www.elsevier.com / locate / bres
Short communication
Nitric oxide is involved in sustained and delayed cell death of rat
retina following transient ischemia
Won-Kyu Ju, Keun-Young Kim, Sung-Jin Park, Dae-Kyoon Park, Cheol-Beom Park,
*
Su-Ja Oh, Jin-Woong Chung, Myung-Hoon Chun
Department of Anatomy, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Socho-gu, Seoul 137-701, South Korea Accepted 8 August 2000
Abstract
We have investigated the role of nitric oxide (NO) in the rat retina following ischemic injury induced by transient increase of intraocular pressure. The thickness of both the inner plexiform layer and inner nuclear layer decreased during early postischemic stages (up to 1 week). In late postischemic stages (2–4 weeks), the thickness of the outer nuclear layer (ONL) decreased markedly. Thus,
G
mechanisms other than excitotoxic ones may contribute to postischemic retinal cell death. Treatment of rats with N -nitro-L-arginine methyl ester, a nitric oxide synthase (NOS) inhibitor, significantly reduced ischemic damage. Our findings suggest that NO is involved in the mechanism of ischemic injury, and plays a key role in the delayed and sustained cell death in the ONL following transient retinal ischemia. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Retina and photoreceptor
G
Keywords: Nitric oxide; N -nitro-L-arginine methyl ester; Ischemia; Retina; Rat
Nitric oxide (NO), a free radical gas with a half-life of a icity triggered by the overactivation of glutamate receptors few seconds, has been shown to play various physiological is considered to be a central component of postischemic and pathophysiological roles in the nervous system [17]. necrosis in nervous tissue, including the retina [3,14,23]. NO is generated by the oxidation of arginine, a reaction The preferential susceptibility of inner retinal neurons to catalyzed by the enzyme nitric oxide synthase (NOS) [18], ischemic damage is in line with this concept, since the which is present in three isoforms, endothelial (eNOS), expression of glutamate receptors is confined to neurons of neuronal (nNOS), and inducible (iNOS). The NO thus the inner retina. However, results from recent studies on generated acts on cells by activating soluble guanylate the rat retina indicate that, with extended postischemic cyclase (sGC), thereby increasing the levels of cyclic GMP survival times, neuronal death becomes significant in the (cGMP), which then mediates the effects of NO on the cell outer nuclear layer (ONL) and may even exceed that [15]. Despite conflicting results, it is generally accepted occurring in the inner retina [12,13]. These observations that generation of excessive NO may cause neuronal suggest that there are other mechanisms, in addition to damage after an ischemic insult [8,21]. excitotoxic processes, that can contribute to cell death in As in other parts of the central nervous system, ischemia the postischemic retina. Thus, differences in the time-in the rettime-ina results time-in a delay time-in the onset of neuronal cell course and extent of postischemic neuronal death in death. Most studies on the postischemic retina have various retinal cell layers could reflect both selective reported that cell death is most pronounced in the inner vulnerability to ischemic injury and different mechanisms retina [5,17,23]. Although the precise mechanisms of of cell death. The sensitivity of retinal neurons to ischemia ischemia-induced neuronal death are unknown, excitotox- might also depend on the specific expression of protective
or death-promoting proteins in different cell types [20].
G
N -nitro-L-arginine methyl ester (L-NAME) is an
iso-*Corresponding author. Tel.:182-25-901-152; fax:182-25-363-110.
E-mail address: [email protected] (M.-H. Chun). form nonselective inhibitor of nitric oxide synthase (NOS)
intraocular pressure, we have examined the effects of retina was profoundly affected; ultimately, at 4 weeks after
L-NAME on the ischemic rat retina in order to determine reperfusion, the outer nuclear layer consisted of only one
whether NO affects specific retinal layers. or two rows of nuclei.
