Anti-Inflammatory Effects of Strawberry
Wine Extracts on LPS-Stimulated RAW
264.7 Macrophage Cells
PRACTICAL TRAINING REPORT
This practical training report is submitted for the partial requirement for Bachelor Degree
By:
Lukas Terry Boedianto 12.70.0044
DEPARTMENT OF FOOD TECHNOLOGY
FACULTY OF AGRICULTURAL TECHNOLOGY
SEOGIJAPRANATA CATHOLIC UNIVERSITY
SEMARANG
ANTI-INFLAMMATORY EFFECTS OF STRAWBERRY WINE EXTRACTS ON LPS-STIMULATED RAW 264.7 MACROPHAGE CELLS
Practical Training at Fu Jen Catholic University, Taiwan
By:
LUKAS TERRY BOEDIANTO Student ID: 12.70.0044 Faculty: Agricultural Technology
This Practical training report has been approved and supported by examiner in Practical Training Exam on July 13th 2015
Semarang, July 13th 2015
Department of Food Technology Faculty of Agricultural Technology Soegijapranata Catholic University
Practical Training Advisor I Practical Training Advisor II
Dr. Tsung-Yu Tsai Dr. Ir. Lindayani, MP.
i PREFACE
Praise the Lord because by His grace and blessing, the author would have the opportunity to undergo the practical training and finish the report. This report is the complete accountability from the practical training which was done in New Taipei City, Taiwan that take place from January 12th until March 12th 2015. During the training, the author did the research with title: Anti-Inflammatory Effects of Strawberry Wine Extracts on LPS-Stimulated RAW 264.7 Macrophage Cells this report was written as a requirement to acquire Bachelor Degree of Food Technology. The author would not be able to finish this task alone and only by support and guidance give by people around the author this report could be finished. Special thanks for:
1. Jesus Christ that always blessed, saved and guided the author in every step of practical training in Taiwan.
2. Dr. Victoria Kristina Ananingsih, ST., MSc. for giving me opportunity to join the internship program.
3. Dr. Tsung-Yu Tsai as my advisor who has advising me and supports me all the time when I did this research.
4. Dr. Ir. Lindayani, MP. as my advisor for taking care of me during this practical training in Taiwan.
5. Jenny, Ajheng, Amber, Gina, Tonny, Cindy, Family and Natasha who alywas give their time to help me to understand the experiment.
6. My family, Mamah, Papah, Raymond, Willy and Alex who always support me in finance and cheers me every day.
7. Last but not least, I would like to give my gratitude to all my beloved friends in Taiwan: Andy, Anne Shih, Joyce, Allisa, Cindy Mama, Yvonne, Anne Fang, Wira Lin, Ian, Garry, Kevin and all other friends that I can not mention one by one that always support and accompany me when I was in Taiwan.
This report is far from perfect, however the author hope this report can still be an inspiration and provide useful information for all the reader.
Semarang, July 13th 2015 Author,
ii
1.1. Background of Practical Training ... 1
1.2. Purpose of Practical Training ... 2
2. INSTITUTE PROFILE ... 3
2.1. Fu Jen Catholic University ... 3
2.2. Food Science Department ... 3
3. RESEARCH PROJECT ... 4
3.1. Research of Research ... 4
3.2. Literature Review ... 5
3.2.1. Strawberry (Fragaria ananassa) Wine ... 5
3.2.2. Characteristics of Inflammation ... 6
3.2.3. Raw 264.7 Macrophage Cell ... 7
3.2.4. Lipopolysaccharide (LPS) ... 8
3.2.5. Trolox ... 9
3.2.6. MTT Assay ... 10
3.2.7. Nitric Oxide (NO) Production Test ... 10
4. RESEARCH METHODOLOGY ... 10
4.1. Time and Place of Practical Training ... 12
4.2. Strawberry Wine Extraction ... 12
a. Materials ... 12
b. Methods ... 12
4.3. Raw 264.7 Macrophage Cell Preparation ... 13
a. Materials ... 13
b. Methods ... 13
4.4. Raw 264.7 Macrophage Cell Counting and Seeding ... 13
a. Materials ... 13
b. Methods ... 14
4.5. Sample Preparation and Injection ... 15
a. Materials ... 15
b. Methods ... 15
b.1. Control, LPS and T100 Sample Preparation ... 15
b.2. Strawberry Wine Extract Sample Preparation ... 15
b.3. Sample Injection ... 15
4.6. MTT Assay ... 16
a. Materials ... 16
iii
b.1. MTT Reagent Preparation ... 16
b.2. MTT Test ... 16
4.7. Nitric Oxide Production Test ... 17
a. Materials ... 17
b. Methods ... 17
b.1. Griess Reagent Preparation ... 17
b.2. NO Standard Curve Preparation ... 17
b.3. NO Production Test ... 17
4.8. Statistical Analysis ... 18
5. RESULT AND DISCUSSION ... 19
5.1. Cell Viability Test ... 19
5.2. Cell Morphology ... 20
5.3. NO Production Test ... 21
6. CONCLUSION AND SUGGESTION ... 24
6.1. Conclusion ... 24
6.2. Suggestion ... 24
8. REFERENCES ... 25
iv
LIST OF TABLES
v
LIST OF FIGURES
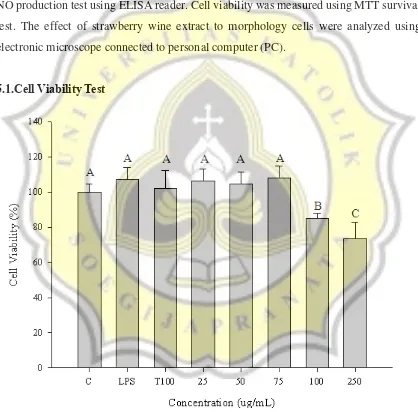
Figure 1. Effect of strawberry wine water extract on the cell viability in LPS-stimulated RAW 264.7 cells. Normal cells as control (C). LPS at 100 ng/mL (LPS). Trolox at 100 μg/mL (T100). Strawberry wine extract at 25-250 μg/mL (25, 50, 75, 100, 250)...19 Figure 2. Raw 264.7 Macrophage Cell Morphology. Normal cells as control (C).
