Half-life of ubiquinone and plastoquinone in spinach cells
Malgorzata Wanke
a,*, Ewa Swiezewska
a, Gustav Dallner
baInstitute of Biochemistry and Biophysics,Polish Academy of Sciences,Pawinskiego5A,02-106Warsaw,Poland bDepartment of Biochemistry,Arrhenius Laboratories for Natural Sciences,Stockholm Uni6ersity,S-106 91Stockholm,Sweden
Received 17 August 1999; received in revised form 28 December 1999; accepted 6 January 2000
Abstract
The half-life of plastoquinone (PQ), ubiquinone-9 (UQ-9) and ubiquinone-10 (UQ-10) in spinach tissue was determined. This was achieved by monitoring the decay of radioactivity incorporated into these lipids from a labeled precursor. The half-life of PQ was 15 h while for UQ-9 and UQ-10 it was longer, i.e. 30 h. The values of half-lives of PQ and UQ suggest a high rate of turnover of these lipids in spinach cells. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Spinacia oleracea; Spinach; Plastoquinone; Ubiquinone; Isoprenoid turnover
www.elsevier.com/locate/plantsci
1. Introduction
The process of isoprenoid biosynthesis in plants provides considerably more products, than in ani-mal tissues [1] including a broad group of iso-prenoid lipids. These lipids are functionally important for many aspects of cell metabolism and also influence membrane structure. In the biosyn-thesis of these compounds in higher plants two pathways were found to be involved: a cytosolic acetate-mevalonate pathway and an alternative Rohmer’s pathway proven to operate for some isoprenoids, e.g. carotenoids, phytol and plas-toquinone (PQ) [2]. The multiple site of localiza-tion of isoprenoid biosynthesis is found both in plant (e.g. isopentenyl diphosphate and farnesyl diphosphate) [3] and animal cells (e.g. farnesyl diphosphate, ubiquinone (UQ) and dolichol [4]. PQ and UQ are isoprenoid lipids and their major portion in plant cells is found in chloro-plasts and mitochondria, respectively. However, relatively high content of both lipids was also
found in the Golgi fraction from spinach cells [5]. This implies that Golgi vesicles could be involved in PQ and UQ biosynthesis and/or transport from the endoplasmic reticulum, the putative site of biosynthesis, to the site of biological deposition. Recent results employing in vivo labeling confirm these observations [6]. However, numerous litera-ture data considered mitochondria as the site of UQ biosynthesis [7] and the presence of a putative mitochondrial transit sequence in the cDNA en-coding methyltranferase involved in biosynthesis of UQ inArabidopsis thaliana [8] suggested that at least this step of UQ synthesis takes place in mitochondria. Similarly PQ biosynthesis was lo-calized to the chloroplast inner envelope mem-brane [9]. Earlier studies on the intracellular flow of isoprenoid intermediates showed that isopen-tenyl diphosphate could be transported into chloroplast and mitochondria [10,11] further serv-ing as a substrate for quinone biosynthesis. The in vivo contribution of this process in PQ and UQ biosynthesis remains to be established.
The level of UQ and PQ in plant cells may be altered under various physiological conditions and developmental states [12] and variation of UQ content in mammalian cells was observed in con-nection with different physiological and
patho-Abbre6iations: ER, endoplasmic reticulum; HPLC, high perfor-mance liquid chromatography; PQ, plastoquinone;t1/2, half-life; UQ,
ubiquinone.
* Corresponding author. Fax: +48-39-121-623.
E-mail address:[email protected] (M. Wanke)
physiological states of the cell as a result of func-tional accommodation [13]. While the major estab-lished functions for both lipids are as electron and proton carriers, i.e. UQ as a redox intermediate of the mitochondrial respiratory chain and PQ as a redox component in photosynthesis, additional functions are also known. Recent studies have demonstrated that PQ is also involved in the chlororespiratory pathway [14]. It was also shown that the reduced forms of UQ (Dr A.M. Wagner, Vrije University, Amsterdam, personal communi-cation) and PQ [15] play an important role as an antioxidant in the cells. The various cellular func-tions in which these compounds are participating may result in complex mechanisms of lipid biosyn-thesis, redistribution and mechanisms of their breakdown. In vivo studies performed by the au-thors [6] have shown that the rate of biosynthesis and the translocation processes for PQ and UQ are different, but the rate of turnover of these lipids in the plant cells has not been studied so far. The aim of the present study was to investigate the half-life of UQ and PQ in order to analyze possible differences in turnover between UQ and PQ.
