Interrelations between
Azospirillum
and
Rhizobium
nitrogen-fixers
and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in
sterile, AMF-free or normal soil conditions
B. Biró
a,∗, K. Köves-Péchy
a, I. Vörös
a, T. Takács
a, P. Eggenberger
b, R.J. Strasser
baResearch Institute for Soil Science and Agricultural Chemistry of the Hungarian Academy of Sciences,
Herman O. ut 15, 1022 Budapest, Hungary
bLaboratory of Bioenergetics, University of Geneva, CH-1254 Jussy, Switzerland
Received 31 May 1999; received in revised form 27 January 2000; accepted 23 March 2000
Abstract
Co-inoculations of the alfalfa (Medicago sativaL.) plants with the associative- and/or the obligate nitrogen-fixing bacteria (Azospirillum brasilense, S;Rhizobium meliloti, R) and/or the vesicular arbuscular mycorrhiza fungus (Glomus fasciculatum, M) were evaluated in a pot experiment under controlled conditions. The effect of these beneficial microbes, as single- (M, R, S), dual- (MR, MS) or multilevel (MRS) inoculation-treatments were assessed in a calcareous loamy chernozem soil, originating from a grass-type natural ecosystem. A range of substrates were used to separate the influence of the indigenous microbes: C, untreated original soil (i.e. including all of the usual microflora); G, gamma-sterilised soil (no competitive microbes); GB, sterile soil (re-suspension of a mycorrhiza-free soil extract). The weight of the host, nodule-number, macro-and microelement contents macro-and the colonisation by the inoculated bacterial macro-and fungal microsymbionts were recorded.
In the gamma-sterilised substrate all of the mono- (M), dual- (MR, MS) or multilevel (MRS) co-inoculations with the selected,Glomus fasciculatumM 107 strain were effective in improving plant growth, nutrient-uptake and abundance of the microsymbionts in the rhizosphere of alfalfa. In contrast a competition from the indigenous microflora in the non-sterilised soil, greatly reduced the functioning of the applied mycorrhizal inoculum. Although the associativeAzospirillumbacteria (MS) slightly reduced effects relative to single mycorrhizal inoculation (M), the multilevel treatments, with both of the diazotrophs (MRS), showed a further enhancement (a synergistic effect) for almost all of the tested parameters and substrates. The functional compatibility of the obligate- and associative diazotrophs in the mycorhizosphere are discussed. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Microsymbionts; Diazotrophs; Mycorrhiza; Competition; Synergism
1. Introduction
The contribution of arbuscular mycorrhizal fungi (AMF) and of associative or obligate nitrogen-fixing
∗Corresponding author.
E-mail address:[email protected] (B. Bir´o)
microsymbionts (Azospirillum- and Rhizobium bac-teria) to soil fertility, productivity and crop yield has been well-documented (Boddey et al., 1991; Jeffries and Dodd, 1991; Biró et al., 1993a; Bethlenfalvay and Schüepp, 1994). The organisms are therefore used to evaluate the functioning of ecosystems (Giller and Cadish, 1995), especially under nutrient unbalanced
conditions (Barea and Azcon-Aguilar, 1983; Barea et al., 1983). Well-known effects of AMF include improved uptake of water (Bethlenfalvay et al., 1987; Sanchez-Diaz et al., 1990; Tobar et al., 1994), and of phosphorus or other macro- and microelements in non-optimal situations (Pacovsky et al., 1985). Rhizosphere–mycorhizosphere systems, can therefore be tailored to help plants to establish and survive in nutrient-deficient, or degraded habitats or during peri-ods of stress (Smith and Bowen, 1979; Sanchez-Diaz et al., 1990).
As a result of the above, seed and soil inoculations are common agricultural practices (Subba Rao, 1985; Champawat, 1990). The success of these technolo-gies, however, depends on the effectiveness and infec-tivity of indigenous microbes and on the interactions between the main participants, such as mycorrhizal fungi, in the rhizosphere (Lindermann, 1983; Puppi et al., 1994). Compatible combinations of the inoculated microbes, such as nitrogen-fixing rhizobium bacteria and AMF, may result in an enhanced effect on plant development (Ames and Bethlenfalvay, 1987; Barea et al., 1988a; Paula et al., 1992; Biró et al., 1993b) in the various microsymbiont–legume systems. Positive influence of the associativeAzospirillum diazotrophs on AM fungal activity has been reported (Subba Rao, 1985; Garbaye, 1994) especially for the monocotyle-donous host plants. In contrast differences in total dry weight were not found in the Glomus+Azospirillum
tripartite system studies by Pacovsky (1988).
Reports on the dual co-inoculations of the AMF with both of the nitrogen-fixing bacteria Rhizobium -and Azospirillum are uncommon. Spores and the hyphosphere of the AMF may, however, harbour so-called “helper bacteria”, such as Burkholderia
or associative diazotrophs (Minerdi et al., 1999; J. Döbereiner, pers. comm.), which can result in benefi-cial effect in a range of soil–plant systems.