In this study 55 adult, male, albino Sprague–Dawley The effects of the NOS inhibitor, L-NAME, on the
rats, weighing between 200 and 250 g, were used; five ischemic retina were confirmed by measuring the thickness animals were used as normal controls and 50 (10 per of the inner plexiform layer (IPL), inner nuclear layer group) for the five experimental groups. Animals were (INL), and ONL. The numbers of cells in the ganglion cell anesthetized with 4% chloral hydrate (1 ml / 100 g body layer (GCL), in the INL, and in the ONL were also weight). The pupils were dilated with 1% tropicamide counted.
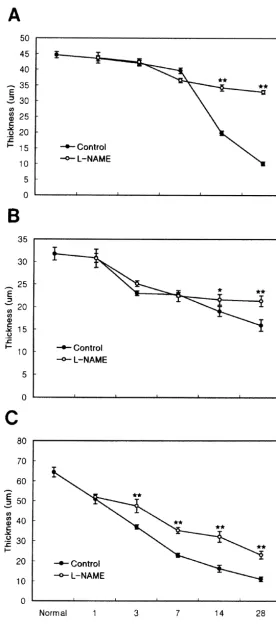
drops. The intraocular pressure (IOP) was raised to 90– In the normal retina, the thickness of IPL, INL, and 120 mmHg by cannulation of the anterior chamber of right ONL was 64.262.39, 31.761.4 and 44.662.4mm, respec-eye with a 30-gauge needle connected to a hydrostatic tively. In the nontreated retina, the thickness of the IPL pressure device; the elevated IOP was maintained for 60 started to decrease 1 day after reperfusion, declining to min. The applied pressure of 90–120 mmHg is in the range 57.4 and 35.6% of control values at 3 days and 1 week of, or slightly above, systemic systolic pressure in the rat. after reperfusion, respectively. Finally, at 4 weeks after Following removal of the cannula, the circulation recomm- reperfusion, the thickness of the IPL was only 16.3% of enced immediately and the IOP decreased to normal values the control value. From 3 days onwards, after reperfusion, within 5 min. Animals were sacrificed by an overdose of the thickness of the INL decreased gradually; however, the chloral hydrate at the following intervals after reperfusion: decrease in thickness was not as severe as that in the IPL 1, 3, 7, 14, and 28 days. The eyes were then enucleated for and ONL. Four weeks after reperfusion, there was an
histological examination. approximately 50% decrease in the thickness of the
L-NAME was dissolved in normal saline. Twenty-five ischemic retina. With respect to the thickness of the ONL,
rats (five per group) received one intraperitoneal injection there was a mild decrease in thickness for up to 1 week, of L-NAME (40 mg / kg) 1 h after cessation of ischemia. corresponding to 88.8% of the control value. However, at
Animals were then sacrificed as described above, at 1, 3, 7, later stages (at 2 and 4 weeks after reperfusion), there was 14, and 28 days after reperfusion. The eyes were also an approximately 55 and 77.4% decrease, respectively.
enucleated for histological examination. Thus, the susceptibility of the IPL to ischemic insult
Enucleated eyes were opened by a circular incision and extended throughout the whole experimental period, the posterior eyecup was immersion-fixed in 4% parafor- whereas the ONL was susceptible only at later stages. maldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 30 In theL-NAME-treated retina, at 1 day after reperfusion,
min. The retina was dissected from the choroid and treated the thickness of the IPL decreased similarly to that in the with the same fixative for another 2 h at 48C. After several untreated, ischemic retina. However, from 3 days onward, washes in PB, small pieces (approximately 1035 mm) the magnitude of the decrease in the treated retina was less were cut from the central retina, and embedded in wax. than that of the ischemic retina. Three days and 1 week Vertical sections (5 mm thick) were cut and stained with after reperfusion, the thickness of the IPL decreased to
hematoxylin and eosin. 73.7 and 54.7% of control values, respectively. Four weeks
The effects of L-NAME on the ischemic retina were after reperfusion, the IPL thickness had decreased to
evaluated quantitatively by measuring the thickness of cell 35.7% of the control. After reperfusion, the extent of the layers in vertical sections from control and experimental decrease of the IPL thickness was 1.3- and 1.5-fold at 3 retinae. Images of each section were recorded using a 403 days and 1 week, respectively, and 2-fold from 2 to 4 objective and 103 projective and processed by an image weeks. Thus,L-NAME treatment reduces the magnitude of
analysis system (BMI-PLUS; Bummi Universe Co., the retinal thickness decrease during the early postischemic Ansan, South Korea). For each experimental condition, 20 stage (3 days onward).
