The impact of season of harvest and duration of
pre-measurement storage impact hydraulic conductance of
stem samples for
Acer rubrum
L. x
saccharinum
L. and
Fraxinus americana
L.
Patricia R. Knight
a,*
,1, Matthew P. Kelting
a,2, J. Roger Harris
a,3,
John R. Seiler
b,4aDepartment of Horticulture,301 Saunders Hall,Virginia Tech,Blacksburg,VA 24061, USA bDepartment of Forestry,324 Cheatham Hall,Virginia Tech,Blacksburg,VA 24061, USA Received 18 March 1999; received in revised form 4 January 2000; accepted 6 January 2000
Abstract
The influence of pre-measurement storage length and season of harvest of stem segment samples on hydraulic conductance and percentage embolism was determined for two tree species because no published guidelines exist concerning storage. Stem sections fromFraxinus americanaL. ‘Autumn Applause’ (white ash) andAcer rubrumL. x
saccharinumL. ‘Autumn Blaze’ (hybrid red maple) were collected from well-established trees in fall 1995 (October), spring 1996 (April), and summer 1996 (July). Ends of stem sections collected in the fall were either covered with wax or left exposed. Entire sections from all dates were placed in closed plastic bags to prevent desiccation during transport and subsequent storage. Stem sections were either analyzed immediately (0 storage) or held at 2°C for 2 or 4 days. Hydraulic conductance before embolisms were cleared with positive pressure (initialkh), hydraulic
conduc-tance after embolisms were cleared (maximum kh), and percentage embolism were similar for all pre-embolism
measurement storage lengths within each of the three seasonal sampling periods for hybrid red maple and spring- and summer-collected white ash. Fall-collected white ash samples with 0 storage had higher initial kh, and percentage
embolism increased if samples were stored. Embolism was greatest for summer-collected samples and lowest for spring-collected samples for hybrid red maple, but values were similar for white ash. Stem covering did not influence measured parameters. Our data indicate that hybrid red maple stem segments can be stored without significant loss of hydraulic conductance for up to 4 days, but white ash should not be stored in the fall. Unless maximum levels of
www.elsevier.com/locate/envexpbot
* Corresponding author. Present address: Mississippi State University, South Mississippi Branch Experiment Station, P.O. Box 193, Poplarville, MS 39470, USA. Tel.: +1-601-7954525; fax: +1-601-7950653.
E-mail address:[email protected] (P.R. Knight). 1Former graduate assistant.
2Former graduate assistant. 3Associate Professor. 4Professor.
native embolism have been reached, as determined from laboratory analysis, stem segments of species on which storage data are not available should be processed as soon as possible. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Red maple; White ash; Embolism; Xylem; Cavitation
1. Introduction
Vessel cavitation results from breaking the con-tinuity of the xylem water column in response to drought or freezing stress and leads to the forma-tion of a water vapor filled discontinuity (Sperry and Tyree, 1988; Tyree and Sperry, 1988). Sperry and Tyree (1988) quantified embolism by measur-ing loss of hydraulic conductance of a stem seg-ment. This technique estimates the presence of all nonrepaired cavitations in a stem segment by measuring hydraulic conductance before and after embolisms are removed using positive pressure. Estimations of reductions in hydraulic conduc-tance as an indicator of embolism is a destructive analysis (Tyree and Sperry, 1989). Additionally, the system described by Sperry et al. (1988) is difficult to transport, requiring the storage of stem samples for transport to the laboratory. Samples may require additional storage prior to embolism measurement due to the limited number of sam-ples that can be analyzed simultaneously.
Previous research utilizing species native to an arid environment indicates that plants with small vessels are less susceptible to embolism (Carlquist, 1982). More recent research suggests that large vessel diameters may only predict greater em-bolism susceptibility within an individual plant, but vessel diameters are not always indicative of embolism susceptibility between multiple plants or even within a species (Dixon, et al., 1988; Sperry and Saliendra, 1994). Specifically, water stress-in-duced cavitation depends on pore diameters in pit membranes, and pore diameters may be corre-lated with vessel size in individual plant organs. Therefore, larger conduits within an individual plant sample would tend to have more perme-ablepit membranes resulting in an increased ten-dency to cavitate at less negative water potentials (Sperry and Tyree, 1990; Sperry and Saliendra, 1994).