The aim of this study was to screen microbial inter-actions, which would take place in the rhizosphere of a target plant, by assessing the functional compatibility of three groups of microorganisms,Rhizobiumand/or
Azospirillumnitrogen-fixers and/or AMF. The concept of single- dual- and/or the multilevel microsymbiont co-inoculations was studied in relation to mycorhizo-sphere effect, and the impact of soil conditions (e.g. in-cluding or exin-cluding the indigenous endomycorrhizal fungal populations).
2. Materials and methods
2.1. Soil treatments and characteristics
In vitro pot experiment was carried out to study the effects of various co-inoculations using alfalfa (Medicago sativa L.) and the following soil treat-ments: C, untreated loamy chernozem soil; this served as an original control soil, where all of the usual rhizosphere microflora (all microbes) were present. G, gamma-irradiated sterile soil; the normal rhi-zosphere microflora was excluded (no microbes), through gamma-sterilisation (15 000 J Co kg−1 soil). GB, gamma-sterilised soil, with the re-addition of a soil extract (80 ml per pot of a soil/water mixture, 2:1 (v/v), filtered through a Jena G 5 (MN, 5mm
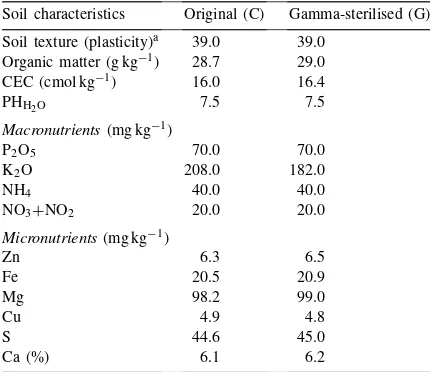
pore-sized paper) to reintroduce the native microbial community, with the exception of propagules of AMF (no indigenous AMF). Some of the main physical and chemical characteristics of the soil before and after the gamma-sterilisation are shown in Table 1.
2.2. Microbial inoculations
Using the above substrates (C, G, GB) plants were grown with or without associative and symbiotic
Table 1
The physical and chemical characteristics of a calcareous cher-nozem soil originating from a natural, grass-type ecosystem (Erd, Hungary) before (C) and after (G) the gamma irradiation (15 000 J Co kg−1 soil)
Soil characteristics Original (C) Gamma-sterilised (G)
Soil texture (plasticity)a 39.0 39.0 Organic matter (g kg−1) 28.7 29.0 CEC (cmol kg−1) 16.0 16.4
PHH2O 7.5 7.5
Macronutrients(mg kg−1)
P2O5 70.0 70.0
K2O 208.0 182.0
NH4 40.0 40.0
NO3+NO2 20.0 20.0
Micronutrients(mg kg−1)
Zn 6.3 6.5
microorganisms, the Azospirillum and Rhizobium
N2-fixers and the AMF (S, R and M, respectively). There were three replicates. The strains used ( Azospir-illum brasilense Km 5,Rhizobium meliloti S 5/7+K 4/1+Lu 41 mixture andGlomus fasciculatumM 107) originated from the Commercial Inoculum Collection of the RISSAC, Budapest. The effect of sixR. meliloti
strains, as candidate inocula was evaluated in a pre-liminary seedling experiment on alfalfa (M. sativa
L.). Sterilised seeds (in 3% chloramin T, washed with five changes of sterile tap water), were germi-nated on Thorton agar and inoculated with 1 ml of rhizobium inoculum (approximately 108CFU ml−1) (Vincent, 1970). Nodule-number and the acetylene reduction activity (ARA) were measured in six repli-cates using the method of Hardy et al. (1973). After 8 weeks of growth, the three most effective rhizo-bia were selected for further inoculations, as 1:1:1 (v:v:v) mixture. Alfalfa plants were inoculated after emergence with 5 ml cell-suspension per pot (approx-imately 108CFU ml−1). The AMF inoculations, 3% of a root–soil mixture (G. fasciculatumand red clover host) for each pot with application made below the seed-layer, prior to sowing.
2.3. Plant growth and measurements
Eight alfalfa seeds (M. sativa L.) were sown into pots containing 250 g of the experimental soil. These were thinned after emergence to five seedlings. The pots were arranged in a randomised block design and supplied with tap water whenever needed. Plants were grown for 90 days under the controlled conditions at 22◦C during the day and 18◦C during the night, with a 18 h photo-period (photosynthetic photon flux density of 600mmol m−2s−1, using metal halide lamps) and
a relative humidity of 70–80%. The dry weights of shoots at the 6th, 9th and 12th weeks of growth and the roots (at the final harvest) were recorded after drying in an oven at 70◦C.
2.4. Estimation of the microbial colonisation and efficiency
Both the total nitrogen content and the nodule-number were used to estimate the efficiency of the rhi-zobium nitrogen-fixers. Nitrogen-free semisolid Nfb
medium (Döbereiner and Day, 1976) was used for the MPN counts of the Azospirillum bacteria (Okon et al., 1977; Tarrand et al., 1978). Washed and cleared 1 g root samples were taken from each treatment and after grinding with sterilised quartz-sand a dilution series was prepared for inoculating the MPN tubes (10 ml of suitable semisolid media in three replicates). Growth records (development of the white, subsurface pellicle) were assessed after 2 days of incubation in 33◦C. Parallel root samples of 1 g (wet weight) were also measured and dried in an oven at 70◦C to give the root moisture percentage.
