 2323
Guidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for MicrobiologyGuidelines on Standard Operating Procedures for Microbiology Guidelines on Standard Operating Procedures for Microbiology
Page
24 Acquired Im munodef iciency Syndrom eAcquired Im munodef iciency Syndrom eAcquired Im munodef iciency Syndrom eAcquired Im munodef iciency Syndrom e 15 7 Collection of sam ple of blood ... 15 8 Quality assurance ... 15 9 Referral ... 1 5 9 Biosafet y ... 16 0 Reporting of result s ... 16 0
25 2525
25 Viral Viral HepatitisViral HepatitisViral Hepatitis Hepatitis 16 3 Collection and t ransport ation of specimens... 16 3 Reporting of result s ... 16 5 Quality assurance ... 16 5
26 PoliomyelitisPoliomyelitisPoliomyelitisPoliomyelitis 16 7 Collection of stool specimen from a case with acute flaccid paralysis (poliom yelitis) 16
7 Reporting of result s ... 16
8 Referral ... 1 6 9 Quality assurance ... 16 9
27 2727
Page Page Page Page x ivx ivx ivx iv
Page Page Page Page
3 Laborat ory diagnosis ... 17 3 Recognizing cases of DF/ DHF/ DSS ... 17
5 Reporting of result s ... 17 6 Quality assurance ... 17 6 Biosafet y ... 17 6 Referral ... 1 7 6
Index IndexIndex
Section A
Pa Pa Pa Page ge ge ge 1111
1. Organization and Functions of
Laboratories
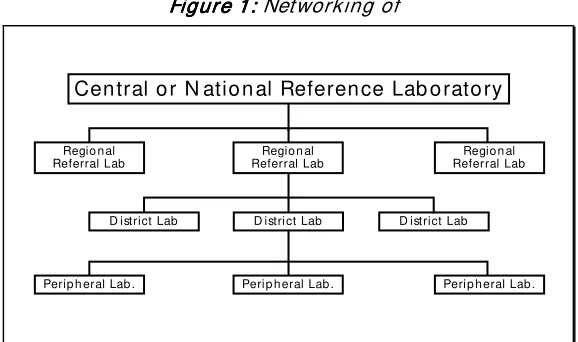
The organizat ion of laboratories in any country is usually a three or four tier system with various possible functional linkages between them. One possible way of networking of laboratories is shown in Fig 1.
Regio n al Referral Lab Regio n al
Referral Lab
D istrict Lab Regio n al
Referral Lab
Peri p h eral Lab . Perip h eral Lab . Peri p h eral Lab .
D istri ct Lab D istrict Lab
Cen tral o r N atio n al Referen ce Lab o rato ry
Peripheral laborat ory services
Peripheral laborat ory services
Peripheral laborat ory services
Peripheral laborat ory services
Peripheral laboratories are located at the point of first contact of patients with the health care services. In m ost developing countries these are available only at prim ary health centre or com m unity health centre (upgraded prim ary health centre) level. These laboratories provide technical support for preventive, curative and prom otive services for the individual as well as the community.
St af f
St af f
St af f
St af f
The staff in peripheral laboratories should include one technician and one laboratory assistant/ attendant.
Space
Space
Space
Space
The space available in peripheral laboratories should include at least one laboratory- cum- office/ record room (approx . 5 m eters x 3 m eters) and one store- room which can be used for other services also (approx . 5 meters x 3 m eters).
Figure 1: Figure 1: Figure 1:
Page Page Page Page 2222
Ot her f acilit ies
Ot her f acilit ies
Ot her f acilit ies
Ot her f acilit ies
Other necessary facilities include
➢
supply of safe water➢
reliable source of energy (battery, electricity, solar or kerosene)➢
sterilization/ disinfection facilities➢
waste disposal facilitiesThere must also be transport and communication facilities between the peripheral and interm ediate laboratories for referral of sam ples and patients, procurem ent of supplies and personal discussion.
Equipm ent and supplies
Equipm ent and supplies
Equipm ent and supplies
Equipm ent and supplies
Necessary equipment and supplies include good microscopes, centrifuges, autoclaves, refrigerators, balances, pH meters, incubators, water bath, t ransport media, glassware, sterile swabs, reagent s for staining (eg. Gram, Albert, Ziehl Neelsen, Romanowsky), reagents for chemical ex amination of urine, kits and reagents for rapid diagnostic tests, sterilized syringes and needles, m icropipettes and tips as well as sterile collection bottles for blood/ serum and water analysis.
Test s t o be perf orm ed
Test s t o be perf orm ed
Test s t o be perf orm ed
Test s t o be perf orm ed
Peripheral laboratories are ex pected to undertake tests of public health as well as clinical relevance. Among the tests of public health relevance, diseases of greater epidem iological im portance should be accorded priority. Testing of environment samples (especially water) also falls into the priorities of public health relevance. Certain rapid serological tests m ay be of use in studying epidem iological patterns of im portant diseases and the sam e can also be perform ed at peripheral laboratories.
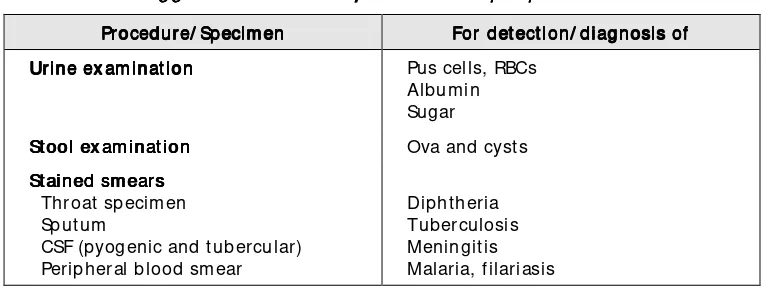
The tests to be perform ed by peripheral laboratories are subject to the availabilit y of resources, manpower, t echnology and prevalence of various diseases in the area catered to by the laboratory. A suggested list is provided in Table 1.