LPS at 100 ng/mL (LPS). Trolox at 100 μg/mL (T100). Strawberry wine extract at 25-250 μg/mL (25, 50, 75, 100, 250)...21 Figure 3. Effect of strawberry wine water extract on the NO production in
vi
LIST OF APPENDICES
1
1. INTRODUCTION
1.1.Background of Practical Training
The most important things in sustainability of human life is food. Nowadays, people not only expect to have delicious food, but they also expect to have a nutritious food to maintain their health. Many food industries with their great technology concern about food quality and safety to search for the best to satisfy consumers. Many developments are being made so that the food will have a better quality. Food technology is very important in order to fulfill every progressive and dynamics changing demands of consumers. Food technology is responsible to every parts of food development. It concerns in many aspects, such as food safety, product development, food quality management, food packaging, food nutrition, food chemistry, food waste management, and food microbiology. In real life work, we as a food technologist will use the skills to be applied in our work.
For that reason, Department of Food Technology, Soegijapranata Catholic University (SCU) sets up a training program to let the student improve their skills and knowldges. In this program, student is given an opportunity either to join a food industry company or
to take a part in-house training (research). Student that is stay in food industry company will know the real bussiness practices and gains a big view about how actual food-related research is executed in the industry. This experience will be helpfull once the student graduates and goes into the working world. Student who chooses in-house training has to go to the selected university to be their facilitator of research. The selected university for this training is Food Science Department, Fu Jen Catholic University (FJU), Taiwan, which is sophisticated in applied biotechnology and microbiology sectors. Through this program, the student is given an opportunity to conduct their research abroad, to experience cultural diversity. This program can held since there is a student exchange mutual agreement between SCU and FJU.
2
Tsung-Yu Tsai, as the Assistant Professor of Food Science Department, Fu Jen Catholic University, Taiwan. The mentor of this research was Jenny as the student of master degree program of Food Science Department, Fu Jen Catholic University, Taiwan.
1.2.Purpose of Practical Training
a. To give experience about doing food science research with the new environtment. b. To give an opportunity to adapt with new circumtances and society in another country
with their own culture.
c. To sharpen and broaden knowledge and experience that could not be learnt in the real industry or scienctist world.
3 2. INSTITUTION PROFILE
2.1. Fu Jen Catholic University
Fu Jen Catholic University (FJU) is a famous private university in Taiwan that is found in 1925 by the Benedictines of Saint Vincent Archabbey. In 1961, FJU was re-established by Society of Jesus, Society Divine World, and Regional Bishop Conference. FJU is located in New Taipei City which is really strategic and has an easy acces to the diversity of cultural and social activities of the capital of Taiwan. FJU is moved by Christian understanding and inspired by the high ideals of Confucian education, and is noted for attracting foreign students from another country and also Indonesia. There are 21,671 undergraduate students, 3,891 graduated students (master program), more than 180,000 alumni that graduated and more than 39 alumni associations worldwide. FJU provides 11 colleges with 48 departments, 47 master program, 23 in-service master program, 11 Ph.D. program, and School of Continuing Education. Seven goals of FJU are human dignity, meaning of life, academic research, community awareness, dialogue with cultures, religious cooperation and spirit of service.
2.2. Food Science Department
4
3. RESEARCH PROJECT
3.1. Background of Research
Oxidative stress has been implicated in human diseases conditions, such as cardiovascular diseases, cancer, aging and neurodegenerative diseases (Bagchi et al., 2000). However, the immune systems in human body is not enough for severe oxidative stress. Hence, certain amounts of exogenous antioxidants and anti-inflammations are required to maintain an adequate immune system in human body.
Strawberry (Fragaria ananassa) is widely cultivated in USA, Spain, Japan, Poland, Korea and Russian Federation. Strawberry are not only available fresh, but also consumed and processed into jams, juices, ice cream, confectionary and other concentration product. The utilization of strawberry can be maximized when strawberry is processed into wine. Recent study has shown that wine has a wide range of phytochemical such as sugars, ethyl alcohol, tannins, aldehydes, esters, amino acids, minerals, vitamins, anthocyanin and other compounds, including phenolic compounds. The phenolic compounds found in strawberry wine are catechin, epicatechin, quercitin and ellagic acid. (Joshi et al., 2005; Sharma, 2000). Phenolic compounds contained in strawberry wine may give a great
protection due to oxidative stress in human body.