To estimate the root-colonisation of AMF an addi-tional subsample of 1 g fresh lateral root was randomly taken and cut for approximately 1 cm segments. They were cleared and stained with acid glycerol trypan blue (Phillips and Hayman, 1970), and mounted on a microscopic slide to estimate AMF colonisations (30 segments in three replicates). The frequency and in-tensity of the mycorrhizal infection (F%; M) and the arbusculum content of the infected parts (a %) were recorded and calculated (Trouvelot et al., 1985), using a five-class system.
2.5. Chemical analysis
Soil physical characteristics were estimated by the method of Buzás (1998). The total element content of the soil was determined for both original (C) and gamma-sterilised (G) samples which were digested with cc. HNO3at 80◦C in a microwave oven. Plant ele-ment contents (Ca, Mg, K, P and S) were assessed after wet digestion with HNO3+H2O2 of air-dried ground plant samples. Elements were measured using induc-tively coupled plasma atomic emission spectrometry (ICP-AES, type: FY-238). Total N content in the shoot biomass was estimated by a modified Kjeldahl method after the wet digestion with cc. H2SO4+H2O2.
2.6. Statistical analysis
Mean data on the MPN counts of theAzospirillum
analysis of variance (two-way ANOVA). Comparison of means was made by using the least significant dif-ferences (LSD=p<0.05). Regression analysis of the
nodulation data and the ARA measurements used the Statgraphics 5.0 program.
3. Results
3.1. Selection of the rhizobium inoculum
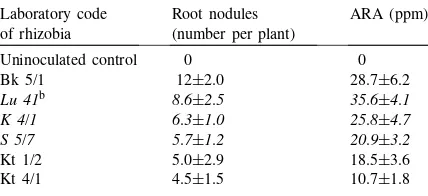
Table 2 shows the result of a preliminary exper-iment, where six candidate R. meliloti strains were used to inoculate sterile alfalfa seedlings, grown in a Thornton agar with six replicates (Vincent, 1970). After 8 weeks of growth, the number of root nodules and the ARA were estimated (Hardy et al., 1973). The number of root nodules on the alfalfa seedlings varied between 4.5 and 12. The data mostly positively correlating with that of ARA (10.7–35.6 ppm ethy-lene h−1per plant). Correlation coefficient (r2)=0.998 (atp=0.0017 level). The equation of the correlation curve wasy=log 1.293x1.137. With strain Bk 5/1, some
small and white nodules developed. This was not significantly related to performance (the ARA mea-surements). The strains Lu 41, K 4/1, S 5/7 showed the highest efficiency. These strains were selected as candidates for further inoculations as a mixed population.
Table 2
A sterile seedling experiment for estimating the efficiency (ARA and nodulating ability) of some R. melilotistrains, as potential candidates for the inoculation of alfalfa in pot experiments (n=6)a Laboratory code
aStrains originated from the Commercial Inocula Collection of the Research Institute for Soil Science and Agricultural Chemistry (RISSAC-MTA TAKI) of the Hungarian Academy of Sciences, Budapest.
bStrains set as italics were selected for the pot experiment, as 1:1:1 (v:v:v) mixed inoculum (approximately 106 CFU ml−1).
3.2. Effect of mono- or multilevel inoculations on the dry matter accumulation
All diazotrophs as dual- (MR, MS) or tripar-tite (MRS) combinations with the AMF (M) in-creased dry matter production in the sterilised (G) and in the re-suspended (GB) treatments, where the other competitive AMF populations were excluded (Table 3).G. fasciculatumM 107, as single inoculum also significantly enhanced growth. This was further increased in the RM dual-inoculated (tripartite) sys-tems. The R. meliloti (R) strain-mixture, which was selected for great effectivity also resulted in the same beneficial effect on the dry matter accumulation in the non-sterilised soil. A much higher increase was found, however, in the multilevel co-inoculation treat-ments, where the AMF was accompanied both by the associative and the symbiotic (Azospirillum and
Rhizobium) nitrogen-fixers (synergistic effect of the MRS treatments).
Re-inoculation of the gamma-sterilised soils with the AM-free soil extract (GB) reconstructed the orig-inal (C) microflora in the rhizosphere. The same dry matter yield was found in the controls in comparison with the non-treated C soil.
In the original non-sterilised soil (C) a uniform ben-eficial effect was found for the plant growth as a re-sult of the single inoculation of microsymbionts, both by the diazotrophs (R, S) and by the AMF (M). The tripartite and the multilevel systems with the Azospir-illum and the Rhizobium diazotrophs (MR, MS and MRS treatments), however, have resulted in the same dry matter accumulation, as the non-inoculated con-trols (Table 3).