This was achieved by monitoring the decay of radioactivity incorporated into these lipids from a labeled precursor, [3H]mevalonate.
2. Materials and methods
Spinach seedlings (Spinacia oleracea cv Meda-nia) were grown for 11 – 14 days in darkness in a growth chamber at 22 – 25°C in vermiculite.
(R,S) [5-3H]mevalonate (specific activity 3.52 Ci/
mmol) was prepared according to Keller [16]. The radiochemical purity of synthesized
[5-3H]mevalonate was 95% (TLC). All other
chemi-cals were purchased from Sigma (St. Louis, MO). PQ standard was prepared from spinach leaves (Collection of Polyprenols, IBB, Warsaw). HPLC solvents were obtained from Baker.
The seedlings (0.5 g fresh weight) without roots were placed in small containers with 0.4 ml of growth medium (5 mM KNO3, 1.5 mM Ca(NO3)2,
1 mM MgSO4, 1 mM KH2PO4, 1 mM NH4Cl, 156 mM EDTA, 72mM FeSO4, 46 mM H3BO3, 6.2mM
MnSO4, 0.8 mM ZnSO4, 0.3 mM CuSO4, 0.7 mM
MoO3, 0.2 mM NH4VO3) [17] supplemented with
0.5 mCi [3H]mevalonate. Labeling was performed
for 24 h at room temperature under continuous laboratory illumination. Afterwards, greening plants were rinsed and placed in a medium devoid of [3H]mevalonate. The incubation was continued
for 61 h under the same temperature and light conditions. Greening of etiolated seedlings ini-tiated with illumination, resulted in totally green plants at the end of experiment. Seedlings were homogenized using a mortar and pestle in 0.25 M sucrose. The homogenates were supplemented with 5 mg ubiquinone-6 (Sigma) as an internal
standard. Lipids were extracted according to Bligh and Dyer [18]. Lower phase was evaporated, dis-solved in hexane and loaded onto a Silica gel column. Single-step elution of lipids with 20% solution of diethyl ether in hexane resulted in separation of fraction containing neutral lipids, after this procedure all the quinones were found in oxidized form. Lipids were protected against light during the whole analytical procedure.
Analysis of lipids was performed by reversed-phase HPLC using a Hewlett-Packard Hypersil ODS 3-mm column. A linear gradient was
em-ployed from the initial methanol:water (9:1) in pump system A to methanol:2-propanol (8:2) in pump system B at flow rate 1.5 ml/min and with a program time of 45 min. The absorbance at 210 nm and radioactivity of the eluate were monitored using a UV-detector and a radioactivity flow de-tector (Radiomatic Instruments, Flow-one Beta), respectively. Radioactive peaks eluted at 12 – 13 min — sterol fraction, 39 min – UQ-9, 41 min – PQ and 43 min – UQ-10 were identified by cochro-matography with unlabeled standards. Chemical reduction of radioactive products supplemented with unlabeled quinones used as internal standards (performed with sodium borohydride), followed by HPLC, further confirmed the identity of PQ and UQs. Incorporation of radioactivity from [3H]mevalonate into isoprenoid lipids was 2.5% of
the radioactivity present in the medium. Ratio of labeled [3H]UQ-10 versus [3H]sterol fraction was
in average 1:10 (and 1:100 for [3H]PQ/[3H]sterol)
in the experimental conditions.
3. Results and discussion
Incorporation of [3H]mevalonate into PQ and
the radioactive precursor. The extensive [3H]mevalonate incorporation into UQ suggests
the presence of an efficient biosynthetic process. In contrast, the rate of PQ labeling was 4 times lower. In tobacco cell culture devoid of functional chloroplasts PQ is biosynthesized via a non-meval-onate pathway [19] and it is likely that this path-way could also be operative in our in vivo system.
Parallel activity of both pathways may explain the lower rate of PQ labeling in comparison with the UQ. Higher content PQ than UQ could also be the reason of this phenomenon. The content of native quinones in the tissue was stable during the experiment and the concentration of PQ was ap-proximately 20mg/g of fresh weight, UQ-9
concen-tration was approximately 3.5 mg/g and UQ-10
was 12 mg/g fresh weight.
Half-life of both UQ-9 and UQ-10 as well as that of PQ were investigated. There is no data available on the breakdown of these quinones in plant cells, whereas the rate of biosynthesis and breakdown of UQ in animal cells has been investi-gated. The half-life of ubiquinone in rat tissues showed limited variations among various organs. In heart, muscle, colon and spleen the t1/2 were
between 50 and 60 h, whereas in stomach, liver and brain they were between 70 and 90 h. In the case of thymus and kidneyt1/2were longer, i.e. 104
and 124 h, respectively [20,21]. The half-lives of phospholipids in rat tissue were around 100 h, of cholesterol 150 h and of dolichol 65 – 140 h [22,23]. It appears therefore that half-life of ubiquinone exhibited similar or lower values than that of other lipids.