sections obtained from different retinae were analyzed. All With L-NAME treatment, the thickness of the INL
values are given as the mean6S.D. Statistical evaluation decreased gradually from 3 days after reperfusion. There
was based on Student’s t-test. was no difference in the decrease of the INL thickness up
Elevation of the IOP for 60 min caused ischemic to 1 week after reperfusion, compared with the INL of the damage to retinal tissues. From 1 to 3 days after reperfu- nontreated retina. However, at 2 and 4 weeks after sion, the thickness of the entire retina declined gradually, reperfusion, as the retinal thickness decreased to 68.1 and reducing to about 30% of the control value after 4 weeks 67.2% of the control value, the magnitude of INL thick-(Figs. 1 and 2). The effects on the individual retinal cell ness decrease was less, being only 1.1- and 1.3-fold, layers were different with respect to the time-course and respectively, compared with the nontreated retina. This extent of reduction in thickness (Fig. 2), in good agree- result indicates that L-NAME treatment reduces the
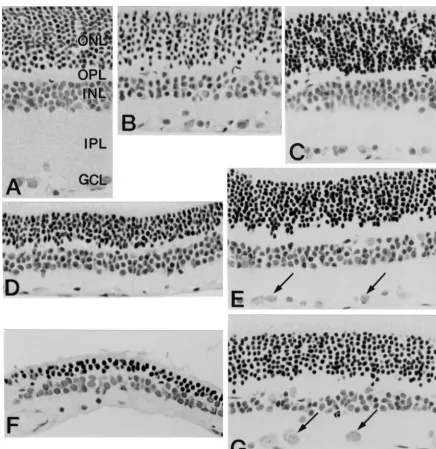
Fig. 1. Morpholgical features of the rat retina taken from different experimental conditions. (A) Normal retina. Five well-organized retinal layers are seen. (B, D and F) Micrographs taken from 5mm thick sections at 1 (B), 2 (D) and 4 weeks (F) after ischemia-reperfusion. In (B), the thickness of the retina is reduced due to disappearance of the inner plexiform layer (IPL). In (D and F), the thickness of the retina is markedly reduced due to loss of photoreceptor cells. Note that the outer nuclear layer (ONL) consists of two or three pyknotic cell layers in (F). (C, E and G) Micrographs taken from 5mm thick sections at 1 (C), 2 (E) and 4 weeks (G) in theL-NAME treated ischemic retina. Compared with those shown in (B, D and F), note the increase in the thickness of the IPL (C) and ONL (E, G). Ganglion cells (arrows) are clearly preserved. OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. Calibration bar: 50mm.
decreased similarly in both L-NAME-treated and non- the findings of the previous report [12], which showed that
treated retinae. However, at 2 and 4 weeks after reperfu- ischemia-reperfusion causes preferential damage to inner sion, the OPL thickness decreased to 76.7 and 73.8% of retinal neurons up to 1 week later, whereas the cell death the control value, respectively. This means that L-NAME occurring in the ONL was both delayed and sustained.
treatment produced a smaller decrease in retinal thickness, The induction of cell death within a few hours of namely 1.7- and 3.3-fold at 2 and 4 weeks after reperfu- ischemia-reperfusion is compatible with an excitotoxic
sion, respectively. mechanism evoked by the release of glutamate, as
course of cell death between the inner retina (GCL and clearly needed to elucidate the actual mechanisms respon-INL) and the outer retina (ONL) seems to indicate that sible for delayed and sustained cell death in the ONL different cell death-inducing mechanisms are at work in following ischemic insult.
