www.elsevier.com / locate / bres
Research report
Short-term ethanol exposure alters calbindin D28k and glial fibrillary
acidic protein immunoreactivity in hippocampus of mice
a ,
*
a a b bIrawan Satriotomo
, Takanori Miki , Masahiro Itoh , Kiyoshi Ameno , Iwao Ijiri ,
a
Yoshiki Takeuchi
a
Department of Anatomy, Faculty of Medicine, Kagawa Medical University, 1750-1 Miki-cho, Kita-gun, Ikenobe, Kagawa 761-0793, Japan
b
Department of Forensic Medicine, Faculty of Medicine, Kagawa Medical University, 1750-1, Miki-cho, Kita-gun, Ikenobe, Kagawa 761-0793 Japan
Accepted 18 July 2000
Abstract
The effects of a short-term ethanol treatment on hippocampus have been studied in mice exhibiting intoxication signs. The alterations of neurons and astrocytes as well as quantitative changes of calbindin D28k-immunoreactivity and glial fibrillary acidic protein-immunoreactivity (GFAP-IR) in selected regions of the dorsal hippocampus were examined using anti-calbindin and anti-GFAP monoclonal anti-body (mAb), respectively. The administration of 6% (v / v) ethanol during first week led to the neuronal death and decrease of the total number of calbindin-IR neurons in the examined brain regions. Moreover, the calbindin positive neurons were shown to have diminished processes following short-term ethanol exposure. These neuronal changes were associated with the increase of the GFAP-IR astrocytes. Hypertrophy of cell bodies and cytoplasmic processes of reactive astrocytes were also seen. In addition, dense, thick and highly-stained GFAP-IR cells with long processes in granular cell layer appeared entering into molecular layer of dentate gyrus. In agreement with the discrepancy percentage of neuronal cell loss and increase of reactive astrocytes detected by calbindin and GFAP-IR using image quantitative analysis, the regional differences in the vulnerability to the neurotoxic effects following short-term ethanol
exposure were found: CA3.CA2.CA1.DG. These findings also illustrate the importance of correlation between calbindin and
GFAP-IR when determining the morphological alteration of neuron and astroglial following short-term ethanol treatment. The disruption of calbindin and GFAP could affect neuronal-astroglial interaction, resulting in disturbance of behaviors dependent on hippocampus.
2000 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behaviour
Topic: Drugs of abuse: alcohol, barbiturates, and benzodiazepines
Keywords: Hippocampus; Short-term ethanol exposure; Intoxication signs; Glial fibrillary acidic protein (GFAP); Calbindin D28k
1. Introduction resulted in a wide variety of behavioral tasks and processes
[7]. Recently it has been recognized that the method used Ethanol exposure has been shown to result in alteration for ethanol exposure may be important, both in the in morphology and function of the central nervous system induction of dependence and degree of damage inflicted on (CNS). Behaviorial studies on the effects of ethanol the CNS. In this context, it has been demonstrated that treatment suggest that the hippocampus, which has been high peak blood ethanol concentrations maintained for particularly identified as one of the targets for neurotoxic relatively short periods of time may be critical in disturb-effects, is more sensitive than other regions and plays a ing brain functions not only in developing brain [28,29] prominent role in memory and learning processes [9]. but also mature brain studies [5,35].
Damage to this structure by acute ethanol treatment Previous studies suggested that glutamate excitotoxicity-like processes and related changes in calcium levels were implied in the toxic effects of alcohol [25]. Such a
*Corresponding author. Tel.: 181-87-891-2087; fax: 1
81-87-891-mechanism is also possible in hippocampus, since
gluta-2088.