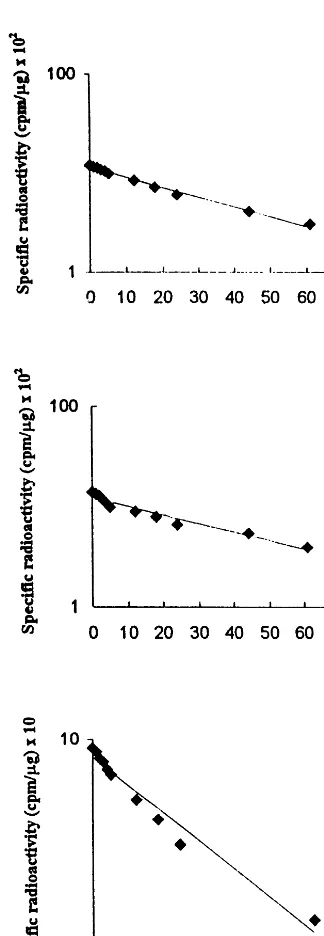
Half-lives of PQ and UQ were monitored by following the decay of specific radioactivity. At initial time-point only traces of free [3H]mevalonate could be detected in the cytosolic
fraction (determined as the amount of radioactiv-ity, data not shown). In this way, the absence of the labeled precursor in the substrate pool ensured that continued incorporation did not occur and the turnover studies were not influenced. Using a semilogarithmic plot the decay of specific radioac-tivity was found to be approximately linear for 60 h. Slightly faster rate of decay observed especially for UQ-9 and UQ-10 during the first few hours might perhaps reflect the differences in the degra-dation processes of these lipids found in various chemical surrounding (membrane bound, protein associated, etc.) at different subcellular compart-ments. More studies are required to support this conclusion.
Half-life of UQ-9 and UQ-10 in spinach seedlings, calculated from the data in Fig. 1A,B were 30 and 32 h, respectively. In the case of PQ thet1/2was considerably shorter — 15 h (Fig. 1C).
The data available concerning half-lives of lipids in plant cells are limited and display different
Fig. 1. Decay of specific radioactivity in ubiquinone (UQ-9) (A), UQ-10 (B) and plastoquinone (PQ) (C). Lipids were isolated from spinach homogenate at various time-points after [3H]mevalonate labeling, and their level of radioactivity
deter-mined. The values are given in cpm/mg of the lipid under
values. In the case of conversion of phosphatidate towards galactosyldiacylglycerol the half-life was found to vary between 2 and 40 min depending on the plant species [24]. Half-life of headgroups of phosphatidylinositols inSpirodela polyrhizaL. was approximately 2 – 5 h [25].
The relatively short half-life time of PQ and UQ indicates the existence of efficient breakdown pro-cesses, which possibly take place at the site of major biological activity of both lipids, i.e. in chloroplasts and mitochondria. The mechanism of breakdown and the enzymes participating in the catabolic process have not yet been investigated in detail in plants or animal tissues. The recently described geranyl-CoA-carboxylase could possibly play a role in acyclic isoprenoid catabolism in plants [26]. Besides their functions as mobile elec-tron and proton carriers in biological membranes PQ and UQ are known to act, similarly to a
-toco-pherol, as efficient endogenous antioxidants. This dual role could explain the high requirements for these lipids in the plant cells.
Acknowledgements
This work was supported by the Polish State Committee for Scientific Research project 6PO4A 046 12 and the Swedish Council for Agricultural and Forestry Research.
References
[1] J.C. Gray, Control of isoprenoid biosynthesis in higher plants, Adv. Bot. Res. 14 (1987) 25 – 91.
[2] H.K. Lichtenthaler, The plants’ 1-deoxy-D-xylulose-5-phosphate pathway for biosynthesis of isoprenoids, Fett/
Lipid. 100 (1998) 128 – 138.
[3] D. McGarvey, R. Croteau, Terpenoid metabolism, Plant Cell 7 (1995) 1015 – 1026.
[4] J. Grunler, J. Ericsson, G. Dallner, Branch-point reac-tions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins, Biochim. Biophys. Acta 1212 (1994) 259 – 277.