Research investigating pre-measurement stor-age durations for stem segments and leaves har-vested for measurement of water potential has yielded mixed results. Karlic and Richter (1979) reported that leaves of several species with vary-ing anatomy may be stored for several days with-out influencing water potential measurements, but only when samples were stored in aluminum bags. Innes and Kelly (1991) indicated that Melicytus
Forst. could be stored in airtight plastic bags for 24 h and Alectryon Gaertn. for 47 h without influencing water potentials. Because each species exhibits a relationship between lower water poten-tials (more negative) and loss in hydraulic conduc-tance (Tyree and Ewers, 1991), these results suggest that stem samples can be stored under certain conditions without influencing embolism measurements.
Although pre-embolism measurement storage may be required, no published guidelines exist concerning the length of time samples may be stored prior to analysis or the influence that sea-son of harvest has on length of storage. Equilibra-tion between the stem segment and the atmosphere inside the plastic bag may result in water loss from the stem. Therefore, storage may have a greater impact onFraxinus americanaL., a ring-porous tree, which has a higher proportion of large vessels compared to Acer rubrum L. x sac
-charinumL., a diffuse-porous tree. The hypothesis examined in this experiment was that season of harvest and duration of storage prior to embolism measurement have an impact on hydraulic con-ductance and percentage embolism forA.rubrum
L. x saccharinum L. andF. americana L.
2. Materials and methods
species,F.americanaL. ‘Autumn Applause’ (white ash), and a diffuse-porous species,A.rubrumL. x
saccharinumL. ‘Autumn Blaze’ (hybrid red maple), on 10 and 15 October, 1995, respectively. Stem sections were terminal and generally consisted of an entire branch to insure minimal introduction of air into cut vessels of the ring-porous tree (Joyce and Steiner, 1995). Stem sections were collected from well-established (3 – 4 m tall) specimens growing at the Urban Horticulture Center (Blacksburg, VA) in a Groseclose silty clay loam (clayey, mixed, Typic Hapludult, pH 6.5). Stem ends were either covered with waxed film (Parafilm, American National Can, Greenwich, CT) or left exposed. Additionally, stem water potentials of subsamples were determined using a pressure chamber (Soilmoisture Equipment Corp., Santa Barbara, CA) just prior to embolism measurement. Both hybrid red maple and white ash stem sections had senescing leaves present. Fifteen terminal stem sections consisting of entire branches were selected on 23 April and 22 July, 1996, from the same trees as fall-collected sections, for spring and summer analysis. Spring-collected hybrid red maple stem sections had fully expanded leaves, but white ash stem sections had not broken bud. Summer-collected stem samples had leaves. All leaves were left intact. Leaves present on the sampled stem portion were covered with moist towels during embolism analysis to minimize transpirational water loss. Stem sections collected in the spring and summer were not covered with waxed film (No difference in coverage response was measured in the fall, and the researchers felt that any effect of the covering was local in nature. Therefore, the effect of the waxed film was negated by the procedure used to harvest the stem section, and stem water potentials were not measured (sample error resulted in invalid values for the fall, yielding no basis for seasonal comparisons). All stem sections were placed in plastic bags to prevent desiccation during transport or subsequent pre-measurement storage. Stem sections were either analyzed immediately or refrigerated at 2°C for 2 or 4 days.