E-mail address: [email protected] (I. Satriotomo). mate is the main excitatory neurotransmitter in the
21
pocampus and the importance of Ca for the functioning diet except that sucrose was substituted isocalorically for and survival of hippocampal cells has been well estab- ethanol. After 4–5 days, the mice which exhibited stage 2 lished. Calcium binding proteins have been implicated as or 3 of intoxication signs according to Freund’s classifica-important regulators of neuronal degeneration in pathologi- tion [10] were selected for morphological observation. The cal process [1]. Calbindin-D28k, which is a member of the intoxication signs consisted of four stages, such as hyper-superfamily of calcium binding proteins is implicated in reactivity and tremor (stage 1); episode of rapid beating the regulation of intracellular calcium and as a marker of a motion of the tail, slow, broad-based gait and rapid neuronal subpopulation [30]. In the adult mammalian backward movements (retropulsion); stereotype or repeti-brain, calbindin was thought to be present only in neurons, tive movement (stage 2); generalized tonic–clonic convul-which is believed to serve a neuroprotective role by its sion (stage 3); and death during convulsion (stage 4). The
calcium-buffering abilities [23]. approval of the Kagawa Medical University Animal
Com-Astrocytes were originally thought to play only suppor- mittee was obtained for this study.
tive roles in the brain. Recently, there have been many The animals were deeply anaesthetized with sodium reports concerning the functions of astrocytes including pentobarbital (40 mg / kg i.p.). To measure the blood neurotransmitter uptake [15], synthesis and secretion of alcohol concentration (BAC) when the animals showed the neurotrophic factors [11]. Analysis targets of astrocytic intoxication signs, blood was taken from the axilla-vessels reaction led the hypothesis that astrocytes are primary of the mice and analyzed by the gas chromatograph targets of chemical injury and mediates the phenotypic (Shimadzu GC-8A, Tokyo, Japan). Four mice of the expression of chemical injury in the CNS [2]. Glial control group and 6 mice of the experimental group were fibrillary acidic protein (GFAP), which is the major protein then perfused transcardially with a fixative of 10% for-of the intermediate filaments for-of astroglial cells, is the most malin neutral buffer solution (pH 7.4, Wako Co. Ltd., commonly used method to examine the distribution of Osaka, Japan) for preparation of paraffin embedded sec-astrocytes and the hypertrophy of sec-astrocytes in response to tions. Serial 7 mm-thick sections were cut coronally and
neural degeneration or injury [21]. stained with cresyl-violet. The remaining animals of each
Our previous report about the effects of short-term group were perfused with 0.02 M phosphate buffered ethanol exposure on the suprachiasmatic nucleus (SCN) of saline (PBS, pH 7.4) followed by a fixative of 4% the hypothalamus of mice demonstrated that there was no paraformaldehyde in PBS. The brains were removed and change on the calbindin D28k-IR with the increase on the cryoprotected by immersion in 30% (w / v) sucrose at 48C GFAP-IR [33]. The question arise that whether similar until the brain sank. Serial 40 mm-thick frozen sections immunoreactivity changes are seen or not in the hippocam- were cut coronally and processed for immunohistochemis-pus. Therefore, the aim of the present study was to try. Free floating sections were immunostained for calbin-investigate the alteration calbindin D28k-IR neurons and din D28k or GFAP using monoclonal antibody. To stain GFAP-IR astrocytes in the hippocampus of mice exhibiting for either GFAP or calbindin D28k, the sections were intoxication signs in response to short-term ethanol treat- incubated in the following solutions and between each ment. To understand which are particularly vulnerable or steps the sections were rinse with PBS: 10% normal goat invulnerable to short-term ethanol exposure, the possible serum in PBS with 0.2% Triton X-100 for 30 min; anti-interaction between short-term sensitivity to ethanol and GFAP rabbit antibody (1:2000 dilution, DAKO, Glostrup, differences in the subfields of hippocampus were also Denmark) or calbindin D28k mouse monoclonal
anti-examined. body (1:500 dilution, Sigma Chemical Company, St.
Louis, MO) overnight; biotinylated anti rabbit IgG or biotinylated anti mouse IgG for 1 h; avidin–biotin complex
2. Materials and methods (Vectastain, Elite ABC Kit, Vector Lab. Burlingame USA)
for 1 h and 0.05% diaminobenzidine–0.03% hydrogen
2.1. Immunohistochemistry procedures peroxidase for 5 min. Stained sections were mounted on
gelatin-coated glass slides and air dried. They were then Thirty male adult mice (7–8 weeks) of BALB / C strain dehydrated through graded (70, 80, 95, and 100%) alcohol from SLC (Shizuoka, Japan), weighing 22–25 g, were to xylene and coverslipped using Entellan (Merck, Ger-housed in separate cages under controlled conditions with many). Control sections omitting the primary antibody a constant temperature (23618C) and kept in 12:12 light / routinely developed to ensure that any observed staining dark cycle. In this study the mice were divided into two was due to calbindin-D28k or GFAP. The morphology and groups, ethanol-fed and pair-fed controls. The experimen- expression of calbindin or GFAP were studied with the aid tal mice (n516) received an unrestricted access liquid diet of a light microscope (Nikon, YTHM, Japan).