[5] E. Swiezewska, G. Dallner, B. Andersson, L. Ernster, Biosynthesis of ubiquinone and plastoquinone in the endoplasmic reticulum-Golgi membranes of spinach leaves, J. Biol. Chem. 268 (1993) 1494 – 1499.
[6] M. Wanke, G. Dallner, E. Swiezewska, Subcellular local-ization of plastoquinone and ubiquinone synthesis in spinach cells. Biochim. Biophys. Acta (2000) 188 – 194. [7] F. Lutke-Brinkhaus, B. Liedvogel, H. Kleining, On the
biosynthesis of ubiquinones in plant mitochondria, Eur. J. Biochem. 141 (1984) 537 – 541.
[8] M.-H. Avelange-Macherel, J. Joyard, Cloning and func-tional expression of AtCOQ3, the Arabidopsis homo-logue of the yeast COQ3 gene, encoding a methyltransferase from plant mitochondria involved in ubiquinone biosynthesis, Plant J. 14 (1998) 203 – 213. [9] J. Soll, G. Schultz, J. Joyard, R. Douce, M. Block,
Localization and synthesis of prenylquinones in isolated outer and inner envelope membranes from spinach chloroplasts, Arch. Biochem. Biophys. 238 (1985) 290 – 299.
[10] D. McCaskill, R. Croteau, Isoprenoid synthesis in pep-permint (Mentha x piperita): development of a model system for measuring flux of intermediates through the mevalonic acid pathway in plants, Biochem. Soc. Trans. 23 (1995) 290S.
[11] J. Chappell, The biochemistry and molecular biology of isoprenoid metabolism, Plant Physiol. 107 (1995) 1 – 6. [12] H. Lichtenthaler, Light stimulated synthesis of plastid
quinones and pigments in etiolated barley seedlings, Biochim. Biophys. Acta 184 (1969) 164 – 172.
[13] L. Ernster, G. Dallner, Biochemical, physiological and medical aspects of ubiquinone function, Biochim. Bio-phys. Acta 1271 (1995) 195 – 204.
[14] T.S. Feild, L. Nedbal, D.R. Ort, Nonphotochemical reduction of plastoquinone pool in sunflower leaves orig-inates from chlororespiration, Plant Physiol. 116 (1998) 1209 – 1218.
[15] T. Hundall, P. Forsmark-Andre`e, L. Ernster, B. An-dersson, Antioxidant activity of reduced plastoquinone in chloroplast thylakoid membranes, Arch. Biochem. Biophys. 324 (1995) 117 – 122.
[16] R.K. Keller, The mechanism and regulation of dolichyl phosphate biosynthesis in rat liver, J. Biol. Chem. 261 (1986) 12053 – 12059.
[17] C.A. Shipton, I. Parmryd, E. Swiezewska, B. Andersson, G. Dallner, Isoprenylation of plant proteins in vivo, J. Biol. Chem. 270 (1995) 566 – 572.
[18] E.G. Bligh, W.J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol. 37 (1959) 911 – 913.
[19] A. Disch, A. Hemmerlin, T.J. Bach, M. Rohmer, Meval-onate-derived isopentenyl diphosphate is the biosynthetic precursor of ubiquinone prenyl side chain in tobacco BY-2 cells, Biochem. J. 331 (1998) 615 – 621.
[20] A. Thelin, S. Schedin, G. Dallner, Half-life of ubiquinone-9 in rat tissues, FEBS Lett. 313 (1992) 118 – 120.
[21] M. Andersson, P.G. Elmberger, C. Edlund, K. Kris-tensson, G. Dallner, Rates of cholesterol, ubiquinone, dolichol and dolichyl-P biosynthesis in rat brain slices, FEBS Lett. 269 (1990) 15 – 18.
[22] C. Edlund, U. Brunk, T. Chojnacki, G. Dallner, The half-lives of dolichol and dolichyl phosphate in rat liver, Biosci. Rep. 8 (1988) 139 – 146.
[23] L.C. Eriksson, G. Dallner, Influence of phenobarbital treatment on the turnover of rat liver microsomal lipids, FEBS Lett. 29 (1973) 351 – 354.
[25] C.A. Brearley, D.E. Hanke, Evidence for substrate-cy-cling of 3-, 3,4-, 4-, and 4,5-phosphorylated phos-phatidylinositols in plants, Biochem. J. 311 (1995) 1001 – 1007.
[26] X. Guan, T. Diez, T.K. Prasad, B.J. Nikolau, E.S. Wurtele, Geranoyl-CoA carboxylase: a novel biotin-con-taining enzyme in plants, Arch. Biochem. Biophys. 362 (1999) 12 – 21.