Table 1: Suggest ed t est s t o be perf orm ed at peripheral laborat ories Suggest ed t est s t o be perf orm ed at peripheral laborat ories Suggest ed t est s t o be perf orm ed at peripheral laborat ories Suggest ed t est s t o be perf orm ed at peripheral laborat ories
Procedure/ Specim en Procedure/ Specim enProcedure/ Specim en
Procedure/ Specim en For det ect ion/ diagnosis ofFor det ect ion/ diagnosis ofFor det ect ion/ diagnosis ofFor det ect ion/ diagnosis of Urine ex am inat ion
Urine ex am inat ion Urine ex am inat ion
Urine ex am inat ion Pus cells, RBCs St ained sm earsearsearsears Throat specim en Sputum
CSF (pyogenic and tubercular) Peripheral blood sm ear
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 3333
Rapid diagnost ic t est s Rapid diagnost ic t est s Rapid diagnost ic t est s
Rapid diagnost ic t est s HIV Hepatitis B surface Ag Syphilis
Meningococcal disease
Int erm ediat e laborat
Int erm ediat e laborat
Int erm ediat e laborat
Int erm ediat e laborat ory services
ory services
ory services
ory services
In m ost developing countries, interm ediate laboratories are located at district or t he regional headquart ers and may act as clinical as well as public healt h laboratories. The following functions are ex pected to be performed by these laboratories:
1. Laboratory support to clinical diagnosis/ public health Quality assurance
Logistic and technical support
Training of staff for peripheral laboratories
2. Supervision and monitoring of peripheral laboratories
Interm ediate laboratories help in the diagnosis and treatm ent of the individual patient and are also used as public health laboratories for epidemiological surveillance and control of diseases in the community. These laboratories also serve as links between peripheral laboratories and the state/ central laboratory for the following:
➢
Collect ion, storage and analysis of dat a.➢
Distribution of reagents, m edia, laboratory m anuals.➢
Purchase of equipment.➢
Supervision of peripheral laboratories.➢
To conduct ex ternal quality assessment scheme (EQAS) for peripheral laboratories.➢
To take part in EQAS organized by the state/ central laboratories.➢
To send sam ples to higher/ reference laboratories for characterization of isolate/ confirmation of diagnosis.St af f
St af f
St af f
St af f
Qualified pathologist/ microbiologist
(Doctor of Medicine/ diploma in clinical pathology) 1
Technicians –
DMLT (diploma in medical laboratory technology) with ex perience 2
Laboratory Assistants (DMLT) 1
Laboratory attendants 2
Cleaner 1 Clerk- cum- storekeeper 1
Page Page Page Page 4444
Space
Space
Space
Space
Microbiology/ Serology laboratory (approx .8 metersx 5 meters) 1 Sterilization, disinfection and media preparation laboratory
(approx . 6 m etersx 4 m eters) 1
Store- room (approx .3 metersx 5 meters) 1 Office (approx . 3 m etersx 5 m eters) 1
Equipm ent
Equipm ent
Equipm ent
Equipm ent
Binocular microscope 2 Colorimeter 1
Dark- field microscope 1 Refrigerator 1
Inoculating chamber 2 Balances 2
Centrifuge 2 pH m eter 1
Autoclave 2 Inspissator 1
Incubator 2 Distil water apparatus 1
Hot air oven 1 Micropipettes as per
workload
Water bath 2 Tips for pipettes as per
workload
VDRL shaker 1
This m anual describes m ost of the tests that have been suggested to be perform ed at interm ediate- level laboratories.
Fu
Fu
Fu
Furt her reading
rt her reading
rt her reading
rt her reading
1. Kum ari S, Bhatia Rajesh, Heuck CC. Qualit y Assurance in Bacteriology and Im m unology. WHO Regional Publication, South East Asia Series No 28, 1998. 2. Kum ari S, Sharm a KB et al. Healt h Laborat ory Services in support of Prim ary Healt h
Page Page Page Page 5555
2.
Collection and Transportation of
Clinical Specim ens
The laboratory diagnosis of an infectious disease begins with the collection of a clinical specimen for ex amination or processing in the laboratory (the right
one, collected at the right time, transported in the right way to the right
laboratory). Proper collection of an appropriate clinical specimen is the first step in obtaining an accurate laboratory diagnosis of an infectious disease. Guidelines for the collection and transportation of specim ens should be m ade available to clinicians in a lucidly written format. The guidelines must em phasize two im portant aspects:
➢
Collection of the specim en before the adm inistration of antim icrobial agent s.➢
Prevention of contam ination of the specim en with ex ternally present organisms or normal flora of the body.General rules for collection and transportation of specimens are sum m arized in Table 1.
Table1: Collect ion and t ransport at ion of specim ensCollect ion and t ransport at ion of specim ensCollect ion and t ransport at ion of specim ens Collect ion and t ransport at ion of specim ens
• Apply strict aseptic techniques throughout the procedure.
• Wash hands before and after the collection.
• Collect the specim en at the appropriate phase of disease.
• Make certain that the specim en is representative of the infectious process (e.g. sputum is the specim en for pneum onia and not saliva) and is adequate in quantity for the desired tests to be perform ed.
• Collect or place the specim en aseptically in a sterile and/ or appropriate container.
• Ensure that the outside of the specim en container is clean and uncontaminated.
• Close the container tightly so that its contents do not leak during transportation.
• Labeland date the container appropriately and com plete the requisition form.
Page Page Page Page 6666
Crit eria for reject ion of specim ens
Crit eria for reject ion of specim ens
Crit eria for reject ion of specim ens
Crit eria for reject ion of specim ens
Criteria should be developed by a laboratory on the basis of which the processing of a specim en m ay not be done by the laboratory. The following are som e ex am ples:
➢
Missing or inadequate identification.➢
Insufficient quantity.➢
Specim en collected in an inappropriate container.➢
Contam ination suspected.➢
Inappropriate transport or storage.➢
Unknown time delay.➢
Haem olysed blood sam ple.Collect ion of specim ens
Collect ion of specim ens
Collect ion of specim ens
Collect ion of specim ens
The clinical state of the patient will not necessarily be reflected by the result of laboratory investigation despite correct laboratory performance unless the specimen is in optimal condition required for the analysis. Some of t he im portant specim ens and their proper collection and transportation m ethods are described here so as to ensure quality.