5
3.2. Literature Review
3.2.1. Strawberry (Fragaria ananassa) Wine
Strawberry is a fruit from genus Fragaria that has red colour with unique shape and flavour. Strawberry is a nutritious fruit because it has various nutrition and also rich in vitamin C which is important for natural antioxidant, iron and other minerals. The phenolic compounds of strawberry is located in major constituents of strawberry water. Strawberry flavour is characterized as fruity, sweet and tart, and the aroma is mainly determined by a complex eters, aldehydes, alcohols and sulfur compounds. Esters, like ethyl and methyl ester, are important for imparting the fruity and floral notes to strawberry. The composition of strawberry fruit in 100 gram can be seen in Table 1.
Table 1. Composition of Strawberry in 100 gram.
Constituents Average Range
Organic acids (mg/100 g) 658-2,162
Total phenols (mg/L) 58-210
Protein (%) 0.23
Total anthocyanin (mg/L) 55-145
Minerals (mg/100 g) 157.8
Source: Sharma, 2000; Spayd & Morris, 1981.
Strawberry fruit are a very delicious fruit and ussualy used for appetizer and dessert. Different utilization of strawberry are used to make jam, juices, puree, confectionary and concentration, and other preserves food, including strawberry wine. Alcoholic beverages
6
A method for strawberry wine making has also been described (Joshi et al., 2006). To make the wine, strawberry should be washed to remove dirts and soils, then crushed into pulps. A palatable strawberry wine made from dilution of pulp with 50% water is suggested available to reduce the acidity (Joshi et al., 2005). Thermovinification (heat treatment) is done to improve colour extraction from weakly colour of strawberry. This process can be done by heating the berries with 50% water at 60-65oC for 5-6 minutes and raising the total suspended solid (TSS) after crushing to improve the sensory quality of wine. The fermentation is done by using yeast (Saccharomyces cereviceae) for 4 days then pressed the fermented wine to dryness (Sims & Bates, 1994).
During fermentation, the physicochemical characteristic of strawberry wine is improved. It contains sugars, ethyl alcohol, higher alcohol, tannin, aldehydes, esters, amino acids, minerals, vitamins, anthocyanin, minor constituent like methanol and a number of flavouring compounds (Joshi et al., 2006; Sharma, 2000). Higher alcohol of strawberry wine are formed due to amino acid biosynthesis from carbohydrates complex or directly from existing amino acids by deamination and decarboxylation. Esters in wine are formed as a result of esterification of alcohols with respective acids (Amerine et al., 1980). While ageing/ maturation of strawberry wine, the phenolic compounds are formed as a result of maceration of anthocyanins. The phenolic compounds in strawberry wine are catechin,
epicatechin, quercitin and ellagic acid (Pilando et al., 1985). Phenolic compounds is important as antioxidant and usually used to reduce oxidative stress such as inflammation in living organisms. Pasteurized and depectinized of strawberry wine is suggested since pectin may cause a bad sensory and make the colour unstable. Depectinized can be done by using 100 ppm of liquid pectic enzyme to breakdown pectin.
3.2.2. Characteristics of Inflammation
7
the rapid movement of plasma and leukocytes from blood into injured tissues as an initial response of body to harmfull stimulus. Chronic inflammation is a prolonged inflammation, which mean the harmfull stimuli leads to a progressive shift in the type of cells present and can be characterized by simultaneous damage (Abbas & Lichtman, 2009).
Table 2. Characteristics of Acute and Chronic Inflammation.
Features Acute Inflammation Chronic Inflammation
Onset Fast: minutes or hours Slow: days
Cellular infiltrate Mainly neutrophilis Monocytes/ macrophages Tissue injury Usually mild and
self-limited
Often severe and progressive Local and systemic signs Prominent Less prominent Source: Cotran et al., 1998.
Inflammation can be defined by the sequential release of mediators such as pro-inflammatory cytokines, NO and prostaglandin E2 (PGE2). Nitric oxide synthase (NOS)
will produce NO by converting L-arginine to L-citruline, accompanied by NO production (Lin et al., 2003). High amount of NO produced by NOS indicates that the cells are in diseases and inflammation. NO may be generated in excess during the host response againts viral and bacterial infections, and also contribute to some pathogenesis by promoting oxidative stress, tissue injury and cancer (Hanada & Yoshimura, 2002; Maeda & Akaike, 1998).
3.2.3. Raw 264.7 Macrophage Cell
Raw 264.7 cell is a macrophage-like monoyte cells that can be found in mouse (Mus musculus). This cells have function as defense and immunity mechanism of the living
8
(Han et al., 2002). As a response of inflammation effects, this cells also will produce NO and the production of NO will endows macrophage cell with cytotoxic activity againts bacteria, viruses, fungi, protozoa and tumor cells. However, high concentration of NO in living host will lead to neuropathogical diseases, rheumatoid arthritis and other disorders. In other words, inflammation mechanism is just a response to a harmful thing but if the healing of wound part of cell take time too long, it will cause many diseases.