(Oriental Yeast Co. Ltd., Tokyo, Japan) containing 6%
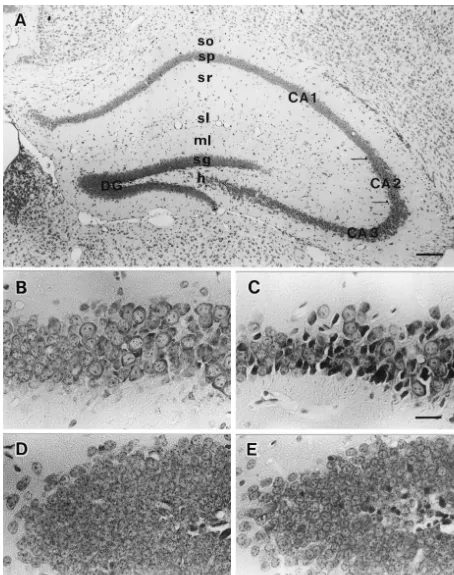
(v / v) ethanol (99.5% ethyl-alcohol, Wako Pure Chemical 2.2. Quantitative procedures Ind. Ltd., Osaka, Japan) as the sole fluid source. The
divided in 4 regions: the fields of the cornu Ammonis processes. The granule cell layer of the fascia dentate (CA1–CA3) and the superior limb of the dentate gyrus contained the smallest (10 mm) neuronal cell bodies and (DG). The cornu Ammonis of CA1 until CA3 was divided was the most densely packed layer in the hippocampus. On into sub-groups of stratum (str.) oriens, str. pyramidale, str. the other hand, the hilus of fascia dentate was composed of radiatum and str. lacunosum moleculare. The dentate gyrus very large (25–35 mm) and loosely packed polymorphic was divided into molecular layers of fascia dentata or str. cells (Fig. 1A). The intake of 6% (v / v) ethanol in the mice oriens, str. granulosum and hilus of fasciae dentata. Our exhibiting intoxication signs altered the neurons in the observations were confined to the dorsal part of hippocam- hippocampus. Neuronal death or lysis was observed in pus, as shown in Fig. 1. The number of labeled cells was fields of the cornu Ammonis (CA1–CA3) and the dentate measured quantitatively using image digital analysis in gyrus (DG) of the experimental group (Fig. 1C and E) sections of the ten mice of both groups. The first of these compared with the control group (Fig. 1B and D). sections which contained dorsal hippocampus was The calbindin-D28k positive neurons were moderately numbered as the first section, with all subsequent sections stained in CA1, CA2 and dentate gyrus subfields of mice being numbered sequentially. For each animal, a number hippocampus. CA1 subfields contained a higher proportion between 1 and 4 was selected randomly in order to of calbindin D28k positive pyramidal cells and were more determine the first section. Every 4th section was picked immunoreactive than the CA2 subfields, whereas pyrami-up and the number of calbindin-IR neurons and GFAP-IR dal cells in the CA3 subfield remained unreactive (Fig. astrocytes (for example, the 2, 6, 10th sections etc. were 2A). The single neurons lying in the str. oriens, str. selected for calbindin-immunohistochemistry (IH) and the radiatum and str. lacunosum-moleculare were intensely 4, 8, 12th sections were chosen for GFAP-IH) were labelled in the control groups (Fig. 2C). These single counted, respectively. About 10 sections from each animal neurons were characterized by the arborizated slender were used in this study. Photographs (3400 for calbindin processes (Fig. 2E). The short-term ethanol exposure led to and 31000 for GFAP) that were taken from selected significant differences when compared with the control sections with a light microscope (Nikon eclips E600) were group (Fig. 2B). The number of calbindin-positive neurons captured by CCD color camera (Inotech, Japan) and was decreased and less intensity of immunostaining was transformed into digits with the help of Viltz Universal seen when compared with the control group (Fig. 2D). software (Vers. 1.5.1, Interwere Corp.) in Power Macintosh Moreover the single neurons that located in str. oriens, str. 4400 / 200 personal computer. Photographs of individual radiatum and lacunosum-moleculare showed a lack of cells were used in order to avoid interference from processes in the experimental group (Fig. 2F). Table 1 overlapping images. The scanned images were placed shows the estimate of the number (mean6S.D.) of
2
permanently on the computer screen then analyzed by calbindin-D28k IR neurons / mm in various subregions in National Institute of Health (NIH) image software. The the hippocampus of ethanol treated and control mice. The area fraction of the total number of IR-cells (proportion of statistical comparison between these groups revealed
sig-4
calbindin-IR area per square measuring fields of 1.35310 nificant differences in all hippocampal subfields, except str. 2
mm and GFAP-IR area per square measuring field of pyramidale of CA1 subfield and str. granulosum of dentate 3 2
1.4310 mm , respectively) were taken. The number of area.