Blood
Blood
Blood
Blood
Whole blood is required for bacteriological ex am ination. Serum separated from blood is used for serological techniques. Skin antisepsis is ex tremely important at the time of collection of the sample. Tincture of iodine (1- 2%), povidone iodine (10%) and chlorhex idine (0.5% in 70% alcohol) are ideal agents. However, som e individuals m ay be hypersensitive to iodine present in some of these. While collecting blood for culture,the following points must be rem em bered:
➢
Collect blood during the early stages of disease since the number of bacteria in blood is higher in the acute and early stages of disease.➢
Collect blood during parox ysm of fever since the number of bacteria is higher at high tem peratures in patients with fever.➢
In the absence of antibiotic adm inistration, 99% culture positivity can be seen with three blood cultures.➢
Small children usually have higher number of bacteria in their blood as com pared to adults and hence less quantity of blood needs to be collected from them (Table 2).Table 2: Volum e of blood t o be collect ed at dif f erent ages: Volum e of blood t o be collect ed at dif f erent ages: Volum e of blood t o be collect ed at dif f erent ages : Volum e of blood t o be collect ed at dif f erent ages
Age AgeAge
Age VoVoVoVo lum e in 2 bot t leslum e in 2 bot t leslum e in 2 bot t leslum e in 2 bot t les
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 7777
2- 5 years 8 m l 6- 10 years 12 m l
> 10 years 20 m l
Cerebrospinal f luid (CSF)
Cerebrospinal f luid (CSF)
Cerebrospinal f luid (CSF)
Cerebrospinal f luid (CSF)
Ex amination of CSF is an essential step in the diagnosis of any patient with evidence of meningeal irritation or affected cerebrum. Almost 3- 10 ml of CSF is collected and part of it is used for biochemical, immunological and microscopic ex amination and remaining for bacteriological or fungal ex am ination. The following im portant precautions need to be taken for CSF collection and transportation:
➢
Collect CSF before antim icrobial therapy is started.➢
Collect CSF in a screw – capped sterile container and not in an injection vial with cotton plug.➢
Do not delay transport and laboratory investigations.➢
Transport in a transport m edium if delay in processing is unavoidable.➢
CSF is a precious specimen, handle it carefully and economically. It m ay not be possible to get a repeat specim en.➢
Perform physical inspection im m ediately after collection and indicate findings on laboratory requisition form.➢
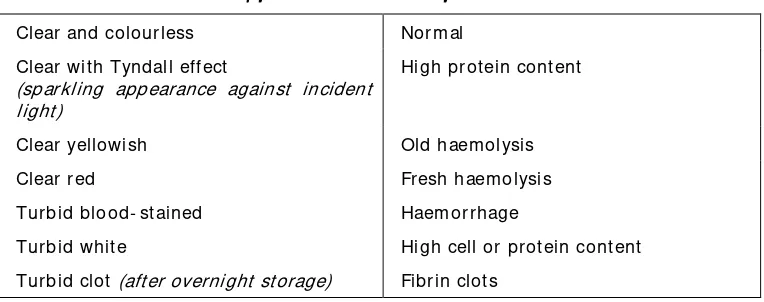
St ore at 37oC, if delay in processing is inevitable.The characteristics of the appearance of CSF are outlined in Table 3.
Table 3: Appearance and int erpret at ions of CSF Appearance and int erpret at ions of CSF Appearance and int erpret at ions of CSF Appearance and int erpret at ions of CSF
Clear and colourless Norm al Clear wit h Tyndall effect
(sparkling appearance against incident light)
High protein content
Clear yellowish Old haem olysis Clear red Fresh haem olysis Turbid blood- st ained Haem orrhage
Turbid whit e High cell or protein cont ent Turbid clot (after overnight storage) Fibrin clots
Sput um
Sput um
Sput um
Sput um
Page Page Page Page 8888
➢
Select a good wide- mouthed sputum container, which is preferably disposable, m ade of clear thin plastic, unbreakable and leak proof m aterial.➢
Give the patient a sputum container with the laboratory serial number written on it. Show the patient how to open and close the container and ex plain the importance of not rubbing off the number written on the side of the container.➢
Instruct the patient to inhale deeply 2- 3 tim es, cough up deeply from the chest and spit in the sputum container by bringing it closer to the mouth.➢
Make sure the sputum sample is of good quality. A good sputum sample is thick, purulent and sufficient in amount (2- 3 ml).Give the patient an additional container with laboratory serial number written on it for an early m orning specim en. Ex plain to the patient to rinse his/ her mouth with plain water before bringing up the sputum.
Urine
Urine
Urine
Urine
Under normal circumstances urine is sterile. The lower part of the urethra and the genitalia are normally colonised by bacteria, many of which may also cause urinary tract infection. Since urine is a good growth medium for all sorts of bacteria, proper and aseptic collection assum es greater im portance for this specim en.
For microbiological ex amination urine must be collected as a “clean catch- m id- stream ” specim en.
Urine specim ens should be transported to the laboratory within one hour for bacteriological ex amination, because of the continuous growth of bacteria
in vitro thus altering the actual concentration of organisms.
St ool
St ool
St ool
St ool
Faecal specim ens for the aetiological diagnosis of acute infectious diarrhoeas should be collected in the early stage of illness and prior to treatment with antim icrobials. A stool specim en rather than a rectal swab is preferred.