To date, raw 264.7 macrophage cell are usually used to do cell culture with growth conditions, protein expression and determination, RNAi, stable cell transfection and also gene transfection (He et al., 2011). The thaw and subculture techniques are very important things to do experiment using raw 264.7 macrophage cells. Pure raw cells should be stored in liquid nitrogen vapour phase to deactivate the cells and prevent the growth of cells. The thaw and subculture process should be done in laminar flow by using aseptic methods.
3.2.4. Lipopolysaccharide (LPS)
Lipopolysaccharide (LPS) is a large molecule consisting of lipid and polysaccharide. LPS can be found in the outer membrane of Gram-negative bacteria which outer and inner core joined by a covalent bond (Hirai et al., 2014). Richard Friedrich Johannes Pfeiffer is a scientist who discovered LPS in Escherichia coli and classified it as an endotoxin. LPS called endotoxin because the toxin is kept within the bacteria outer membrane and will only released to the environment when there is a destruction of bacteria membrane. LPS plays a major role in bacteria because it contributes greatly on cell structure formation, give protection from harmful chemical attack and also may stimulates strong response of immune system (Rietschel et al., 1994).
LPS is a complex molecule which composed of three major parts, the parts are O polysaccharide, core oligosaccharide and lipid A.
a.O polysaccharide (O antigen) comprises the outer domain of LPS molecule and
9
penetrable cell membranes to medicine like antibiotic because smooth LPS is less hydrophobic (Rittig et al., 2003; Raetz & Whitfield, 2002).
b.Core oligosaccharide attached to O polysaccharide and contains simple sugar molecule
like heptose and 3-deoxy-D-mannooctulosonic acid and also contain other components like amino acids, phosphate and ethanolamine (Hershberger & Binkley, 1968).
c.Lipid A is a phosphorylated glucosamine disaccharide attached with various fatty acids.
Since this part attach with lipid, then the characteristic is hydrophobic. Lipid A is responsible to the toxicity level of Gram-negative bacteria and the toxicity level is depend on how much lipid A is contained on bacteria. Once the bacteria cells is destroyed by immune system, lipid A will be released to the environment and cause diseases like diarrhea, fever and also fatal endotoxic shock (Tzeng et al., 2002).
The injection of LPS which contain lipid A in mammalian cells may cause uncontrolled immune systems and will stimulate to produce inflammatory mediators (Kilar et al., 2013). The mediators of inflammation system is Toll-like receptor 4, which is responsible to activate the immune cell system. Damage in endothelial layer of blood vessel caused by Toll-like receptor 4 can lead to capillary leak syndrome, dilation of blood vessels and decrease in cardiac functions. The high concentration of LPS injected to mammalian cells may cause further effects like disseminated intravascular coagulation (DIC) with loss of organs function such as kidneys, adrenal glands, lungs and heart (Stephens et al., 2007).
3.2.5. Trolox
Trolox (C14H18O4) is an analog of vitamin E. International Union or Pure and Applied
10
3.2.6. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) Assay
MTT assay is a test based on the conversion of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) into formazan crystals by living cell. Mitochondiral activity is used to determines the formazan crystal formation in living cells. The number of the cell populations are related to the number of cell viability (Van Meerloo et al., 2011). Cell viability assay developed for a 96-wells format that suitable for high throughput screening. Viable cells can convert MTT reagent into purple colored formazan product that can be detected by using 570 nm wavelengths (Marshall et al., 1995). A dead cell will lose the ability, thus unable to convert MTT into formazan. Formazan can be absorbed and precipitated by the cells surfaces and the color of the cells will change into dark purple. Formazan should be soluble when recording the absorbance using absorbance-reader equipment (Tada et al., (1986). There are various liquid to soluble the formazan such as dimethylformamide, SDS, acidified isoporopanol, dimethyl sulfoxide (DMSO) and also combination of detergent and organic solvent. DMSO is the one of the most common solvent that used to dissolve hydrophobic substances, such as formazan crystals, for in vivo and in vitro purposes (Bartsch, et al., 1976).
3.2.7. Nitric Oxide (NO) Production Test
Nitric Oxide (NO) plays a major roles in plants, bacteria and even in mammalian life (Moncada et al., 1991). Multiple physiological and pathophysiological function of NO are achieved by using various classes of nitric oxide synthase (NOS). The excess or lack of NO production in cells can cause nitrosative stress, leading damages of protein (DNA) and cell injury and death (Murphy, 1999). Wagner and coworkers established that the excess amount of NO in cells indicates that inflammation have occured in these cells (Wagner et al., 1984). This shown that NO is involved in many of human diseases and need to be regulated because it plays multiple role in living organisms (Lum et al., 2002).
11
12
4. RESEARCH METHODOLOGY
4.1. Time and Place of Practical Training
The practical training is conducted in Food Science Department, Fu Jen Catholic University, Xinzhuang District, New Taipei City, Taiwan (Figure 1.). This activity take place between January 12th to March 12th 2015.