One stem section consisting of 1- or 2-year-old wood from each segment was selected, and stem ends were recut under water to a length of 20 cm
to prevent induction of air into cut stem ends (Sperry et al., 1994). Stem segments were then fitted with gaskets, retrimmed with razor blades, and placed in parallel manifolds in the system (Sperry and Tyree, 1988). The presence of embolisms was indicated by reduction in hydraulic conductance (Sperry et al., 1988). Hydraulic conductance (kh) is
defined as kh=6/(dP/dl) where 6is the flow rate
(kg s−1) and dP/dlis the pressure gradient (MPa
m−1
). Initial khand maximum khwere measured
for each stem section. Fall stem segments were sequentially perfused with tap water using a hydraulic head of 3 – 5 KPa for measurement of initialkh(Sperry et al., 1988). After initialkhwas
measured, stem segments were flushed with pressurized tap water at 150 KPa for 15-min cycles until maximumkhwas achieved. Perfusing duration
did not exceed 4 h. (Sperry et al., 1994). Spring and summer stem segments were sequentially perfused with a 10 mmol l−1oxalic acid solution (Tyree and
Sperry, 1988) with a hydraulic head of 3 – 5 KPa for measurement of initial kh. After initial kh was
measured, stem segments were flushed at 150 KPa with a 10 mmol l−1oxalic acid solution for 15-min
cycles until maximumkhwas achieved. Reduction
in hydraulic conductance (percentage embolism) was calculated as percentage embolism= (maxi-mum kh−initial kh)/maximum kh×100. Oxalic
acid was used during the spring and summer sampling period to prevent microbial growth resulting from increased use of the apparatus.
The fall experimental design was a completely random design with 3 (storage time)×2 (end coverage) factorial consisting of six replications. The spring and summer experimental design was a completely random design consisting of five replications. All data were subjected to analysis of variance and Scheffe’s Multiple Comparison Procedure (PB0.05, SAS, 1988).
3. Results and discussion
Hybrid red maple. Initial kh, maximum kh, and
local in nature. Therefore, failure of end coverage to influence measured parameters can be at-tributed to the length of stem sections (20 cm) utilized for sampling and laboratory handling of excised stem samples. Each stem section harvested in the field generally consisted of an entire branch segment. Stem segments utilized for analysis of hydraulic conductance were sampled from interior portions of the stem sections. Additionally, stem sections were recut under water to prevent the induction of air into the cut portions of the stem using the method described by Sperry et al. (1988).
Initialkh was highest for spring-collected stem
segments and lowest for summer-collected stem samples (Table 1). Embolism levels reportedly increase as the growing season progresses (Sperry et al., 1994), and by definition, reductions in initial kh are reported as percentage embolism.
High initial kh measurements in spring-collected
stems indicated refilling of freeze – thaw induced
embolisms that typically occur over the winter for some diffuse-porous species. Refilling of em-bolisms for diffuse-porous species has been docu-mented by several researchers using a variety of plant material (Sperry et al., 1994; Tognetti and Borghetti, 1994). Additionally, new xylem growth may have occurred (Mauseth, 1988).
Maximum levels of kh were also highest for
spring-collected stems (Table 1). There were no differences in maximum kh for summer- and
fall-collected stems. Differences between measure-ments of maximum kh can be attributed to
phy-siological and anatomical changes in the plants over the course of the growing season. New growth of xylem would occur in the spring, while any vessels that embolize would be susceptible to infiltration by fungi or growth of tyloses (Mauseth, 1988). Xylem blockage due to fungal growth or tyloses could not be removed using the Sperry apparatus, resulting in diminished maxi-mum kh measurements in summer and fall
col-lected stems.
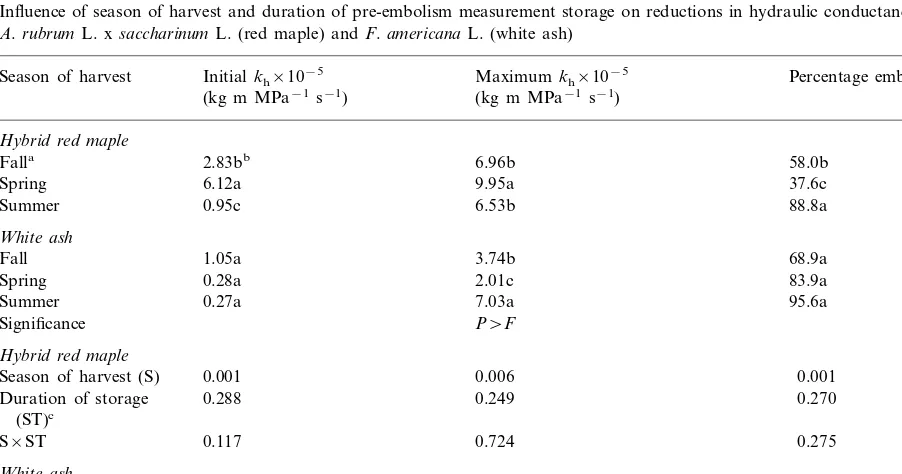
Table 1
Influence of season of harvest and duration of pre-embolism measurement storage on reductions in hydraulic conductance (kh) for
A.rubrumL. xsaccharinumL. (red maple) andF.americanaL. (white ash)
Initialkh×10−5 Percentage embolism
Season of harvest Maximumkh×10−5
(kg m MPa−1s−1) (kg m MPa−1s−1)
Hybrid red maple
2.83bb 6.96b
Falla 58.0b
Spring 6.12a 9.95a 37.6c
88.8a 6.53b
0.95c Summer
White ash
1.05a
Fall 3.74b 68.9a
Spring 0.28a 2.01c 83.9a
Summer 0.27a 7.03a 95.6a
P\F Significance
Hybrid red maple
Season of harvest (S) 0.001 0.006 0.001
Duration of storage 0.288 0.249 0.270
(ST)c
0.117
S×ST 0.724 0.275
White ash
Season of harvest (S) 0.001 0.001 0.001
0.001 0.243
0.002 Duration of storage (ST)
0.002 0.484
S×ST 0.018
aN=36 for fall-collected stem samples, andn=18 for spring- and summer-collected stem samples.