➢
The faeces specim en should not be contam inated with urine.➢
Do not collect the specim en from bed pan.➢
Collect the specim en during the early phase of the disease and as far as possible before the adm inistration of antim icrobial agents.➢
1 to 2 gm quantity is sufficient.➢
If possible, subm it m ore than one specim en on different days.Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 9999
➢
St ore at 2- 8oC.➢
Modified Cary and Blair m edium (see chapter 5) is recom m ended as a good transport m edium. It is a very stable m edium and can be stored for use in screw – capped containers. It is a sem i- solid transport m edium. At least two swabs should be inoculated. Most pathogens will survive for up to 48 hours at room temperature. Specimens are unacceptable if the m edium is held for m ore than one week or if there is detectable drying of the specim en.Alternative transport media are Venkataraman- Ramakrishnan m edium (V- R fluid) or alkaline peptone water. VR fluid should be prepared in 30 m l (1 oz) screw capped bottles (MacCartney bottles). It preserves vibrios for m ore than six weeks and has also proved to be a very convenient m edium for transportation as it can be kept at room tem perature after collection of the specim en.
Throat swab
Throat swab
Throat swab
Throat swab
➢
Depress the tongue with a tongue blade.➢
Swab the inflam m ed area of the throat, pharynx or tonsils with a sterile swab taking care to collect the pus or piece of m em brane.➢
Transport in sterile transport tube.Bone m arrow
Bone m arrow
Bone m arrow
Bone m arrow
Bone m arrow is collected by a doctor who is well trained in this procedure
➢
Decontam inate the skin overlying the site from where specim en is to be collected with 70% alcohol followed by 2% tincture of iodine.➢
Aspirate 1 m l or m ore of bone m arrow by sterile percutaneous aspirat ion.➢
Collect in a sterile screw- cap tube.➢
Send to laboratory im m ediately.Rect al swab
Rect al swab
Rect al swab
Rect al swab
➢
Insert swab at least 2.5 cm beyond t he anal sphinct er so t hat it enters the rectum.➢
Rotate it once before withdrawing.➢
Transport in Cary and Blair or ot her t ransport medium.Transport at ion of specim ens
Transport at ion of specim ens
Transport at ion of specim ens
Transport at ion of specim ens
Page Page Page Page 10101010
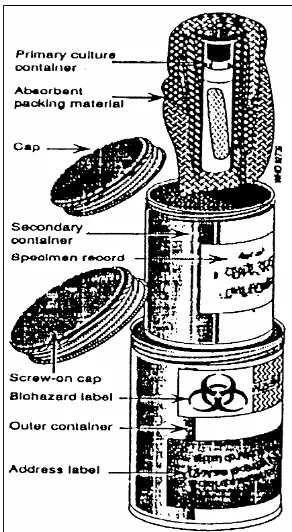
over a short distance, the specim en should be placed upright in appropriate racks. For long distance transportation, it should be placed in three containers, i.e:
➢
A prim ary cont ainerA prim ary cont ainerA prim ary cont ainerA prim ary cont ainer which has the specim en and is leakproof with a screw- cap.➢
A secondary cont ainerA secondary cont ainerA secondary cont ainerA secondary cont ainer which is durable, waterproof and m ade of metal or plastic with a screw- cap. It should have enough absorptive material to absorb the contents of the primary container should the latter break or leak. On its outside, the details of the specim en should be pasted.➢
A t ert iary cont ainerA t ert iary cont ainerA t ert iary cont ainerA t ert iary cont ainer is usually made of wood or cardbox . It should be capable of withstanding the shocks and traum a of transportation. Dry ice can be kept between this and the secondary container along with sufficient absorbents and provision for the escape of carbondiox ide to prevent a pressure build- up inside (Fig 1).Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 11111111
Figure 1: Figure 1: Figure 1:
Figure 1: Transportation container
A continuous effort m ust be m ade in order to ensure proper collection and transportation of clinical specimens. Full cooperation of nursing staff and others concerned with specim en collection is required and can be achieved once they are m ade aware of the principles involved and the significance of what they are being asked to do.
Furt her reading
Furt her reading
Furt her reading
Furt her reading
1. Lennette HE, Balows A, Hauser WJ et al. Collection, Handling and Processing of Specim en. In Manual of Clinical Microbiology, 4th Ed, ASM, Washingt on, DC, 73-98, 1985.
Page Page Page Page 11111111
3. Sterilization
St erilizat ion St erilizat ion St erilizat ion
St erilizat ion is defined as the destruction or removal (by filtration) of all m icroorganism s and their spores, whereas disinf ect ion disinf ect ion disinf ect ion is the destruction of disinf ect ion many microorganisms but not usually the bacterial spores. Sterilization is usually achieved with the help of heat whereas chem ical agents are em ployed to effect disinfection.
St erilizat ion and disinfection are part of the daily routine of microbiological laboratories and constitute a vital activity which ensures that cultures, containers, media and equipment are treated in such a way that only the inoculated organisms will grow while all others will be eliminated.
St erilizat ion by heat
St erilizat ion by heat
St erilizat ion by heat
St erilizat ion by heat
This can be achieved by autoclaving, by ex posing articles to dry heat in hot air ovens or boiling.
Aut ocla
Aut ocla
Aut ocla
Aut oclaving
ving
ving
ving
Autoclaves can sterilize anything that can withstand a temperature of 121oC for 30 m inutes. A pressure cooker used in hom es for cooking purposes can also be used as a makeshift aut oclave.
The containers having clinical m aterial are subjected to heat treatm ent in the autoclave after which these are em ptied and washed and put back into service.
Only autoclaves designed for laboratory work and capable of dealing with a mix ed load should be used. Porous load and bottle fluid sterilizers are rarely satisfactory for laboratory work. There are two varieties of laboratory aut oclaves:
➢
Pressure cooker type.➢
Gravity displacem ent m odels with autom atic air and condensate discharge.Pressure
Pressure
Pressure
Pressure---- cooker t ype laborat ory aut oclaves
cooker t ype laborat ory aut oclaves
cooker t ype laborat ory aut oclaves
cooker t ype laborat ory aut oclaves
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 12121212
gauge and a safety valve are fitted in the lid. Water in the bottom of the autoclave is heated by ex ternal gas burners, an electric im m ersion heater or a steam coil.