3.3. Nutrient uptake of the inoculated plants among the various soil conditions
Table 3
Shoot dry weight (Sh), root dry weight (Rt) and the total dry matter production (Sh+Rt) of alfalfaa(M. sativaL.) Strainsb Substrates and dry matter yield (g per pot)
C G GB
Sh Rt Sh+Rt Sh Rt Sh+Rt Sh Rt Sh+Rt
Control 1.56a 1.72a 3.28a 0.97a 1.04a 2.35a 1.66a 1.80a 3.46a
M 1.72b 2.51b 4.23b 2.33b 2.84b 5.17b 2.49c 2.33b 4.82b
MR 1.60a 1.59a 3.19a 2.07b 3.93b 5.25c 2.27b 2.94b 5.21c
MS 1.71b 2.10a 3.81a 2.01b 2.71b 4.72b 2.10b 2.76b 4.86b
MRS 1.59a 2.38a 3.97a 2.50b 3.38b 5.87c 2.04b 3.29c 5.33c
R 1.73b 2.53b 4.30b n.d.c n.d. n.d. n.d. n.d. n.d.
S 1.73b 2.50b 4.22b n.d. n.d. n.d. n.d. n.d. n.d.
RS 1.74b 2.64b 4.37b n.d. n.d. n.d. n.d. n.d. n.d.
aPlants were grown for 3 months in either the original- (C), gamma-sterilised- (G) or AM-free-resuspended (GB) chernozem soil with single-, dual- or multilevel microbial treatments. Dry matter was collected at the 6th, 9th and 12th weeks and is summarised here (g DW per pot). Each value is the mean of three pots. n.d.: not determined.
bM: mycorrhiza (G. fasciculatumM 107), R: rhizobium (R. melilotiS 5/7+Lu-41+K 4/1), S: spirillum (A. brasilenseKm5) or their combinations, respectively.
cNot determined.
In relation to nitrogen uptake among the original (non-sterilised) soil conditions (C), positive effects of rhizobium inoculation were found in all of the combi-nations (R, RS or MRS). In case of theAzospirillum
diazotroph bacteria an enhanced N content was found in the MS treatment; the mycorrhizal inoculation re-sulted in an additive positive effect.
Uptake of P and K were also increased by the in-oculatedR. meliloti strain-mixture as single- (R), or
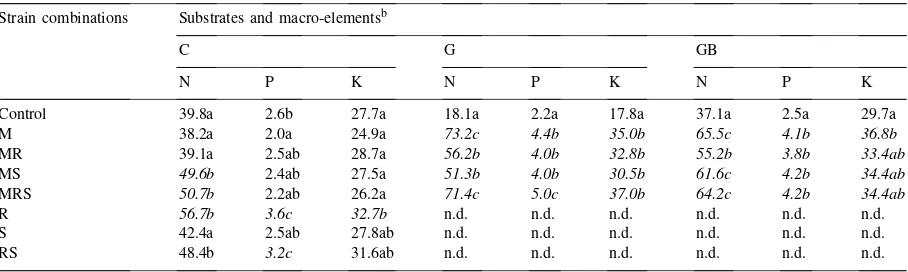
Table 4
The quantity of the main nutrients (N, P, K) in the shoot biomass of alfalfa in the pot experimenta
Strain combinations Substrates and macro-elementsb
C G GB
N P K N P K N P K
Control 39.8a 2.6b 27.7a 18.1a 2.2a 17.8a 37.1a 2.5a 29.7a
M 38.2a 2.0a 24.9a 73.2c 4.4b 35.0b 65.5c 4.1b 36.8b
MR 39.1a 2.5ab 28.7a 56.2b 4.0b 32.8b 55.2b 3.8b 33.4ab
MS 49.6b 2.4ab 27.5a 51.3b 4.0b 30.5b 61.6c 4.2b 34.4ab
MRS 50.7b 2.2ab 26.2a 71.4c 5.0c 37.0b 64.2c 4.2b 34.4ab
R 56.7b 3.6c 32.7b n.d. n.d. n.d. n.d. n.d. n.d.
S 42.4a 2.5ab 27.8ab n.d. n.d. n.d. n.d. n.d. n.d.
RS 48.4b 3.2c 31.6ab n.d. n.d. n.d. n.d. n.d. n.d.
aPlants were grown in a non-sterilised calcareous chernosem soil (C: all microbes), in a gamma-sterilised soil (G: no microbes) and in a sterilised substrate, to which a mycorrhiza-free soil extract was added (GB: no mycorrhiza). The effect of the single- (M, R, S), dual-(MR, MS, RS) and multilevel (MRS) inoculations were assessed by using bacterial and fungal microsymbionts. M:G. fasciculatumM 107, R:R. melilotiS 5/7+Lu 41+K 4/1, S:A. brasilenseKm 5. n.d.: not determined.
bValues denoted by the same letter are not significantly different atp
5% level. Significant differences are given in italics.
dual systems with the other nitrogen-fixing bacteria (A. brasilenseKm 5-RS). With the mycorrhizal (M,
G. fasciculatumM 107) partner (Table 4) the effect was smaller.
was found, as a result of the gamma-sterilisation. The effect of the additional AM-free rhizosphere mi-croflora depended on the microelement, studied. Any microbial re-inoculation (GB) decreased the Mg, Zn and Cu and S content of the alfalfa shoot-biomass, compared to the gamma-sterilised substrate (G). The concentration of Fe was increased by the re-suspension of the AM-free soil extract in the control substrates.