the different regions [23]. The relatively late onset and the long duration (4 weeks or more) of the process of cell
death in the ONL, indicate that cell death is not induced by Acknowledgements excess levels of excitatory transmitters released during
ischemia or reperfusion. This is to be expected, since both This work was supported by a grant of the Korea Health ionotropic and metabotropic glutamate receptors are wide- 21 R&D Project (HMP-00-B-21300-0209), Ministry of ly expressed in neurons of the inner retina, but are not Health and Welfare by Brain Korea 21, Republic of Korea.
found in the ONL [1]. We are grateful to H.-D. Rho and B.-W. Hong for their
In the present study, we found that L-NAME, a non- technical assistance.
selective NOS inhibitor, was able to protect the IPL and INL of the rat retina against postischemic damage in the early postischemic stages, whereas the ONL was more
References protected at later stages. Our result, that cell damage in the
ONL was protected by a single dose of L-NAME given
¨ ¨
[1] J.H. Brandstatter, P. Koulen, H. Wassle, Diversity of glutamate
immediately after the ischemic insult, is an interesting receptors in the mammalian retina, Vision Res. 38 (1998) 1385– result. The mechanism responsible for protection of ONL 1397.
damage is not known. However, if we consider that [2] D.S. Bredt, S.H. Snyder, Nitric oxide mediates glutamate-linked enhancement of cGMP leveles in the cerebellum, Proc. Natl. Acad.
damages of IPL and INL are protected from early stages,
Sci. USA 86 (1989) 9030–9033.
protection of the ONL may be secondary consequences
[3] D.W. Choi, Ischemia-induced neuronal apoptosis, Curr. Opin.
Neuro-following protection of the inner retina. The fact that NO is
biol. 6 (1996) 667–672.
a neurotoxin in the inner retina in the early stages of [4] V.L. Dawson, T.M. Dawson, D.A. Bartley, G.R. Uhl, S.H. Snyder, ischemia-reperfusion, is in agreement with a previous Mechanisms of nitric oxide-mediated neurotoxicity in primary brain
cultures, J. Neurosci. 13 (1993) 2651–2661.
report [7], which showed that N-nitro-L-arginine, a NOS
[5] A. Hayashi, A.W.A. Weinberger, H.C. Kim, E. de Juan Jr, Genistein,
inhibitor, was able to protect against the ischemic damage
a protein tyrosine kinase inhibitor, ameliorates retinal degeneration
occurring in the inner retina of the rat. Our result, namely
after ischemia-reperfusion injury in rat, Invest. Ophthalmol. Vis. Sci.
that NO selectively induces photoreceptor cell death at 38 (1997) 1193–1202.
later stages, strongly suggests that NO is a key free radical, [6] K.A. Hossmann, Glutamate-mediated injury in focal cerebral
is-which induces delayed and sustained cell death in the chemia: the exitotoxin hypothesis revised, Brain Pathol. 4 (1994) 23–36.
ONL. Taken together, our results indicate that NO may be
[7] O. Geyer, J. Almog, M. Lupu-Meiri, M. Lazar, M. Lazar, Y. Oron,
involved in retinal toxicity over different time intervals,
Nitric oxide synthase protect rat retina against ischemic injury,
and that different mechanisms of cell death clearly exist in FEBS Lett. 374 (1995) 399–402.
the postischemic retina. In early postischemic stages, the [8] I.M. Goldstein, P. Ostwald, S. Roth, Nitric oxide: a review of its role
over-release of glutamate is the primary event that induces in retinal function and disease, Vision Res. 36 (1996) 2979–2994. [9] O. Goureau, D. Hicks, Y. Courtois, Human retinal pigmented
cell death in the inner retina, with NO being more involved
epithelial cells produce nitric oxide in response to cytokines,
in secondary degeneration. Support for this hypothesis
Biochem. Biophys. Res. Commun. 198 (1994) 120–126.
comes from the following facts: (i) once released, gluta- [10] C. Iadecola, F. Zhang, S. Xu, R. Casey, M.E. Ross, Inducible nitric mate binds to and stimulates glutamate receptors, increas- oxide synthase gene expression in brain following cerebral ischemia,
ing intracellular calcium concentration and stimulating J. Cereb. Blood Flow Metab. 15 (1995) 378–384.