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
➢
Ensure that there is sufficient water inside the cham ber.➢
Load the autoclave and fasten the lid keeping the discharge tap open.➢
Adjust the safety valve to the required tem perature and turn the heat on.➢
Allow the mix ture of air and steam to pass out freely till all air has been discharged.➢
Close the air discharge tap and let the steam pressure rise within the chamber till it attains a temperature of 121oC (1.5 kg/ cm2).➢
Hold on the pressure for 15 m inutes.➢
Turn off the heat and let the autoclave cool.➢
Slowly open the air and steam discharge taps after the pressure gauge has reached zero.➢
Allow the m aterial to cool before these are handled (usually agar bottles take hours before these becom e safe to handle).Aut oclave wit h air discharge by gravit y displacem ent
Aut oclave wit h air discharge by gravit y displacem ent
Aut oclave wit h air discharge by gravit y displacem ent
Aut oclave wit h air discharge by gravit y displacem ent
These are usually rectangular in shape and arranged horizontally. These autoclaves have a jacket around the chamber.
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
➢
Bring the jacket of the autoclave to operating tem perature.➢
Load the cham ber, close the door and open the steam valve so that steam can freely enter the top of the cham ber. Air and condensate shall automatically flow out through the drain at the bottom.➢
When the drain therm om eter reaches the required tem perature, allow further period for the load to reach that tem perature (this has to be determ ined initially and periodically for each autoclave).➢
Continue the autoclave cycle for the holding period.Page Page Page Page 13131313
➢
Gradually and softly open the autoclave enabling the steam to escapeand allow the load to cool further.
Hot air oven
Hot air oven
Hot air oven
Hot air oven
A hot air oven is electrically operated and should be equipped with a fan to ensure uniform temperature inside. The required temperature for sterilization is generally 160oC for one hour.
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
Operat ing inst ruct ions
➢
Arrange the material to be sterilized loosely and evenly on the racks of the oven allowing free circulation of air and thereby even heating of the load.➢
Do not pack the load tightly since air is a poor conductor of heat.➢
Switch on the power supply and control the tem perature of the oven by adjusting the therm ostat.➢
Note the tim e when the desired tem perature is reached (heating- up time).➢
Hold the load in the oven at this tem perature for a definite period of time (holding period). This is usually 60 minutes at 160oC.➢
Do not overheat since it would char the cotton plugs and paper wrappings.Autoclaves and hot air ovens can be used for disinfection of infectious waste before it is discarded. In addition, waste can be disposed of by boiling in detergent or by burial.
Boiling in det ergent
Boiling in det ergent
Boiling in det ergent
Boiling in det ergent
In the absence of an autoclave, m ost specim en containers can be boiled in water having detergents to decontaminate. This process kills the vegetative bacteria but fails to destroy the spores and certain viruses. The easiest way to get best results is to add washing powder or sodium carbonate crystals, 60 gram s to one litre of water in a big container and boil specim en containers in it for a minimum of 30 minutes.
Disinf ect ion
Disinf ect ion
Disinf ect ion
Disinf ect ion
Commonly-Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 14141414
used chemical disinfectants include chlorine releasing compounds; ethyl and isopropyl alcohol, quaternary ammonium compounds and gluteraldehyde.
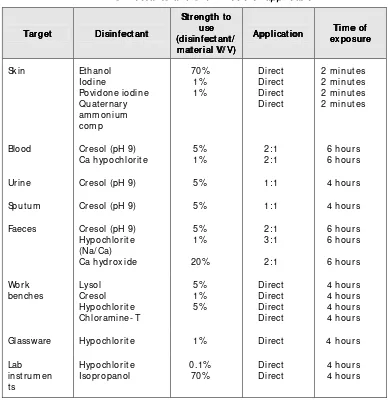
The synopsis of a few com m only- used disinfectants is given in Table 1.
Preferred methods of sterilization for common articles are given in Table 2.
Page (disinf ect ant /(disinf ect ant / (disinf ect ant / Glassware Hypochlorite 1% Direct 4 hours Lab
* Based upon: Basics of quality assurance: WHO/ EMRO, 1992, page 162
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page
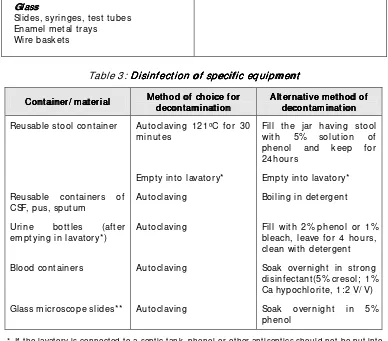
Slides, syringes, test tubes Enam el m etal trays
Wire baskets
Table 3: Disinf ect ion of specif ic equipm ent Disinf ect ion of specif ic equipm ent Disinf ect ion of specif ic equipm ent Disinf ect ion of specif ic equipm ent
Cont ainer/ m at erial Cont ainer/ m at erial Cont ainer/ m at erial
Cont ainer/ m at erial Met hod of choice f or Met hod of choice f or Met hod of choice f or Met hod of choice f or decont am inat iondecont am inat ion decont am inat ion Reusable stool container Autoclaving 121oC for 30
m inutes
Fill t he jar having stool with 5% solut ion of phenol and keep for 24hours
Em pty int o lavat ory* Em pty int o lavat ory* Reusable containers of
CSF, pus, sputum
Aut oclaving Boiling in det ergent
Urine bot t les (aft er em ptying in lavat ory*)
Aut oclaving Fill wit h 2% phenol or 1% bleach, leave for 4 hours, clean wit h det ergent Blood containers Autoclaving Soak overnight in strong
disinfectant(5% cresol; 1% Ca hypochlorite, 1:2 V/ V) Glass m icroscope slides** Aut oclaving Soak overnight in 5%
phenol
* If the lavatory is connected to a septic tank, phenol or other antiseptics should not be put into the lavatory.
** Glass m icroscope slides which have been used for the diagnosis of tuberculosis should be discarded after keeping them soaked in detergent overnight.