Percent embolism was highest for summer-col-lected stems and lowest for spring-colsummer-col-lected stems (Table 1). High levels of embolism measured in summer-collected stem samples may be related to xylem tensions occurring in actively growing twigs. Zimmermann (1983) suggested that distal twigs were subject to lower water potentials, mak-ing those plant parts more susceptible to em-bolism. Additionally, Tyree and Sperry (1989) reported that loss of hydraulic conductance for red maple occurred over a relatively small reduc-tion in water potential. This leads to speculareduc-tion that embolism levels in the trunks could be much lower than embolism levels in the twigs sampled for this study. In our experiment, 1995 fall-col-lected twigs had lower embolism levels compared to 1996 summer-collected stem samples. Em-bolism levels may have been lower in 1995 for all sampling dates compared to embolism levels in 1996 or some recovery in embolism levels may have occurred between summer and fall. Em-bolism recovery between seasons may occur be-cause new stem diameter growth in plants moved down from stem tips to the trunk (Harris, 1983). This new caliper growth may have provided a reservoir of new vessels that rehydrated stem por-tions sampled in the fall for this experiment. Fall levels of embolism corresponded to embolism lev-els achieved by other hybrid red maple samples during winter (unpublished data), indicating that fall stem samples reached their maximum loss of hydraulic conductance before the fall harvest date in October.
Initial kh, maximum kh, and percentage
em-bolism of hybrid red maple were not influenced by duration of pre-embolism measurement storage, regardless of season of harvest (data not shown). Lack of response for initial kh and embolism to
duration of pre-measurement storage can be at-tributed to the relatively low number of large vessels present in diffuse-porous wood (Mauseth, 1988). Kramer and Boyer (1995) reported that large early-wood vessels were most susceptible to embolism, diffuse-porous vessels were moderately susceptible, and tracheids were least susceptible to cavitation resulting from tensions in xylem. Large vessels typically have larger pit membrane pore diameters which help determine a vessel’s
suscep-tibility to embolism within an individual plant (Sperry and Tyree, 1988). Small vessels and subse-quently smaller pit membrane diameters increase resistance for water loss to the environment just as they increase resistance to air-seeding when plants are intact in the environment (Sperry and Tyree, 1988; Zimmermann, 1983). Additionally, Bates and Niemiera (1996) reported that Norway maple has high levels of stem suberization, and this suberization might also be present in red maple and would therefore reduce water loss dur-ing storage. Because maximum kh was not
influ-enced by storage time, reductions in initial khcan
be presumed to be an accurate measure of per-centage embolism and not due to microbial growth which may result when vessels embolize (Mauseth, 1988).
Red maple trees slightly more than 1 m in height had sap flow approaching 100 mg cm−2
h−1 MPa−1 (Knight, 1997). When initial k h
val-ues are converted to units having the same magni-tude as those reported for sap flow and divided by the approximate leaf area of sampled stem seg-ments, sampled stem segments were found to have flow rates approaching 324 mg cm−2
h−1
MPa−1
. These estimations suggest that adequate water flow was present to support plant growth in spite of reduced hydraulic conductance.