4.2. Strawberry Wine Extraction
a.Materials
Materials and tools for the extraction was strawberry wine (11% of alcohol), aquadest, condensator, freezer, sonicator, centrifuge, centrifuge tube, freeze dryer and electric balance.
b.Methods
Strawberry wine with 11% of alcohol was condensed at 40±3oC. Put sample into tube and
13
4.3. Raw 264.7 Macrophage Cell Preparation
a.Materials
Materials and tools for this preparation was raw cell 264.7 (bought from Food Industry Research and Development Institute, Bioresource Collection and Research Center, Hsinchu, Taiwan), PBS, TryplE, DMEM medium with 5% of FBS, alcohol, laminar flow, micropipette, tips, volume pipette, auto pipette, dish, centrifuge, tissue paper, high pressure suction unit, incubator and falcon tube.
b.Methods
Laminar air flow was wiped with 75% of ethanol and UV light was turned on for 15 minutes before use. Medium was warmed using waterbath until the temperature reached 37 oC. B16 cells were taken out from the liquid nitrogen tank and warmed using waterbath
until the temperature reached 37 oC. Cells were added by 5.5 ml medium and centrifuged
at 1000 rpm for 3 minutes. Dish was prepared and added by 7 ml medium. After centrifuged, medium was removed using high pressure and the cells were diluted using 1 ml medium. The cells were added to dish plate and incubated at 37 oC for 24 hours. The
medium was removed after incubated and washed using 2 ml phosphate buffer saline (PBS). PBS was removed and then 7 ml medium were added to the dish plate. The cells were incubated at 37oC for 24 hours (Hirai et al., 2014).
4.4.Raw 264.7 Macrophage Cell Counting and Seeding
a.Materials
14
b.Methods
Cells were taken out from incubator and removed the medium. Cells were washed using 2 ml PBS and removed the PBS. 1.5 ml TryplE were added to the dish and removed it. The cells were incubated at 37oC for 3 minutes. After 3 minutes , the dish was knocked and then the cells were collected using 7 ml medium. The solution was put into falcon tube and then centrifuged at 1,000 rpm for 3 minutes. The supernatant (medium) was removed and the cells were collected using 10 ml medium. Three micro tubes were prepared and 900 μl medium were added to second micro tube. One ml of cells were taken and put into first micro tube. First micro tube 100 μl were added to the second micro tube (10 fold dilution). Second micro tube 20 μl was taken and put into the third micro tube. Twenty μl of trypan blue stain (0.4%) were added to third micro tube (2 fold dilution). Sample 20 μl was injected to cell counting plate using micro pipette. The cell was counted in 5 squares of two sides of cell counting plate using microscope. The number of cells and preparation proportion was calculated. The medium and cells were mixed using low speed vortex.
𝑦
𝑎 × 𝑏 × 𝑐 × 𝑑 ÷ 𝑒 × 𝑓 = 𝑥 × 𝑔
Preparation proportion = 1 ml medium with cells ∶ x − 1 ml medium without cells
Known:
y = cell number d = 2 fold dilution g = cell seeding number a = two side of counting plate e = 10μl of sample
b = number of square f = Coefficient c = 10 fold dilution x = Total medium (ml)
The 96 well-plate and 8-channel pipette were prepared. The solution of cells were put
15
4.5.Sample Preparation and Injection
a.Materials
Materials and tools for this preparation was strawberry wine extract, LPS, trolox, DMEM medium with 5% of FBS, tip, micropipette, alcohol, laminar flow, 96-well plates, micropipette, tips, volume pipette, auto pipette, dish, tissue paper, high pressure suction unit, incubator, vortex and falcon tube.
b.Methods
b.1. Control, LPS and T100 Sample Preparation
Control was made by using 200 μl medium. LPS was made by mixing 1 ml medium with 1μl LPS. T100was made by mixing 100 μl of trolox with 900 μl of medium, then mix 100 μl of solution with 900 μl medium.
b.2. Strawberry Wine Extract Sample Preparation
There are 5 variable of strawberry wine extract sample used in this experiment; 25, 50,
75, 100 and 250 μg/ml. Ten μl of strawberry extract was added to 990 μl of medium
(hundred-fold dilution). A 100 μl amount of hundred-fold dilution was added to 900 μl medium (become thousand-fold dilution). Concentration at 25, 50, 75, 100 and 250 μg/ml was made by mixing 25, 50, 75, 100 and 250 μl of thousand fold dilution with 975, 950, 925, 900 and 750 μl medium. One μl of LPS was added to T100, concentration 25, 50, 75, 100 and 250 μg/ml. All variable sample was homogenized using vortex.
b.3. Sample Injection
16
added to the 96-well plates carefully and then incubated at 37oC for 24 hours (Hirai et al., 2014).
4.6.MTT Assay
a.Materials
Materials and methods for this test was MTT reagent, DMEM medium with 0% FBS, tip, micropipette, alcohol, laminar flow, 96-well plates, micropipette, tips, volume pipette, auto pipette, dish, tissue paper, high pressure suction unit, incubator, vortex and falcon tube.
b.Methods
b.1. MTT Reagent Preparation
A 2.5 ml amount of MTT reagent were mixed using 22.5 ml of DMEM medium without FBS and vortex.
b.2. MTT Test
The medium in 96-well plates was removed using high pressure suction unit. The solution
17
4.7.Nitric Oxide Production Test
a.Materials
Materials and methods for this test was Griess reagent, sulfanilamide, phosphoric acid, aquadest, naphthylethylenediamine dihydrochloride, NaNO2, DMEM medium without
FBS, tip, micropipette, alcohol, laminar flow, 96-well plates, micropipette, tips, volume pipette, auto pipette, dish, tissue paper, high pressure suction unit, incubator, vortex and falcon tube.