In the original normal soil (C), the content of some elements was unaffected by the inoculated microsym-bionts. With Ca slightly increased, but not significant, uniform uptake occurred with the single- or dual di-azotroph treatments C+R, C+S and C+RS, respec-tively (Table 5).
3.4. Abundance of the inoculated microbes in the rhizosphere of the alfalfa
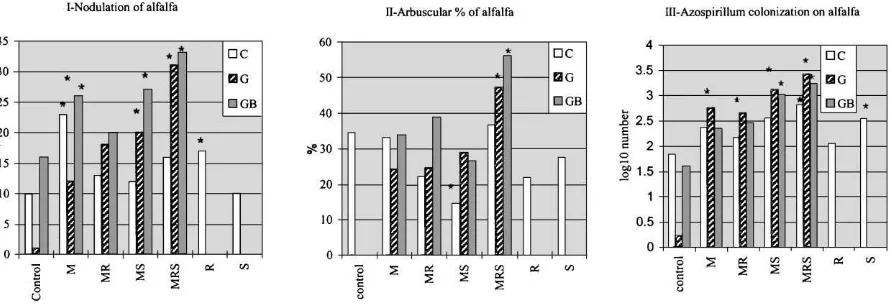
For the AMF, the infection frequency (F, %) in the targeted roots was high (all near to the 100%) and was not significantly changed for in the different microbial treatments (data not shown). The arbuscular content of the infected parts (a, %), however, was a more vari-able as a function of the single-, dual- or multilevel co-inoculations (Fig. 1). Addition of the AM-free soil suspension (the normal rhizosphere constituents) to the gamma-sterilised substrates (G, GB) increased the arbusculum content of the alfalfa root system. A
syn-Fig. 1. Nodule-number (I), arbusculum content (II) and abundance of the associative diazotrophs (III) on the alfalfa roots after 3 months of growth in a calcareous loamy chernozem soil (C: all microbes), in the same gamma-sterilised soil (G: no microbes) and in the sterilised substrate, resuspended with mycorrhiza-free soil extract (GB: no mycorrhiza). The effect of the single- (M, R, S), dual- (MR, MS) and multilevel (MRS) coinoculations were assessed after 3 months of growth in a pot experiment among controlled conditions. M: vesicular AMF (G. fasciculatumM 107), R:R. melilotiS 5/7+K 4/1+Lu 41 mixture), S:A. brasilenseKm 5) and their combinations, respectively.
Table 5
The amount of Ca, Mg, Fe, Zn, Cu and S in the shoot biomass of the alfalfa in a pot experimenta
Treatments Element contentb (mg per pot)
Ca Mg Fe Zn Cu S
C 2.23a 3127a 124.7a 22.5a 7.23a 4026.7a G 3.30c 4330c 139.4a 39.4c 10.91b 7483.3b
GB 3.34c 3543b 237.5b 28.6b 7.98a 4656.7a
C+M 2.20a 3086a 92.4a 22.6a 6.62a 3670.0a C+MR 2.19a 2856a 97.9a 22.7a 6.48a 3596.7a C+MS 2.25a 2880a 113.5a 22.7a 6.95a 4374.3a C+MRS 2.01a 3063a 91.4a 23.8a 6.97a 3390.0a C+R 2.42b 3120a 129.0a 26.7a 6.78a 4073.3a C+S 2.37b 2943a 120.4a 20.9a 6.92a 3847.7a C+RS 2.44b 2890a 100.4a 24.8a 7.37a 3390.0a aPlants were grown in a non-sterilised calcareous chernosem soil (C: all microbes), re-inoculated in a gamma-sterilised soil (G: no microbes) and in the sterilised substrate, which was re-suspended with a mycorrhiza-free soil extract (GB: no mycor-rhiza). The effect of the single- (M, R, S), dual- (MR, MS, RS) and the multilevel (MRS) co-inoculations were assessed by using bacterial and fungal microsymbionts. M:G. fasciculatumM 107, R:R. melilotiS 5/7+Lu 41+K 4/1, S:A. brasilenseKm 5.
bValues sharing the same letter in a column do not differ significantly atp<0.05. Significant differences are given in italics.
(G. fasciculatum M 107) and Azospirillum (A. brasilenseKm 5) co-inoculations (C+MS).
For nodule-number, after the 3 months of plant growth, a beneficial effect was found for all mi-crobial inoculations in the sterile or AM-free soils (Fig. 1). A further enhancement was developed with the multilevel co-inoculations (MRS). In the origi-nal soil (C) a significantly increased nodule-number was also recorded with the mycorrhizal (G. fascicu-latum M 107) inoculation. Dual treatments with the obligate- and the associative diazotrophs (Rhizobium+
Azospirillumbacteria, RS) also resulted in a synergis-tically increased nodule-number (nn=27 per pot, data not shown). This was the highest rate in the control soil.