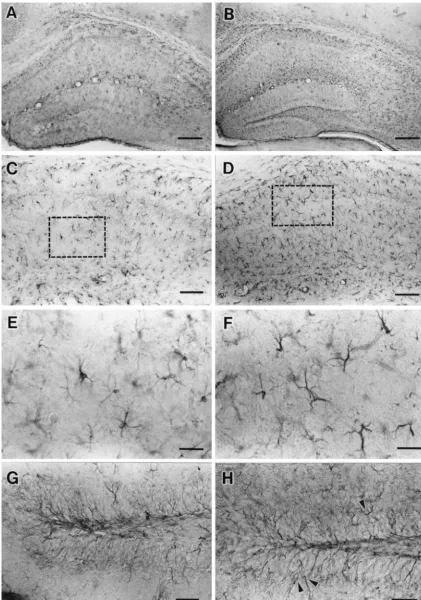
IR-positive cells / mm-square tissue from each region of the In the control mice, GFAP-IR perikarya and processes hippocampal sections of the alcohol treated animals was were observed throughout the hippocampal formation calculated and then compared with the respective control including CA1–CA3 and DG cells of the hippocampus group (Tables 1 and 2). The statistical analysis of data (Fig. 3A). Both pyramidal and granular cell layers were from each region were performed using Statview-J-4.5 clearly distinguished from adjacent layers, because only (Abacus Concepts, CA) with Student’s t-test (P,0.05 was fine GFAP-IR were seen in these layers (Figs. 3C and G).
taken as being significant). The GFAP-IR astrocytes were observed to have small
bodies with the short processes in the control group (Fig. 3E). The short-term ethanol exposure resulted in distinct
3. Results alteration of GFAP-IR astrocytes in the dorsal
hippocam-pus (Fig. 3B). In alcohol treated mice, the numbers of The analysis by the gas chromatography showed the GFAP-IR astrocytes were increased, whereas the area and BAC ranged from approximately 3.0 to 4.8 mg / ml with intensity of GFAP-IR were marked in CA1–CA3 as well means 4.1260.85 (mean6S.D.) mg / ml. These values as DG subfields of hippocampus (Fig. 3D). Hypertropic coincided with Freund’s result, which showed a similar astrocytes with the longer processes were also observed BAC (3.0–6.5 mg / ml) [10]. In all sections examined, the (Fig. 3F). In addition, dense, thick and highly-stained pyramidal cell layer of CA1 contained small round type GFAP-IR cells of processes of granular cell layers ap-neurons (10–15 mm) which were tightly packed. CA2– peared entering into the molecular layer of DG (Fig. 3H). CA3 layers had various sizes of neurons (20–30 mm) The estimates of the number (mean6S.D.) of GFAP-IR
2
Table 1
3 2 a
Data on the number of calbindin-IR neurons (310 cells / mm ) in the various regions of mice hippocampus following short-term ethanol treatment
Subfields n Ammon’s horn
St. Granulosum E 10 31.4465.58
c
C 10 32.1567.28
a
The values are presented as mean6S.D.
b
E: Experiment groups.
c
C: Control groups.