b.Methods
b.1. Griess Reagent Preparation
Griess reagent was made by mixing 3 ml of 0.1% naphthylethylenediamine
dihydrochloride with 3 ml of 1% sulfanilamide with 5% of phosphoric acid.
b.2. NO Standard Curve Preparation
Sample for standard curve of NO test was made by mixing 69 gram of NaNO2 with 1 ml
aquadest, then 10 μl of the solution was added to 10 ml aquadest (1,000 μM). There were 8 different concentration of NaNO2; 0, 10, 20, 30, 40, 50, 60 and 80 μM. Concentration at 0 μM was made by using 1,000 μl of aquadest. Concentration at 10, 20, 30, 40, 50, 60 and 80 μM was made by mixing 1,000 μM NaNO2 with 990, 980, 970, 960, 950, 940 and 920 μl aquadest. Standard curve of NO test was made by mixing 100μl of any different
concentration of NaNO2 with 100 μl Griess reagent in 3 repeats. The NO value was
analyzed using ELISA-reader at 550 nm (Hirai et al., 2014; Kim et al., 2014).
b.3. NO Production Test
18
into another 96-well plates. A 100 μl amount of Griess reagent was added to 96-well plates and then the NO value of strawberry was analyzed using ELISA-reader at 550 nm (Hirai et al., 2014; Kim et al., 2014).
4.8.Statistical Analysis
19 5. RESULT AND DISCUSSION
Anti-inflammatory effect of strawberry wine extract on LPS-induced RAW 264.7 macrophage cells were analyzed. Strawberry wine extract was made by using water extraction method. LPS was injected to RAW 264.7 macrophage cells to stimulate the inflammation condition of the cells. To know the anti-inflammatory effect of strawberry wine extract, different concentrations of strawberry wine extract was injected to the cells and trolox was used as the positive control. Anti-inflammatory effect was measured by NO production test using ELISA reader. Cell viability was measured using MTT survival test. The effect of strawberry wine extract to morphology cells were analyzed using electronic microscope connected to personal computer (PC).
5.1.Cell Viability Test
20
Cell viability test is used to know wether the sample is suitable for the cells or not. To analyze the cell viability, MTT assay is used as a method using ELISA reader. Viable cells in the bottom of the plate have the skill to absorb and convert MTT reagent into formazan crystals which have purple color, but death cells are not. Formazan is dissolved in DMSO solvent and change the color of solution into purple that can be detected by using 550 nm wavelength of ELISA reader. Amount number of viable cell will determine the color of solution and also the absorbance of ELISA reader (Marshall et al., 1995; Tada et al., 1986; Bartsch et al., 1976).
Based on Figure 1. we can see that cell viability of LPS-stimulated RAW 264.7 cells were depend on extract concentration. LPS, T100, 25, 50 and 75 μg/mL were not significantly different with control. This means the addition of LPS and T100 were not hurt the cells, thus the number of the cells in wells could be mantained. The same thing happened in the concentration of 25, 50 and 75 μg/mL because it proved that the cell viability at these concentrations are not significantly different from the control. This means that these concentrations does not lead to cell death. However, cell viability at 100 and 250 μg/mL of extract were decreased signicantly. This was happen because the high concentration of extract could kill the cells and resulted in low cell viability. Strawberry wine extract contains alcohol. The higher concentration of sample was added, the higher alcohol will
be. Alcohol is a damaging agent for all cells, from the liver to nerveous system (Blasiak et al., 2000; Luo & Miller, 1997). High concentration of alcohol, especially ethanol, may
lead to necrotic cell death, mediated by a high production of reactive oxygen species (ROS) (Peri et al., 2005).
5.2.Cell Morphology
21
Figure 2. Raw 264.7 Macrophage Cell Morphology. Normal cells as control (C). LPS at 100 ng/mL (LPS). Trolox at 100 μg/mL (T100). Strawberry wine extract at 25-250 μg/mL (25, 50, 75, 100, 25-250)
The control cells had round shape with low degree of spreading (Figure 2.C.). Cell with 100 ng/mL of LPS significantly changed, the shape was not round anymore (Figure 3.T100.). LPS induced cell spreading and also pseudopodia formation (Figure 2.LPS.). This means that LPS may cause the cells into inflammation state and NO will be produced as a response (Harmey et al., 2002). When the strawberry wine water extract was added with various concentration into LPS-induced RAW 264.7 cells, it can prevent the cell from spreading and also the pseudopodia formation.
5.3.NO Production Test
In order to assess the anti-inflammatory activities of strawberry wine water extract, RAW 264.7 cells were stimulated with LPS in the presence or absence of the extract and the NO production contained in medium was measured. The inhibition of NO production was observed in cells with addition of the strawberry wine water extract (25-250 μg/mL).