The most probable number method (MPN) for cal-culating the abundance of the associative nitrogen-fixing bacteria was also carried out for the rhizo-sphere of the alfalfa host. These data showed that the cell-number of theAzospirillumdiazotrophs increased due to the mono- or multilevel inoculations. The high-est rates were found in case of the single- or dual my-corrhizal co-inoculations (M, MS, MRS) especially in the sterile or in the AM-free soils (G, GB). In the original substrate (C) the lowest cell numbers were recorded in theAzospirillum-non-amended combina-tions, such as the M, R and MR treatments. All of the mycorrhizal co-inoculations increased the abundance of the associative diazotrophs. In the GB substrate, only the dual- or multilevel co-inoculations of the MS or (MRS) treatments were effective.
4. Discussion and conclusions
Associative and symbiotic nitrogen-fixing bacteria and AMF are common beneficial microbes of the monocotyledonous- and leguminous- plants; about 80–90% of the higher plants, respectively. Artificial seed and soil inoculation techniques were, therefore, started over 100 years ago; as a simple application of a mixed nodule extracts (Hiltner, 1895). Inoculated mi-crobes, however, compete with the native microflora of the soil. This is the main reason for failed inocula-tion experiments (Graham, 1992; Giller and Cadish, 1995). Effects of abiotic environmental stress factors (temperature, drought, acidity, etc.) are also common (Smith and Bowen, 1979; Graham, 1992; Bayoumi
et al., 1995). The final effect of any microbial inocu-lation of plant-rhizosphere functioning is, therefore, the result of a complex of interactions between the plants, the rhizosphere inhabitants and the different microbial and environmental components involved (Barea et al., 1988b; Postma et al., 1989). To study antagonistic and synergistic effects, especially among the so-called beneficial microorganisms is crucial to plant growth and sustainability (Bethlenfalvay et al., 1985; Höflich, 1993).
In the present study the effect of the separated single-, dual- or multilevel mycorrhizal co-inoculations have been demonstrated for alfalfa, grown in normal, sterilised and AM-free soil conditions. Detection of the impact of associative and symbiotic nitrogen-fixers and of AMF on the plant growth and development was possible with this technique. Sterilisation pro-cedures have been used to study the effect of some abiotic factors on the introduced microbes (Bayoumi et al., 1999), or to eliminate the influence of soil-borne plant pathogens (Postma et al., 1989). Separation of the AMF and the other usual rhizosphere components (mainly bacteria and other micromycetes) is possible using a sterilisation and re-inoculation procedure. In the GB treatments a successful reconstruction of the original rhizosphere microflora occurred; evidence by plant growth and the element-content of the host (Table 2).
In all of the applied substrates (C, G, GB) benefi-cial, synergistic effect of the multilevel co-inoculations of the obligate- and associative diazotrophs and the AMF (MRS) were found. Other measurements, such as chlorophyll-fluorescence analysis also indicated a stress buffer effect of AMF (Strasser, 1996).
The dry matter production and Rhizobium nodu-lation data showed a significant synergistic effect between the AMF (M) and the separately applied di-azotrophs (MR or MS), and between the obligate and associative nitrogen-fixing bacteria (RS) used. The effects of diazotroph bacteria are rarely considered in studies of legumes.Azospirillumbacteria are used mainly for mono-cotyledonous hosts (Döbereiner and Day, 1976; Subba Rao, 1985; Pacovsky, 1988; Belimov et al., 1999). In the other dual-inoculated systems the AMF and theRhizobiumnitrogen-fixing bacteria are normally used for inoculating the legu-minous hosts (Ames and Bethlenfalvay, 1987; Cham-pawat, 1990). Synergistic effect of the associative and obligate nitrogen-fixers should be a consequence of the clear separation of their functioning (e.g. by the root-nodules) inside the plant-rhizosphere.
Several experiments have been published detailing the success and failure of Azospirillum inoculations for agricultural crops, such as the wheat and the maize (Biró, 1992a,b). Simultaneous AMF treat-ment seems to result in better establishtreat-ment of the nitrogen-fixers. It is less common for co-inoculated of phosphorus-mobilising microorganisms (such as the Agrobacterium or Flavobacterium sp.) (Barea et al., 1988b; Belimov et al., 1999).
All of theR. melilotiisolates used originated from the root-nodules of the alfalfa. This is grown widely in Hungary. Strains were selected for their perfor-mance as single treatments with sterile substrates. Under non-sterile-conditions (C), however, a high competitive ability with the indigenous populations is needed. A mixture of three efficient nodulatingR. melilotistrains were therefore used.
The microsymbionts have distinct habitats in the rhizosphere, such as the nodules (for theRhizobium) and the root-interior for the associative diazotrophs and the AMF. Both nitrogen-fixers inhabit the interia of the root-systems or the mycelial network. Func-tional interrelations must exist between them and the AMF (Bethlenfalvay et al., 1985; Subba Rao, 1985; Champawat, 1990; Paula et al., 1992). In case of the Rhizobium bacteria the nitrogen-fixing ability is separated to the inside of the root nodules. Gener-ally competition seems not to occur between rhizobia and AMF (Ames and Bethlenfalvay, 1987). Associa-tive diazotrophs, on the other hand are not separated from the mycelia of the endomycorhizal fungi. These
nitrogen-fixers can function both inside and outside AMF structures. This may result in a competition for nutrients between the two microsymbionts. The success and the failure of the Azospirillum and AM fungal co-inoculations may therefore depend on the physiological stage of the host, the time of the infec-tions or on the nutrient demands of the microsymbiont partners.