Biohaz
Biohaz
Biohaz
Biohazard wast e m anagem ent
ard wast e m anagem ent
ard wast e m anagem ent
ard wast e m anagem ent
Waste is defined as any solid, liquid or gaseous material that is no longer used and will either be recycled, disposed of or stored in anticipation of treatm ent and/ or disposal.
St orage
St orage
St orage
St orage
Page Page Page Page 17171717
Disposal opt ions
Disposal opt ions
Disposal opt ions
Disposal opt ions
There are three m ain disposal options:
➢
render the waste noninfectious by autoclaving and dispose it in the general waste stream . If autoclaving is not possible, decontam inate with chemical disinfectants or by boiling for 20 minutes before disposal.➢
on- site incineration, if possible.➢
transportation of locally- generated waste to a distant appropriate facility.Incineration is the preferred disposal option. Not only does this m ethod render the waste noninfectious but it also changes the form and shape of the waste. Sterilization is an effective method for decontaminating waste, but it does not alter the appearance of the waste. Steam sterilization in an autoclave
at a t emperat ure of 121oC for at least 15 m inutes destroys all form s of microbial life, including high numbers of bacterial spores. This type of complete sterilization can also be accomplished using dry heat which requires
a tem perature of 160- 170oC for 2- 4 hours. However, it m ust be ensured that heat com es in contact with the m aterial to be rendered sterile. Therefore, bottles containing liquid material should have loosened caps or cotton plug caps to allow for steam and heat ex change within the bottle. Biohazard bags containing waste should be tied loosely. Once sterilized, biohazardous waste should be sealed in appropriate containers, labelled as disinfected waste and disposed of in an approved facility.
Biological waste should be clearly labelled prior to disposal and com plete records should be maintained.
Burial
Burial
Burial
Burial
It is not a decontaminating process per se. However, it does prevent the infectious material from becoming a reservoir of infection if properly buried. It requires digging a pit of alm ost 5 m eters depth and 2 m eters width and having a tightly fitted heavy lid on top. Disposable containers with clinical m aterial are thrown daily into it and the lid is replaced im m ediately after throwing the specim ens. Once a week, the refuse is covered with a layer of quicklim e. If quicklim e is not available, the refuse is covered with alm ost 10 cm thick layer of dried leaves once a week.
Furt her reading
Furt her reading
Furt her reading
Furt her reading
Page Page Page Page 17171717
4. Staining Techniques
Staining of the clinical m aterial or the bacteria from colonies on laboratory m edia provide a direct visualization of the m orphology of the organism s as well as their reactions to the chemicals present in stains. This is an invaluable and easy- to- use tool for establishing the identity of various m icroorganism s. Some of the commonly- used staining techniques are:
➢
Methylene blue staining➢
Gram staining➢
Albert staining➢
Ziehl Neelsen staining (Acid fast staining)➢
India ink staining➢
Iodine staining for ova and cysts in faecesMet hylene blue st aining
Met hylene blue st aining
Met hylene blue st aining
Met hylene blue st aining
Ingredient s and preparat ion
Ingredient s and preparat ion
Ingredient s and preparat ion
Ingredient s and preparat ion
Methylene blue 0.3 gm
Distilled water 100 m l
Dissolve the dye in water. Filter through a filter paper.
St aining procedure
St aining procedure
St aining procedure
St aining procedure
➢
Make a sm ear on a glass slide, dry in air and fix by passing it over the flam e of a burner 3- 4 tim es.➢
Stain for one m inute by pouring m ethylene blue solution over the sm ear.Page
The stain is used to m ake out clearly the m orphology of the organism s e.g.
Yersinia pestis in ex udate, Haemophilus influenzae in CSF and gonococci in urethral pus.
Gram st aining
Gram st aining
Gram st aining
Gram st aining
This is the m ost ex tensively used differential stain that divides bacteria into two major groups. Those which retain crystal violet dye after treatment with iodine and alcohol appear purple or bluish purple and are designated as Gram positive. Those bacteria which lose the crystal violet show the colour of the counter stain employed. The commonly- used counter stain is saffranin which gives a pink/ red colour to bacteria and these organism s are labelled as Gram negative.
Ingredient s and preparat ion
Ingredient s and preparat ion
Ingredient s and preparat ion
Ingredient s and preparat ion
Distilled water 80 ml
Mix solutions A and B. Store for 24 hours before use.
Gram iodine Gram iodine Gram iodine Gram iodine
Iodine crystals 1.0 gm
Potassium iodide 2.0 gm
Distilled water 300 m l
Grind the dry iodine and potassium iodide in a m ortar. Add water, a few m l at a tim e, and grind thoroughly after each addition until the iodine and iodide dissolve. Rinse the solution into an am ber glass bottle with the rem ainder of the distilled water.
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page
Distilled water 90 ml
St aining procedure
St aining procedure
St aining procedure
St aining procedure
➢
Make a thin sm ear on a clean glass slide, dry it in air and fix by passing through flam e of a burner.➢
Cover the sm ear with crystal violet, keep for one m inute.➢
Wash the slide with water, then cover with Gram iodine and let it stand for one m inute.➢
Wash the slide with water.➢
Decolour with acetone/ alcohol, rocking the slide gently for 10- 15 seconds till the violet colour comes off the slide.➢
Wash with water im m ediately.➢
Counterstain with saffranin. Let the counterstain stand for 30 seconds.➢
Wash with water, blot dry and ex am ine under the oil im m ersion lens of a microscope.Uses
Uses
Uses
Uses
Widely used in diagnostic bacteriology m ainly to differentiate organism s on the basis of m orphology and Gram reaction.
Albert st aining
Toluidine blue 0.15 gm
Malachite green 0.20 gm
Glacial acet ic acid 1.0 ml
Alcohol(95%) 2.0 ml
Distilled water 100 m l
Grind and dissolve the dyes in alcohol, add water and then add acetic acid. Let the m ix ture stand for 24 hours and then filter.
Page Page Page Page 20202020
Iodine 2.0 gm
Potassium iodide 3.0 gm
Distilled water 300 m l
Dissolve iodine and potassium iodide in water by grinding in a m ortar with a pestle. Filter through a filter paper.