d
NR: no reaction, n, number of animals examined. *P,0.05, **P,0.01, ***P,0.001 Student’s t-test.
pocampus are presented. The statistical comparison of both 4. Discussion groups revealed a significant increase of GFAP-IR in all
regions of the hippocampus of alcohol treated mice. The In this study we provide evidence that short-term quantitative determination of decrease in the total number ethanol exposure alters the morphology and the numbers of of calbindin positive neurons and increase in the total calbindin-D28k-IR neurons and GFAP-IR astrocytes in the number of reactive astrocytes in the dorsal hippocampal hippocampus of the mice exhibiting intoxication. It is formation showed regional differences in the vulnerability interesting that our present result differs from our previous to the neurotoxic effect of short-term ethanol treatment: study on the SCN. Our previous study showed that there
CA3.CA2.CA1.DG (Fig. 4). was no change on the calbindin D28k-IR with the increase
Table 2
3 2 a
Data on the number of GFAP-IR astrocytes (310 cells / mm ) in the various regions of mice hippocampus following short-term ethanol treatment
Subfields n Ammon’s horn
Molecular ly. E 10 5.0960.83***
c
C 10 3.3160.90
b
St. Granulosum E 10 5.1061.18***
c
The values are presented as mean6S.D.
b
E: Experiment groups.
c
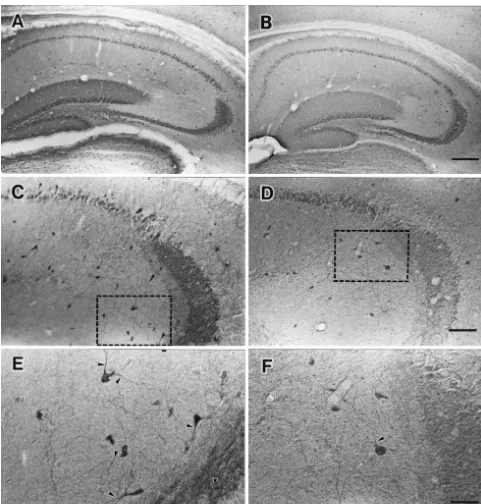
Fig. 2. Photomicrographs of calbindin D28k-IR in the hippocampus of control and ethanol treated mice. Calbindin-IR neurons revealed pyramidal cells of CA1–CA2 and granular cells of dentate gyrus (DG) have intensive staining however pyramidal cells of CA3 have no staining (A, C). Weak staining and the decrease of calbindin-positive neurons of the hippocampus were observed following short-term ethanol exposure (B, D). The subfields of the hippocampus (framed area) of the control mice in (C) and experimental mice (D) are magnified in (E) and (F), respectively. Note that the short ethanol exposure produced the poor dendritic arborization of calbindin D28k-IR neurons (black arrowheads) compared to the control group. Scale bars, 400mm (A and B), 200mm (C and D), and 50mm (E and F).
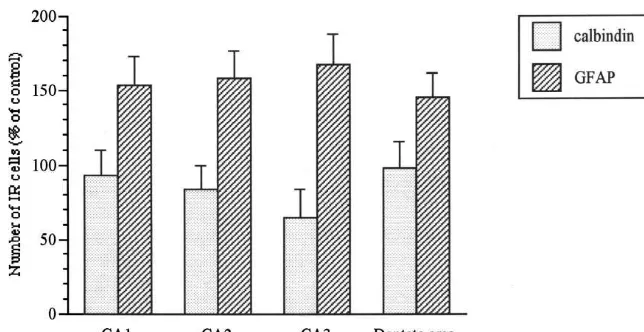
Fig. 4. Mean value (6S.D.) of the total number of calbindin-D28k IR neurons and GFAP-IR astrocytes in subfields of hippocampus from ethanol-treated mice, expressed as a percentage of controls. The values for each ethanol group represent a mean of count from ten mice. The counts for each subfield of hippocampus within each group were taken from the same set of ten mice. Note that the decrease of calbindin positive neuron is associated with the increase of GFAP-IR astrocytes in the dorsal hippocampus of ethanol treated mice.