C LPS T100 25
22
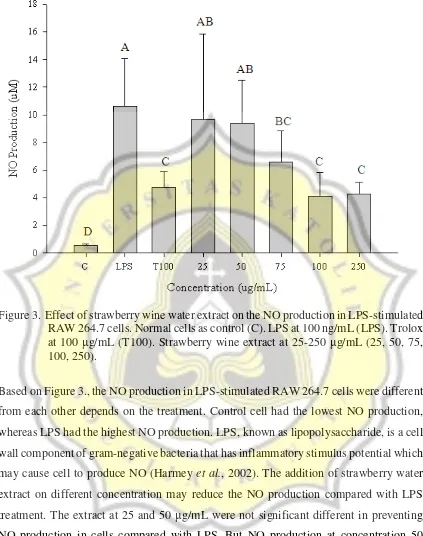
Figure 3. Effect of strawberry wine water extract on the NO production in LPS-stimulated RAW 264.7 cells. Normal cells as control (C). LPS at 100 ng/mL (LPS). Trolox at 100 μg/mL (T100). Strawberry wine extract at 25-250 μg/mL (25, 50, 75, 100, 250).
Based on Figure 3., the NO production in LPS-stimulated RAW 264.7 cells were different from each other depends on the treatment. Control cell had the lowest NO production,
whereas LPS had the highest NO production. LPS, known as lipopolysaccharide, is a cell wall component of gram-negative bacteria that has inflammatory stimulus potential which
may cause cell to produce NO (Harmey et al., 2002). The addition of strawberry water extract on different concentration may reduce the NO production compared with LPS treatment. The extract at 25 and 50 μg/mL were not significant different in preventing NO production in cells compared with LPS. But NO production at concentration 50
23
T100 is positive control and contains trolox which is a water soluble substances and analog of vitamin E used to reduce oxidative stress or damage in cells (Moulin et al., 1998). T100 should has the lowest NO production among the other LPS-induced cells because it may inhibit the production of NO in LPS-induced cells. NO production in cells with 25 and 50 μg/mL of extract were significant different compared to T100, however, at concentration 75-250 μg/mL were not. NO production at concentration 100 and 250 µg/mL of strawberry wine water extract were lower than T100 respectively. It means the inhibition level of NO production in cell with 100 and 250 µg/mL was higher than in T100. This is because the number of the cell in each well is not the same. Some of the cells in concentration 100 and 250 µg/mL cannot survive and may influence the NO production level.
NO production at concentration 25, 50 and 75 µg/mL decreases with the increasing of strawberry wine water extract concentration. The extract may reduce the level of NO production because strawberry contains so many phenolic compounds, such as anthocyanins, ellagic acid, ellagic acid glycosides and ellagitannins (Clifford, 2004). Those phenolic compounds can inhibit NO production, thus strawberry wine may reduce inflammation proccess in cells. The extract at 75 µg/mL showed significantly higher
24
6. CONCLUSION AND SUGGESTION
6.1.Conclusion
The optimum concentration of strawberry wine that the cells still can survive and also can reduce oxidative stress in LPS-induced Raw 264.7 macrophage cell is 75 µg/mL.
6.2.Suggestion
25
7. REFRENCES
Abbas, A.B. and Lichtman A.H. (2009). “Ch. 2 Innate Immunity”. In Saunders (Elsevier). Basic Immunology. Functions and Disorders of the Immune System 3rd Ed.
Amerine, M.A., Kunkee K.E., Ough C.S., Singleton V.L. and Webb A.D. (1980). Tehcnology of Wine Making. AVI Publ. Co. Inc. Westport. C.T. p.794
Bagchi, D., Bagchi, M., Stohs, S. J., Das, D. K., Ray, S. D., Kuszynski, C. A. (2000). Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology Vol. 148, 187–197.
Berg, Robin V.D., Guido R.M.M.H., Henk V.D.B and Aalt. B. (1999). Applicability of an Improved Trolox Equivalent Antioxidant Capacity (TEAC) Assay for Evaluation of Antioxidant Capaity Measurements of Mixtures. Food chemistry Vol. 66: 511-517.
Blasiak, J., Trzeciak A., Malecka-Panas E., Drzewoski J., and Wojewodzka M. (2000). In Vitro Genotoxicity and The Gastrointestinal Tract Mucosa Cells. Toxicology in
Vitro. Vol. 14: 287-295.
Clifford, M.N. (2004). Diet-derived Phenols in Plasma and Tissues and Their Implicatios for Health. Planta Med. Vol. 12: 1103-1114.
Cotran, Kumar and Collins. (1998). Robbins Pathologic Basis of Disease. Philadelphia. W.B. Saunders Company.
Fuhrman, B., Nina V., Amram S., and Michael A. (2001). White Wine with Red Wine-like Properties: Increased Extraction of Grape Skin Polyphenols Improves the Antioxidant Capacity of the Derived White Wine. J. Agric. Food. Chem. Vol. 49: 3164-3168.
Freshney, R. I. (2006). Basic Principles of Cell Culture. Centre for Oncology and Applied Pharmacology, Cancer Research UK Beatson Laboratories, Garscube Estate, Bearsden, Glasgow G61 IBD, Scotland, UK.
Green, L. C.; Wagner, D. A.; Glogowski, J.; Skipper, P. L.; Wishnok, J. S.; Tannenbaum, S. R. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological samples.
Anal. Biochem. Vol. 126: 131- 138
26
Hirai, S., Sho H., Yoshiaki M., Shin O., Yuki S., Kenji S. and Yukari E. (2014). Anti-inflammatory Effect of Pyroglutamyl-Leucine on Lipopolysaccharide-stimulated RAW 264.7 Macrophages. Life Science Vol. 117: 1-6.