St aining procedure
St aining procedure
St aining procedure
St aining procedure
➢
Cover the heat- fix ed sm ear with Albert stain I. Let it stand for two minutes.➢
Wash with water.➢
Cover the sm ear with Albert stain II. Let it stand for two m inutes.➢
Wash with water, blot dry and ex am ine.Uses
Uses
Uses
Uses
To demonstrate metachromatic granules in C.diphtheriae. These granules appear bluish black whereas the body of bacilli appear green or bluish green.
India ink st aining
India ink st aining
India ink st aining
India ink st aining
St aining procedure
St aining procedure
St aining procedure
St aining procedure
➢
Place a loopful of India ink on the side of a clean slide.➢
A sm all portion of the solid culture is suspended in saline on the slide near the ink and then em ulsified in the drop of ink, or else, m ix a loopful of liquid culture of specimens like CSF with the ink.➢
Place a clean cover slip over the preparation avoiding air bubbles.➢
Press down, or blot gently with a filter paper strip to get a thin, even film.➢
Ex am ine under dry objectives followed by oil im m ersion.Use
Use
Use
Use
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 21212121
Ziehl Neelsen st aining
Ziehl Neelsen st aining
Ziehl Neelsen st aining
Ziehl Neelsen st aining
Ingredient s and preparat ions
Ingredient s and preparat ions
Ingredient s and preparat ions
Ingredient s and preparat ions
Carbol fuchsin 1%
Sulphuric acid 25%
Methylene blue 0.1%
➢
Select a new, unscratched slide and label the slide with a Laboratory Serial number.➢
Make a smear from yellow purulent portion of the sputum using a bam boo stick. A good sm ear is spread evenly, 2 cms x 3 cms in size and is neither too thick nor too thin. The optimum thickness of the sm ear can be assessed by placing the sm ear on a printed m atter, the print should be readable through the sm ear.➢
Let the sm ear air- dry for 15- 30 m inutes.➢
Fix the sm ear by passing the slide over the flam e 3- 5 tim es for 3- 4 seconds each time.➢
Place the fix ed slide on the staining rack with the sm eared side facing upwards.➢
Pour filtered 1% carbol fuchsin over the slide so as to cover the entire slide.➢
Heat the slide underneath until vapours start rising. Do not let carbol fuchsin to boil or the slide to dry. Continue the process up to five minutes.➢
Allow the slide to cool for 5- 7 m inutes.➢
Gently rinse the slide with tap water to rem ove the ex cess carbol fuchsin stain. At this point, the smear on the slide looks red in colour.➢
Decolor the stained slide by pouring 25% sulphuric acid on the slide and leaving t he acid for 2- 4 minut es.➢
Lightly wash away the free stain. Tip the slide to drain off the water.➢
If the slide is still red, reapply sulphuric acid for 1- 3 minutes and rinse gently with tap water.➢
Counter stain the slide by pouring 0.1% m ethylene blue solution onto the slide and let it stand for one m inute.➢
Gently rinse the slide with tap water and tip the slide to drain off the water.Page Page Page Page 22222222
➢
Ex am ine the slide under a m icroscope using 40 x lens to select the suitable area of the slide and ex amine under 100 x lens using a drop of immersion oil.Uses
Uses
Uses
Uses
Distinguishes acid fast bacilli such as Mycobacterium tuberculosis and
M.leprae from other non- acid fast bacilli.
Iodine st aining f or ova and cyst s
Iodine st aining f or ova and cyst s
Iodine st aining f or ova and cyst s
Iodine st aining f or ova and cyst s
➢
On a clean glass slide place one drop of norm al saline and one drop of 2% iodine solution at two different sites.➢
Mix a portion of stool first with norm al saline and then with iodine solution with the help of a wire loop or applicator.➢
Place coverslips on both the em ulsions.➢
Ex am ine the preparations under 10x and 40x of the m icroscope for various ova and cysts.Qualit y cont rol of st ains
Qualit y cont rol of st ains
Qualit y cont rol of st ains
Qualit y cont rol of st ains
Test all stains at appropriate intervals for their ability to distinguish positive and negative organism s and document the results. The perform ance standards for Ziehl- Neelsen and Gram staining are as given below:
St ain Ziehl- Neelsen Mycobacterium
spp.
* If no standard strains are available, known laboratory strains should be used as controls.
The quality control procedure for stains needs to be perform ed on a weekly basis and also as and when a new lot of reagent s for staining is procured/ prepared.
Furt her reading
Furt her reading
Furt her reading
Furt her reading
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or MicrobiologyGuidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 23232323
Page Page Page Page 23232323
5. Bacteriological Media
The role of suitable quality culture media for cultivation of microorganisms cannot be over emphasised. On it depends t he very success of isolat ion of aetiological agents. Only in ex ceptional cases, can an organism be identified on the basis of its morphological characteristics alone.
Types of m edia
Types of m edia
Types of m edia
Types of m edia
Bacteriological m edia can be broadly sub- divided into four categories.
(1) Ordinary cult ure m edia
(1) Ordinary cult ure m edia
(1) Ordinary cult ure m edia
(1) Ordinary cult ure m edia
These are routinely em ployed in a laboratory e.g. nutrient broth, nutrient agar, infusion brot h and lysat e media.
(2) Enriched m edia
(2) Enriched m edia
(2) Enriched m edia
(2) Enriched m edia
Certain organism s do not grow on ordinary nutrient m edia. They require growth- promoting ingredients such as blood, glucose, serum, egg, etc. The media containing ingredients which enhance their growth- promoting qualities are enriched m edia e.g. blood agar, chocolate agar and Loeffler m edium.
(3) Enrichm ent m edia
(3) Enrichm ent m edia
(3) Enrichm ent m edia
(3) Enrichm ent m edia
Enrichment media are liquid media containing chemical constituents which inhibit som e norm al flora and allow pathogens which m ay be present in very small number in the specimen, to grow unhampered and thus enriching them. Isolated colonies of these organism s m ay be obtained by subculturing onto solid m edia. An ex am ple of enrichment m edia is selenite broth used for prim ary isolation of enteric bacteria.