Although the neuronal damage can be correlated with the sure. These results are similar to earlier reports on animals total amount of ethanol administrated, these results indi- chronically treated with ethanol [18,19] and on human cate a smaller dose can actually be more harmful than the alcoholics [3]. The calbindin-IR staining has confirmed larger one, provided the smaller dose is consumed in a these findings, significant alterations of calbindin-IR neu-pattern that produces correspondingly higher blood ethanol rons were also observed in experimental mice. Our study concentrations. In fact, there is evidence that the binge also showed the hippocampal neurons appear to be dif-exposure (i.e., consuming a given amount of ethanol over a ferentially susceptible to toxic effects of ethanol both shorter period of time) reduces the amount of ethanol within and between cell types. The resistance or selective needed to produce brain damage in developing [4,12] or vulnerability of different hippocampal neuron populations mature rats [5,35]. It appears that ethanol intoxication can seemed to be inversely correlated with the calbindin-D28K trigger directly neuronal degeneration. The neurotoxicity contents of cells.
of ethanol has been widely discussed physiologically [13] It has been assumed that neurons containing certain and morphologically [8,31], as well as in in vivo studies intracellular calcium binding proteins may have a greater
21
[6]. Ethanol intoxication is associated with changes in the capacity to buffer Ca and therefore would be more activity of neurons in the central nervous system. How- resistant to degeneration [14]. A previous study demon-ever, the cellular and molecular mechanisms underlying strated that cultured hippocampal neurons expresses cal-these changes are still poorly understood. The disturbance bindin D28k-IR resistant to neurotoxicity induced either by
21
of Ca homeostasis has been proposed as a common step glutamate or calcium ionophore [23]. It is of interest that in in the development of cytotoxicity and / or cell dysfunction our results, the CA3 is more vulnerable than the other
21
in the CNS. A sustained increase in cytosolic Ca subfields of hippocampus, since the immunostaining re-concentration, different from the rapid and transient sults showed that the CA3 fails to show the evidence of changes occurring in physiological condition, is invariably granular cells containing calbindin-IR neuron. The other associated with neuronal damage [26,27]. The increase in fact, CA1 cells are more resistant to the neurotoxic effect
21
Ca appears to mediate the toxicity of several known of ethanol than CA2 cells because the CA1 subfield neurotoxic agents (cyanide, chlordecone, various heavy contains a higher proportion of calbindin D28k positive metals), which induce either alterations in the physical pyramidal cells than the CA2 subfield. Rami et al. [32] integrity of the plasma membrane or mitochondrial impair- also showed CA1 cells are more immunoreactive than the ment and consequent ATP depletion [16]. These results CA2 cells and the pyramidal cells of the CA2 subfield are suggest that ethanol induces one or more of the mecha- more vulnerable than CA1 to ischemia. Compared to nisms involved in the cytosistolic buffering of intracellular rodents, a number of clinical and experimental reports on
21 21
Ca . Thus intoxication elevated intracellular Ca levels epileptic damage indicates that calcium-binding proteins may participate in adaptation of the central nervous system are concentrated in the CA2 area of the monkey or human to ethanol and / or to occurrence of ethanol induced neuro- hippocampus, so called the ‘resistant zone’ [17,34].
nal damage. Quantitative estimations of changes in calbindin
rat cerebrocortical and olfactory regions during subchronic ‘binge’
decrease in the calbindin-D28K contents of these neurons
intoxication with ethanol: possible explanation for olfactory deficits
was accompanied by cell damage. According to our
in alcoholics, Alcohol. Clin. Exp. Res. 20 (1996) 284–292.
results, we suggest that the decrease of calbindin-positive [6] D.L. Davies, W.E. Cox Dela, Delayed growth and maturation of neurons reflected the loss of neurons. Our present data astrocytic cultures following exposure to ethanol: electron
micro-demonstrated that the observed decrease in the total scopic observations, Brain Res. 547 (1991) 53–61.
[7] L.D. Devenport, R.L. Hale, Contributions of hippocampus and
number of calbindin-positive neurons is also directly
neocortex to the expression of ethanol effects, Psychopharmacology
related to the increased amount of reactive astrocytes
(Berl.) 99 (1989) 337–344.
among the subfields. The increase in the number of GFAP- [8] D. Durand, J.A. Saint-Cyr, N. Gurevich, P.L. Carlen, Ethanol-IR astrocytes in hippocampal subfields might reflect in- induced dendritic alterations in hippocampal granule cells, Brain creases in GFAP synthesis. New recent evidence has Res. 477 (1989) 373–377.