Hsueh, R. and Tamara Roach. (2008). Passage Procedure for RAW 264.7 Cells. AfCS Procedure Protocol.
Joshi, V.K. Sharma S. and Kumar K. (2006). Technology for Production and Evaluation of Strawberry Wine. Bev. Food World. Vol. 33 (1): 77-78.
Kilar, A., Dornyei A. and Kocsis B. (2013). Structural Characetrization of Bacterial Lipopolysaccharides with Mass Spectrometry and On- and Off- Line Separation Techniques. Mass Spectrom. Rev. Vol. 32 (2): 90-117.
Kim, J.H., Yon-Suk K., Jin-Woo H., Young-Ki H., Jung-Suck L., Se-Kwon K., You-Jin J., Sang-Ho M., Byong-Tae J., Young Y.B. and Pyo-Jam P. (2014). Sulfated Chitosan Oligosaccharides Suppress LPS-Induced NO Production via JNK and NF-KB Inactivation. Molecule Vol. 19: 18232-18247.
Kleschyov, A. L.; Munzel, T. (2002). Advanced spin trapping of vascular nitric oxide using colloid iron diethyldithiocarbamate. Methods Enzymol. Vol. 359: 42-51.
Kojima, H.; Nakatsubo. N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirata, Y.; Nagano, T. (1998). Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. Vol. 70: 2446-2453.
Lin, S.; Wei X.; Xu Y.; Yan C.; Dodel R.; Zhang Y.; Liu J.; Klaunig J.E.; Farlow M. and Du Y. (2003). Minocycline Blocks 6-hydroxydopamine-induced Neurotoxicity and Free Radical Production in Rat Cerebellar Granule Neurons. Life Science Vol. 72: 1635-1641.
Lum, H. K.; Butt, Y. K.; Lo, S. C. (2002). Hydrogen peroxide induces a rapid production of nitric oxide in mung bean (Phaseolus aureus). Nitric Oxide Vol. 6: 205-213.
Luo, J. and Miller M.W. (1997). Basic Fibroblast Growth Factor- and Platelet-derived Growth Factor-Mediated Cell Proliferation in B104 Neuroblastoma Cells: Effect of Ethanol on Cell Cycle Kinetics. Brain Res Vol. 770 (1-2): 139-150.
Moncada, S.; Palmer, R. J. M.; Higgs, E. A. (1991). Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. Vol. 43: 373-376.
27
Murphy, M. P. (1999). Nitric oxide and cell death. Biochimica et Biophysica Acta Vol. 1411: 401-414.
Peri, L., Pietraforte D., Scorza G., Napolitano A., Fogliano V., and Minetti M. (2005). Apples Increase Nitric Oxide Production by Human Saliva at the Acidic pH of the Stomach: A New Biological Function for Polyphenols with a Catechol Group. Free
Rad Biol Med Vol. 39: 668-681.
Pilando, L.S., Wrolstad R.E. and Heatherbell D.A. (1985). Influence of Fruit Composition, Maturity and Mold Contamination on the Colour and Appearance of Strawberry Wine. J. Food Sci. Vol. 50: 1121-1125.
Raetz, C.R. and Whitfield C. (2002). Lipopolysaccharide Endotoxins. Annu. Rev.
Biochem. Vol. 71: 635-700.
Rana, R.S., Vyas K.K. and Joshi V.K. (1986). Studies on Production and Acceptability of Cider from Himachal Apples. Indian Food Packer. Vol. 90 (6): 56-61.
Rietschel, E.T., Kirikae T., Schade F.U. Mamat U., Schmidt G. And Loppnow H. (1994). Bacterial Endotoxin: Molecular Relationships of structure to Activity and Function.
FASEB J. Vol. 8 (2): 217-25.
Rittig, M.G., Kaufmann A., Robins A., Shaw B., Sprenger H., and Gemsa D. (2003). Smooth and Rough Lipopolysaccharide Phenotypes of Brucella Induce Different Intracellular Trafficking and Cytokine/ Chemokine Release in Human Monocytes.
J. Leukoc. Biol. Vol. 74 (6): 1045-1055.
Sharma, S. (2000). Preparation and Evaluation of Strawberry Wine. PhD Thesis. Postharvest Technology. Nauni. Solan.
Spayd, S.E. and Morris J.R. (1981). Influence of Immature Fruits on Strawberry Jam Quality and Storage Stability. J. Food Sci. Vol. 46: 406-414.
Stephens, D.S., Greenwood B. and Brandtzaeg P. (2007). Epidemic Meningitis, Meningococcaemia and Neisseria meningitidis. Lancet Vol. 369 (9580): 2196-2210.
28
Tzeng, Y.L., Datta A., Kolli V.K., Carlson R.W. and Stephens D.S. (2002). Endotoxin of
Neisseria meningitis Composed Only Intact Lipid A: Inactivation of the
Meningococcal 3-Deoxy-D-Mannooctulosonic Acid Transferase. J. Bacteriol. Vol. 184 (9): 2379-2388.
29
8. APPENDICES
Appendices 1. Research Schedule
December January February March April Note
4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 Week(s) Searching references. Practice cell sub culture,
strawberry wine concentration. Experiment execution.