White ash. Initialkh, maximumkhand
percent-age embolism of white ash were not influenced by end coverage for the fall sampling period (data not shown). Initial kh and percentage embolism
among season of harvest was similar among treat-ments. Maximum kh was highest for
summer-col-lected samples, and spring-colsummer-col-lected stems had the lowest levels of maximum kh (Table 1). Several
Table 2
Influence of season of harvest and pre-embolism measurement storage duration on reductions in hydraulic conductance forF.
americanaL. (white ash)a
Percentage Initialkh×10−5
Treatment
(kg m MPa−1s−1) embolism
Fall, 0 days 2.18ab 58.8b storage
Fall, 2 days 0.79b 63.3ab storage
0.20b
Fall, 4 days 89.5a
storage
Spring, 0 days 0.18b 83.0ab storage
0.41b
Spring, 2 days 81.7ab
storage
0.26b
Spring, 4 days 87.1ab
storage
0.24b
Summer, 0 96.7a
days storage
Summer, 2 0.23b 93.6a
days storage 0.35b
Summer, 4 96.6a
days storage
aN=12 for fall-collected stem samples, and n=6 for spring- and summer-collected stem samples.
bMeans followed by the same letter within columns are not different according to Scheffe’s Multiple Comparison Proce-dure (PB0.05).
longer functional or necessary for plant growth (Romberger and Hejnowicz, 1993).
Both initial and maximum kh were low in the
spring, indicating that new large vessels were not present and previous large vessels may have been filled with tyloses. Because white ash grows rapidly in the late spring (Mauseth, 1988), high embolism levels in terminal growing tissue may be an indication of high water demand and growth levels. Distal stems may be highly susceptible to water stress-induced embolism, thereby exhibiting higher embolism levels than might be expected in the central trunk (Zimmermann, 1983). High em-bolism levels in the summer were measured after leaf and shoot expansion ceased. Therefore, water demand would be primarily for maintenance and not shoot extension, allowing the plant to exist at higher embolism levels. High embolism levels cou-pled with slow growth might signal the plant of impending dormancy. Additionally, hydraulic re-strictions in a terminal twig would only limit water availability to a small portion of the tree.
There was an interaction between season of harvest and storage duration for initial kh and
percentage embolism for white ash (Table 1). Stem segments collected in fall without storage had higher initial kh values compared to other
stem segments, regardless of storage (Table 2). Corresponding percentage embolism levels also only changed within season of sampling for fall samples. This change occurred at day 4.
4. Conclusions
Results of this experiment demonstrated that hybrid red maple, a diffuse-porous tree species, can be stored prior to measurement during the fall (October), spring (April), or summer (July) for at least 4 days without affecting hydraulic conduc-tance or percentage embolism during the particu-lar season of storage. Conversely, white ash, a ring-porous tree species, can be stored prior to embolism measurement in the spring and summer without affecting hydraulic conductance or per-centage embolism, but cannot be stored for 4 days in the fall without affecting embolism. Fall stor-age results in lowered initial kh if stored for 2 or
and maximum kh measurements taken in the
spring might be expected to be lower than mea-surements taken in the summer after differentia-tion of new xylem. Lower kh levels in the fall
might reflect the development of tyloses over the course of the growing season. In addition, Tyree et al. (1991) reported that estimates of xylem vulnerability may be artificially high since flushing may initiate water flow through damaged inter-vessel pit membranes that would not be actively involved in water transport. Therefore, high em-bolism values could be a result of artificially high maximumkhvalues that result from the
introduc-tion of water into pathways rendered nonfunc-tional by means other than embolism. Additionally, embolized early-wood vessels from prior years may contribute little water for transpi-ration, but may influence maximum kh values
4 days and an increase in percentage embolism if stored for 4 days. These results are similar to those of Davis and Potter (1982) who examined the stem water potentials of Rhododendron and reported that plants with smaller vessels could be stored for extended periods of time. Our research indicates samples used for embolism determina-tion should not be stored unless testing has deter-mined that storage will not influence the level of embolism measured.