Acknowledgements
Grants of Hungarian Research Fund (OTKA-T0235-43), the COST Actions 8.30, 8.38, the Swiss National Foundations (31-46.860.96/31-52.541.97) and of the Soc. Acad. Genève to RJS are highly acknowledged.
References
Ames, R.N., Bethlenfalvay, G.J., 1987. Localised increase in nodule activity, but no competitive interaction of cowpea rhizobia due to pre-establishment of vesicular-arbuscular mycorrhiza. New Phytol. 106, 207–215.
Barea, J.M., Azcon-Aguilar, C., 1983. Mycorrhizas and their significance in nodulating nitrogen-fixing plants. In: Brady, N.C. (Ed.), Advances in Agronomy, Vol. 36. Academic Press, New York, pp. 1–54.
Barea, J.M., Bonis, A.F., Olivares, J., 1983. Interactions between
Azospirillumand VA mycorrhiza and their effects on growth and nutrition of maize and ryegrass. Soil Biol. Biochem. 15, 706–709.
Barea, J.M., Azcon-Aguilar, C., Azcon, R., 1988a. Vesicular arbuscular mycorrhiza improve both symbiotic N2-fixation and N-uptake from soil as assessed with a N technique under field conditions. New Phytol. 106, 717–725.
Barea, J.M., Azcon-Aguilar, C., Azcon, R., 1988b. The role of mycorrhiza in improving the establishment and function of theRhizobium-legume system under field condition. In: Beck, D.P., Materon, L.A. (Eds.), Nitrogen Fixation by Legumes in Mediterranean Agriculture. Martinus Nijhoff, The Hague, pp. 153–162.
Bayoumi, H.E.A.F., Biró, B., Balázsy, S., Kecskés, M., 1995.
Rhizobiumand Bradyrhizobiumstrains affected by inhibitory environmental factors. Acta Microbiol. Immunolog Hung. 42, 61–69.
Bayoumi, H.E.A.F., Abdorhim, H., Khalif, A., Godwar, H., Ködöböcz, L., Jevcsák, I., Biró, B., Kecskés, M., 1999. Soil physical factors affecting the survival of Rhizobium leguminosarumbv.viceaepopulations in vivo. Sci. Bull. North Univ. Baia Mare Ser. C 13, 8–28.
Bethlenfalvay, G.J., Schüepp, H., 1994. Arbuscular mycorrhizas and agrosystem stability. In: Gianinazzi, S., Schüepp, H. (Eds.), Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Birkhauser, Berlin, pp. 117–131. Bethlenfalvay, G.J., Brown, M.S., Stafford, A.E., 1985.Glycine–
Glomus–Rhizobiumsymbiosis. II. Antagonistic effects between mycorrhizal colonization and nodulation. Plant Physiol. 79, 1054–1058.
Bethlenfalvay, G.J., Brown, M.S., Newton, W.E., 1987. Photo-synthetic water- and nutrient-use efficiency in a mycorrhizal legume. In: Mycorrhizae in the Next Decade. Practical Applications and Research Priorities. Proceedings of the Seventh NACOM, Gainesville, FL, pp. 231–233.
Biró, B., 1992a. N2-fixing, plant-growth promotingAzospirillum
bacteria — a review. Agrokem. Talajtan 39, 363–367 (in Hungarian).
Biró, B., 1992b. Possibility ofAzospirillumplant inoculations — a review. Agrokem. Talajtan 41, 390–399 (in Hungarian). Biró, B., Köves-Péchy, K., Szili-Kovács T., Szegi, J., 1993a. Effect
of fertilizer on spontaneousRhizobiuminfection in Hungarian soils. In: Szabolcs, I. (Ed.), Soil Resilience and Sustainable land Use. Agrokem. Talajtan 42, 207–212.
Biró, B., Vörös, I., Köves-Péchy, K., Szegi, J., 1993b. Symbiont effect ofRhizobiumbacteria and AM fungi onPisum sativum
in recultivated mine spoils. Geomicrobiol. J. 11, 275–284. Boddey, R.M., Urquiaga, S., Reis, V., Döbereiner, J., 1991.
Biological nitrogen fixation associated with sugar cane. Plant Soil 137, 111–117.
Buzás, I. (Ed.), 1998. Handbook of Soil and Agricultural Practical Studies, Vol. 1. Soil Physical and Chemical Characteristics (in Hungarian). Mezõgazdasági Kiadó, Budapest, 242 pp. Champawat, R.S., 1990. Response of chickpea (Cicer arietinum)
to Rhizobium and vesicular arbuscular mycorrhiza dual inoculation. Acta Microbiol. Pol. 39, 163–169.
Döbereiner, J., Day, J.M., 1976. Associative symbioses in tropical grasses: characterization of microorganisms and dinitrogen-fixing sites. In: Newton, W.E., Nyman, C.J. (Eds.), Proceedings of the First International Symposium on N2-fixation, Vol. 2. Washington University Press, Pullman, WA, pp. 518–538.