(4
(4
(4
(4 ) Dif f erent ial and select ive m edia
) Dif f erent ial and select ive m edia
) Dif f erent ial and select ive m edia
) Dif f erent ial and select ive m edia
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 24242424
fermenting colonies can be differentiated from the pale non- lactose fermenting colonies.
Selective media will selectively permit the growth of pathogens and inhibit the com m ensals. In addition, it m ay differentiate the pathogen from com m ensals that grow by the colour and opacity of the colonies e.g. blood tellurite medium for C.diphtheriae.
In addition, transport m edia are also frequently used to sustain the viability of organisms when a clinical specimen is to be transported from the periphery to laboratory. The transport m edium prevents the outgrowth of cont aminant s during t ransit and sustains t he pat hogen. Cary and Blair and Stuart m edia are two ex am ples of this group of m edia.
Preparat ion of m edia and checking of pH
Preparat ion of m edia and checking of pH
Preparat ion of m edia and checking of pH
Preparat ion of m edia and checking of pH
Presently, a wide range of culture m edia are available com m ercially in the form of dehydrated m edia. These m edia are sim ply reconstituted by weighing the required quantities and by adding distilled water, as per the manufacturer’s instructions.
The pH determ ination can be conveniently done with the use of Lovibond com parator with phenol red indicator disc.
➢
Take two clean test tubes and add 5 m l of the m edium to each of the tubes. One serves as a blank while phenol red indicator is added to the other tube.➢
Com pare the colour of the m edium with the phenol red indicator at the appropriate pH m arking.➢
Add N/ 10 NaOH or N/ 10 HCl, drop by drop till the colour of the m edium m atches the colour of the disc at the required pH reading.➢
Calculate the volum e of the NaOH or HCL of 1/ 10 strength for 5 m l of the m edium to get the required pH.➢
Based on the calculation, the volum e of 1N NaOH or IN HCl required for the total volum e of m edium can be calculated and added.➢
Check the pH of the m edium once again before use.The quantity of agar given in the form ulae of m edia m ay have to be changed depending upon the quality of agar used. The concentration varies from bat ch t o bat ch and should be such t hat will produce a sufficient ly firm surface on solidification. This can be tested by streaking with inoculating wire.
Page Page Page Page 25252525
Nut rient brot h
Nut rient brot h
Nut rient brot h
Nut rient brot h
Meat ex tract 10.0 gm
Peptone 10.0 gm
Sodium chloride 5.0 gm
Distilled water 1000 m l
Mix the ingredients and dissolve them by heating in a steam er. When cool, adjust the pH to 7.5- 7.6.
Nut ri
Nut ri
Nut ri
Nut rient agar
ent agar
ent agar
ent agar
To the ingredients as in nutrient broth, add 15 gm agar per litre. Dissolve the agar in nutrient broth and sterilize by autoclaving at 121oC for 15 minutes. Prepare plates and slopes as required.
Glucose brot h
Glucose brot h
Glucose brot h
Glucose brot h
Nutrient broth 900 m l
Glucose (10% solution) 100 ml
➢
Dissolve 9 gm glucose in distilled water and sterilize by tyndallisation.➢
Add l00 m l of the glucose solution to 900 m l of sterile nutrient broth.➢
Dispense 60 m l each in 100 m l pre- sterilized culture bottles.➢
Sterilize by open steaming at l00oC for one hour.Blood agar
Blood agar
Blood agar
Blood agar
Nutrient agar 100 ml
Sheep blood (defibrinated) 10 m l
➢
Melt the sterile nutrient agar by steam ing, cool to 45oC.➢
Add required am ount of sheep blood aseptically with constant shaking.➢
Mix the blood with m olten nutrient agar thoroughly but gently, avoiding froth formation.➢
Im m ediately pour into petri dishes or test tubes and allow to set.Chocolat e agar
Chocolat e agar
Chocolat e agar
Chocolat e agar
Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology Guidelines on St andard Operat ing Procedures f or Microbiology
Page Page Page Page 26262626
➢
Melt the sterile nutrient agar by steam ing and cool to about 75oC.➢
Add blood to the m olten nutrient agar and allow to rem ain at 75oC after gently mix ing till it is chocolate brown in colour.➢
Pour in petri dishes or test tubes for slopes as desired.XLD agar
XLD agar
XLD agar
XLD agar
Xylose 3.5 gm
1 – lysine 5.0 gm
Lactose 7.5 gm
Sucrose 7.5 gm
Sodium chloride 5.0 gm
Yeast ex t ract 3.0 gm
Sodium desox ycholate 2.5 gm Sodium thiosulphate 6.8 gm Ferric ammonium citrate 0.8 gm
Phenol red 0.08 gm
Agar agar 15.0 gm
Water 1000 ml
Weigh the ingredients into a flask and add distilled water. Mix the contents well and steam it for 15 minutes (do not autoclave). Cool to 56oC and pour in plates.
Buf f ered glycerol saline
Buf f ered glycerol saline
Buf f ered glycerol saline
Buf f ered glycerol saline
Glycerol 300 ml
Sodium chloride 4.2 gm
Disodium hydrogen phosphate 10.0 gm Na2 H PO4 Anhydrous 15.0 gm Phenol red aqueous solution 0.02 per cent 15.0 m l
Water 700 ml
➢
Dissolve NaCl in water and add glycerol.➢
Add disodium hydrogen phosphate to dissolve.➢
Add phenol red and adjust pH to 8.4.➢
Distribute 6 m l in universal containers (screw - capped bottles of 30 ml capacity). Autoclave at 115oC for 15 m inutes.Loef f ler serum m edium
Loef f ler serum m edium
Loef f ler serum m edium
Loef f ler serum m edium
Nutrient broth 100 m l
Serum (sheep or horse or ox ) 300 m l