Acknowledgements
Mention of trade names does not imply en-dorsement of products named nor criticism of similar products not used.
References
Bates, R.M., Niemiera, A.X., 1996. A comparison of morpho-logical features affecting water loss in Norway maple and Washington hawthorn stems. J. Environ. Hort. 14, 71 – 76. Carlquist, S., 1982. Wood anatomy of Illicium (Illiciaceae): phylogenetic, ecological, and functional interpretations. Am. J. Bot. 69, 1587 – 1598.
Davis, T.D., Potter, J.R., 1982. Storing unrootedRhododen
-dron cuttings: effects on water potential, carbohydrate levels, and root formation. Hort. Sci. (Abstr.) 17, 491. Dixon, M.A., Butt, J.A., Murr, D.P., Tsujita, M.J., 1988.
Water relations of cut greenhouse roses: the relationship between stem water potential, hydraulic conductance and cavitation. Sci. Hort. 36, 109 – 118.
Harris, R.W., 1983. Arboriculture: Care of Trees, Shrubs, and Vines in the Landscape. Prentice-Hall, Englewood Cliffs, NJ.
Innes, K.P.C., Kelly, D., 1991. Some woody vegetation sam-ples may be stored for 24 hours without affecting measured water potential. NZ J. Bot. 29, 345 – 347.
Joyce, B.J., Steiner, K.C., 1995. Systematic variation in xylem hydraulic capacity within the crown of white ash (Fraxinus americana). Tree Physiol. 15, 649 – 656.
Karlic, H., Richter, H., 1979. Storage of detached leaves and
twigs without changes in water potential. New Phytol. 83, 379 – 384.
Knight, P.R., 1997. Influence of transplanting practices on growth and embolism levels for urban tree species. PhD Dissertation. Virginia Polytechnic Institute and State Uni-versity, Blacksburg, VA.
Kramer, P.J., Boyer, J.S., 1995. Water Relations of Plants and Soils. Academic Press, San Diego, CA.
Mauseth, J.D., 1988. Plant Anatomy. Benjamin/Cummings Publishing Co., Menlo Park, CA.
Romberger, J.A., Hejnowicz, Z., 1993. Plant Structure: Func-tion and Development. Springer – Verlag, Berlin.
SAS Institute Inc., 1988. SAS/STAT User’s Guide, release 6.03 edn. SAS Institute Inc., Cary, NC.
Sperry, J.S., Saliendra, N.Z., 1994. Intra-plant and inter-spe-cific variation in xylem cavitation in Betula occidentalis. Plant Cell Environ. 17, 1233 – 1241.
Sperry, J.S., Tyree, M.T., 1988. Mechanisms of water stress-in-duced xylem embolism. Plant Physiol. 88, 581 – 587. Sperry, J.S., Tyree, M.T., 1990. Water stress induced xylem
embolism in three species of conifers. Plant Cell Environ. 13, 427 – 436.
Sperry, J.S., Donnelly, J.R., Tyree, M.T., 1988. A method for measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 11, 35 – 40.
Sperry, J.S., Nichols, K.L., Sullivan, J.E.M., Eastlack, S.E., 1994. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of northern Utah and interior Alaska. Ecology 75, 1736 – 1752.
Tognetti, R., Borghetti, M., 1994. Formation and seasonal occurrence of xylem embolism inAlnus cordata. Tree Phys-iol. 14, 241 – 250.
Tyree, M.T., Ewers, F.W., 1991. Tansley review no. 34: The hydraulic architecture of trees and other woody plants. New Phytol. 119, 345 – 360.
Tyree, M.T., Sperry, J.S., 1988. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Answers from a model. Plant Phys-iol. 88, 574 – 580.
Tyree, M.T., Sperry, J.S., 1989. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Phys. Mol. Biol. 40, 19 – 38.
Tyree, M.T., Snyderman, D.A., Wilmot, T.R., Machado, J.-L., 1991. Water relations and hydraulic architecture of a tropical tree (Schefflera morototoni): data, models, and a comparison to two temperate species (Acer saccharumand
Thuja occidentalis). Plant Physiol. 96, 1105 – 1113. Zimmermann, M.H., 1983. Xylem Structure and the Ascent of
Sap. Spring – Verlag, New York.