[9] H. Franke, H. Kittner, P. Berger, K. Wirkner, J. Schramek, The
suggested that astrocytes have important roles in the CNS
reaction of astrocytes in the hippocampus of adult rats during
function including synthesis and / or secretion neurotrophic
chronic ethanol treatment and correlations to behaviour
impair-factors [11], and aid repair of injured neuronal tissue [22]. ments, Alcohol 14 (1997) 445–454.
The data quoted above demonstrated that short-term [10] G. Freund, Alcohol withdrawal syndrome in mice, Arch. Neurol. 21 ethanol exposure produced distinct alteration in calbindin (1969) 315–320.
[11] S. Furukawa, Y. Furukawa, E. Satoyoshi, K. Hayashi, Synthesis and
D28k-IR neurons and neighboring GFAP-IR astrocytes.
secretion of nerve growth factor by mouse astroglia cells in culture,
The diminished processes of calbindin positive neurons
Biochem. Biophys. Res. Commun. 136 (1986) 57–63.
might reflect in a retraction of the neuronal dendritic arbor
[12] C.R. Goodlett, B.L. Marcussen, J.R. West, A single day of alcohol
which play crucial role in cell-to-cell communication. A exposure during the brain growth spurt induces brain weight previous study of targeted deletion in astrocyte inter- restriction and cerebellar Purkinje cell loss, Alcohol 7 (1990) 107–
114.
mediate filament suggested that GFAP is important for
[13] D.L. Gruol, J.G. Curry, Calcium signals elicited by quisqualate in
astrocytes-neuronal interactions and astrocytes processes
cultured Purkinje neurons show developmental changes in
sensitivi-play a vital role in modulating synaptic efficacy in the
ty to acute alcohol, Brain Res. 673 (1995) 1–12.
CNS [24]. Thus, it should be further emphasized in the [14] C.W. Heizmann, Calcium signaling in the brain, Acta Neurobiol. present study that short-term administered ethanol disrup- Exp. (Warsz) 51 (1993) 15–23.
tion of GFAP and calbindin could affect neuronal-astrogli- [15] H.K. Kimelberg, D.M. Katz, High-affinity uptake of serotonin into immunocytochemically identified astrocytes, Science 228 (1985)
al interaction, resulting in disturbance of behaviors
depen-889–891.
dent on hippocampus. The link established here between
[16] H. Komulainen, S.C. Bondy, Increased free intracellular Ca21 by
calcium binding protein such as calbindin-IR and GFAP- toxic agents: an index of potential neurotoxicity?, Trends Phar-IR provide valuable evidence for discoveries concerning macol. Sci. 9 (1988) 154–156.
neuronal-astrocytes interactions. [17] C. Leranth, C.E. Ribak, Calcium binding protein are concentrated in the CA2 field of the monkey hippocampus: a possible key to this region’s resistance to epileptic damage, Exp. Brain Res. 85 (1991) 129–136.
Acknowledgements [18] N.V. Lukoyanov, M.D. Madeira, M.M. Paula-Barbosa, Behavioral
and neuroanatomical consequence of chronic ethanol intake and
This work was supported by a Grant-in-Aid for Sci- withdrawal, Physiol. Behav. 66 (1999) 337–346.
[19] C. Lundqvist, C. Alling, R. Knoth, B. Volk, Intermittent ethanol
entific Research from the Ministry of Education, Science,
exposure of adults rats: hippocampal cell loss after one month of
Sport and Culture of Japan (09307018). We would like to
treatment, Alcohol Alcohol. 30 (1995) 737–748.
thank Dr. Gail S. Tucker for her constructive comments [20] M.D. Madiera, J.P. Andrade, A.R. Lieberman, N. Sousa, O.F.X. and suggestions, and Mr. Kensaku Miyamoto and Mrs. Almeida, M.M. Paula-Barbosa, Chronic alcohol consumption and
Mizue Fukutomi for their technical assistance. withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels, J. Neurosci. 17 (1997) 1302–1319.