Garbaye, J., 1994. Helper bacteria: a new dimension to the mycorrhizal symbiosis. New Phytol. 128, 197–210.
Giller, K.E., Cadish, G., 1995. Future benefits from biological nitrogen fixation: an approach to agriculture. Plant Soil 174, 255–277.
Graham, P.H., 1992. Stress tolerance in Rhizobium and Brady-rhizobiumand nodulation under adverse soil conditions. Can. J. Microbiol. 38, 475–484.
Hardy, R.W.F., Bums, R.C., Hosten, R.D., 1973. Application of the acetylene–ethylene assay for measurement of nitrogen fixation. Soil Biol. Biochem. 5, 47–81.
Hiltner, A., 1895. Zur frage der stickstoffernahrung in Pflanzen. Landw. Vers. Statist. 51, 92–97.
Höflich, G., 1993. Effect of variety and environmental factors on the photo-effectivity of bacterial inoculations in peas. Zentralbl. Mikrobiol. 148, 315–324.
Jeffries, P., Dodd, C.J., 1991. The use of mycorrhizal inoculants in forestry and agriculture. In: Arora, D.K., Rai, B., Mukerji,
K.G., Knudsen, G.R. (Eds.), Handbook of Applied Mycology, Vol. 1, Soil and Plants. Marcel Dekker, New York, pp. 77–129. Lindermann, R.G., 1983. Mycorrhizal interactions with the rhizo-sphere microflora: the mycorhizorhizo-sphere effect. Phytopathology 78, 366–371.
Minerdi, D., Fani, R., Bonfante, P., 1999. Nitrogen fixation genes in a Burkholderia, living in symbiosis with an arbuscular mycorrhizal fungus. In: Proceedings of the Sixth Symposium on Bacterial Genetics and Ecology, Florence, Italy. Abstract of Papers, p. 80.
Okon, Y., Albrecht, S.L., Burris, R.H., 1977. Methods for growing
Spirillum lipoferumand for counting it in pure culture and in association with plants. Appl. Environ. Microbiol. 33, 85–88. Pacovsky, R.S., 1988. Influence of inoculation withAzospirillum
brasilenseandGlomus fasciculatumon sorghum nutrition. Plant Soil 110, 283–287.
Pacovsky, R.S., Fuller, G., Paul, E.A., 1985. Influence of soil on the interactions between endomycorrhizae and Azospirillum on sorghum. Soil Biol. Biochem. 17, 521–531.
Paula, M.A., Urquiaga, S., Siqueira, J.O., Döbereiner, J., 1992. Synergistic effects of vesicular-mycorrhizal fungi and diazotrophic bacteria on nutrition and growth of sweet potato (Ipomea batatas). Biol. Fert. Soils 14, 61–66.
Phillips, J.M., Hayman, D.S., 1970. Improved procedures for clearing roots and staining parasitic and VAM fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 55, 158–161. Postgate, J.R., 1969. Viable counts and viability. In: Norris,
J.R., Ribbons, D.W. (Eds.), Methods in Microbiology, Vol. 1. Academic Press, New York, pp. 611–618.
Postma, J., Hok-A-Hin, H., Oude Voshaar, J.H., 1989. Influence of the inoculum density on the growth and survival of
Rhizobium leguminosarumbiovartrifoliiintroduced into sterile and non-sterile loamy sand and silt loam. FEMS Microbiol. Ecol. 73, 49–58.
Puppi, G., Azcón, R., Höflich, G., 1994. Management of positive interactions of arbuscular mycorrhizal fungi with essential groups of soil microorganisms. In: Gianinazzi, S., Schüepp, H., (Eds.), Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Birkhäuser, Basel, pp. 201–215.
Sanchez-Diaz, M., Pardo, M., Antolin, M., Pena, J., Aguirreola, J., 1990. Effect of water stress on photosynthetic activity in the
Medicago–Rhizobium–Glomus symbiosis. Plant Sci. 71, 215– 221.
Smith, S.E., Bowen, G.D., 1979. Soil temperature, mycorrhizal infection and nodulation inMedicago truncatulaandTrifolium subterraneum. Soil Biol. Biochem. 11, 469–473.
Strasser, R.J., 1996. Stress as driving force for adaptation and evolution: Mycorrhiza as stress-buffer. In: Abiotic Stress Allevation by Arbuscular Mycorrhizal Fungi. Workshop of COST Action 8.21, Budapest. Abstract of Papers, p. 32. Subba Rao, N.S., 1985. Effect of combined inoculation of
Azospirillum brasilense and vesicular arbuscular mycorrhiza on pearl millet (Pennisetum americanum). Plant Soil 81, 283– 286.
Azospirillumgen. nov. and two species,Azospirillum lipoferum
(Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 24, 967–980.
Trouvelot, A., Kough, J.L., Gianinazzi-Pearson, V., 1985. Mesure du Taux de mycorrhization VA d’un systeme radiculaire. In: Symposium Europeen Sur Les Mycorrhizes, INRA, Paris, pp. 217–221.
Tobar, R., Azcon, R., Barea, J.M., 1994. Improved nitrogen uptake and transport from15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol. 126, 119–122.