[11] W.-K. Ju, K.-Y. Kim, H.-D. Hofmann, I.-B. Kim, M.-Y. Lee, S.-J.
nNOS and eNOS to produce NO [2]; and (ii) the NO
Oh, M.-H. Chun, Selective neuronal survival and upregulation of
produced by activation of glutamate receptors induces
PCNA in the rat inner retina following transient ischemia, J.
neurotoxicity in cortical neurons [4]. Neuropathol. Exp. Neurol. 59 (2000) 241–250.
At later stages, the mechanism by which NO causes an [12] I.-B. Kim, K.-Y. Kim, C.K. Joo, M.-Y. Lee, S.-J. Oh, J.-W. Chung, ¨
almost complete loss of ONL neurons could be attributed M.-H. Chun, Reaction of Muller cells after increased intraocular pressure in the rat retina, Exp. Brain Res. 121 (1998) 419–424.
to a direct effect of the nitric oxide radical on
photo-[13] R.L. Handy, P.K. Moore, A comparison of the effects ofL-NAME,
receptor cells, or to over-activation of photoreceptor cells
7-NI and L-NIL on carrageenan-induced hindpaw oedema and NOS
by NO itself. This interpretation is supported by the activity, Br. J. Pharmacol. 123 (1998) 1119–1126.
following evidence: (i) activation of iNOS occurs long [14] P. Louzada-Junior, J.J. Dias, W.F. Santos, J.J. Lachat, H.F. Bradford,
after the onset of cerebral ischemia [11]; (ii) iNOS is J. Coutinho-Netto, Glutamate release in experimental ischaemia of the retina: An approach using microdialysis, J. Neurochem. 59
¨
expressed in both Muller cells and pigment epithelial cells
(1992) 358–363.
following various stimuli [9]; and (iii) NO increases the
[15] S. Moncada, R.M. Palmer, E.A. Higgs, Nitric oxide; physiology,
level of cyclic guanosine monophosphate, which opens pathophysiology, and pharmacology, Pharmacol. Rev. 43 (1991)
1
light-sensitive Na channels and induces dark currents in 109–142.
amino acids and ischemia on rat retinal choline acetyltransferase- in the brain pathology of heat stress, Prog. Brain Res. 115 (1998) containing cells, Invest. Opthalmol. Vis. Sci. 36 (1995) 1692–1700. 297–333.
¨
[18] R.M.J. Palmer, D.S. Ashton, S. Moncada, Vascular endothelial cells [22] B.K. Siesjo, The role of calcium on cell death, in: D.L. Price, H. synthesize nitric oxide from L-arginine, Nature (Lond.) 333 (1988) Thoenen, A.J. Aguayo (Eds.), Neurodegenerative Disorders:
Mecha-664–666. nisms and Prospects for Therapy, Wiley, New York, 1991, pp.
[19] B. Peruche, J. Krieglstein, Mechanisms of drug actions against 35–59.
´
neuronal damage caused by ischemia – an overview, Prog. Neuro- [23] M.E. Szabo, M.T. Droy-Lefaix, M. Doly, C. Carre, P. Braquet, psycholpharmacol. Biol. Psychiat. 17 (1993) 21–70. Ischemia and reperfusion-induced histologic changes in the rat [20] D.M. Rosenbaum, P.S. Rosenbaum, H. Gupta, M. Singb, A. Aggar- retina: Demonstration of a free radical-mediated mechanism, Invest.