[21] P.M. Martin, J.P. O’Callaghan, A direct comparison of GFAP
References immunocytochemistry and GFAP concentration in various regions
of ethanol-fixed rat and mouse brain, J. Neurosci. Methods 58 [1] M.S. Airaksinen, J. Eilers, O. Garaschuk, H. Thoenen, A. Konnerth, (1995) 181–192.
M. Meyer, Ataxia and altered dendritic calciums signaling in mice [22] A.J. Mathewson, M. Berry, Observation on astrocytes response to carryng a targeted null mutations of calbindin D28k gene, Proc. Natl the cerebral wound in adult rats, Brain Res. 327 (1985) 61–69. Acad. Sci. USA. 94 (1997) 1488–1493. [23] M.P. Mattson, B. Rychlik, C. Chu, S. Christakos, Evidence for [2] M. Aschner, R.M. LoPachin Jr., Astrocytes: Targets and mediators calcium-reducing and excitoprotective roles for the calcium-binding of chemical-induced CNS injury, J. Toxicol. Environ. Health 38 protein-D28k in cultured hippocampal neurons, Neuron 6 (1991)
(1993) 329–342. 41–51.
[3] O. Bengochea, L.M. Gonzalo, Effect of chronic alcoholism on [24] M.A. McCall, R.G. Gregg, R.R. Behringer, M. Brenner, C.L. human hippocampus, Histol. Histopathol. 5 (1990) 349–357. Delaney, E.J. Galbreath, C. Zhang L, R.A. Pearce, S.Y. Chiu, A. [4] D.J. Bonthius, J.R. West, Ethanol induced neuronal lost in the Messing, Targeted deletion in astrocytes intermediate filament developing rats: increased brain damage with binge exposure, (GFAP) alters neuronal physiology, Proc. Natl. Acad. Sci. USA 93 Alcohol. Clin. Exp. Res. 14 (1990) 107–118. (1996) 6361–6366.
molecular events involved intoxicity, Alcohol. Clin. Exp. Res. 14 mice: Effect on hippocampal cells and synapses, Exp. Neurol. 80
(1990) 819–826. (1983) 218–226.
[26] P. Nicotera, G. Bellomo, S. Orrenius, Calcium mediated mechanisms [32] A. Rami, A. Rabie, M. Thomasset, J. Krieglstein, Calbindin-D28k in chemically induced cell death, Ann. Rev. Pharmacol. Toxicol. 32 and ischemic damage of pyramidal cells in rat hippocampus, J.
(1992) 449–470. Neurosci. Res. 31 (1992) 89–95.
[27] S. Orrenius, P. Nicotera, The calcium ion and cell death, J. Neural [33] I. Satriotomo, T. Miki, M. Itoh, Q. Xie, K. Ameno, Y. Takeuchi, Transm. 43 (1994) 1–11. Effect of short-term ethanol exposure on the suprachiasmatic [28] D.R. Pierce, J.R. West, Alcohol-induced microcephaly during the nucleus of hypothalamus: immunohystochemical study in mice,
third trimester equivalent: relationship to dose and blood alcohol Brain Res. 847 (1999) 124–129.
concentration, Alcohol 3 (1986) 185–191. [34] R.S. Sloviter, A.L. Sollas, N.M. Barbaro, K.D. Laxer, calcium [29] D.R. Pierce, J.R. West, Blood alcohol concentration: a critical factor binding protein (calbindin-D28k) and parvalbumin immunocytoch-for producing fetal alcohol effects, Alcohol 3 (1986) 269–272. emistry in the normal and epileptic human hippocampus, J. Comp. [30] B. Pfeiffer, A.W. Norman, B. Hamprecht, Immunocytochemical Neurol. 308 (1991) 381–396.
characterization of neuron-rich rat brain primary cultures: calbindin [35] J.Y. Zou, D.B. Martinez, E.J. Neafsey, M.A. Collins, Binge ethanol– D28k as marker of neuronal subpopulation, Brain Res. 476 (1989) induced brain damage in rats: effect of inhibitors of nitric oxide
120–128. synthase, Alcohol. Clin. Exp. Res. 20 (1996) 1406–1411.