Functional detection of chemopreventive glucosinolates in
Arabidopsis thaliana
Heidrun B. Gross
1, Tim Dalebout
2, C. Douglas Grubb, Steffen Abel *
Department of Vegetable Crops,Uni6ersity of California-Da6is,One Shields A6enue,Da6is,CA95616,USA
Received 18 May 2000; received in revised form 20 July 2000; accepted 20 July 2000
Abstract
Natural isothiocyanates, derived from glucosinolates by myrosinase-catalyzed hydrolysis, are potent chemopreventive agents that favorably modify carcinogen metabolism in mammals by inhibiting metabolic activation of carcinogens and/or by inducing carcinogen-detoxifying enzymes. Methylsulfinylalkyl isothiocyanates are potent selective inducers of mammalian Phase 2 detoxifi-cation enzymes such as quinone reductase [NADP(H):quinone-acceptor oxidoreductase, EC 1.6.99.2]. Members of the Cruciferae family, including the model plant speciesArabidopsis thaliana(L.) Heyhn, synthesize methylsulfinylalkyl glucosinolates. We have adapted a colorimetric bioassay for quinone reductase activity in Hepa 1c1c7 murine hepatoma cells as a versatile tool to rapidly monitor methylsulfinylalkyl glucosinolate content inA.thalianaleaf extracts. Using wild type plants and mutant plants defective in the synthesis of 4-methylsulfinylbutyl glucosinolate (glucoraphanin), we have demonstrated thatA.thaliana(ecotype Columbia) is a rich source of Phase 2 enzyme inducers and that methylsulfinylalkyl glucosinolates, predominantly glucoraphanin, account for about 80% of the quinone reductase inducer potency of Columbia leaf extracts. We have optimized leaf extraction conditions and the quinone reductase bioassay to allow for screening of large numbers of plant extracts in a molecular genetic approach to dissecting glucosinolate biosynthesis inA.thaliana. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Arabidopsis thaliana; Bioassay; Chemopreventive glucosinolates; Phase 2 detoxification enzymes; Quinone reductase; Sulforaphane www.elsevier.com/locate/plantsci
1. Introduction
Glucosinolates are a diverse class of sulfur- and nitrogen-containing secondary metabolites that have long been the defining chemotaxonomic char-acter of the order of Brassicales and that are mainly found in members of the Brassicaceae (Cruciferae) and in several other families of di-cotyledonous angiosperms [1]. Glucosinolates con-sist of a common glycone moiety and a variable aglycone side-chain derived from amino acids,
most frequently from methionine, phenylalanine, tyrosine, and tryptophane. The glycone is charac-terized by a thioglucose and a sulfonated oxime group, both attached to the a-carbon of the parental amino acid [2]. Upon tissue disruption, glucosinolates are rapidly hydrolyzed by myrosi-nase (b-thioglucoside glucohydrolase, EC 3.2.3.1) to unstable intermediates that, as dictated by chemical conditions, spontaneously rearrange to isothiocyanates, thiocyanates, or nitriles [3,4]. Al-though the primary biological function of glucosi-nolates in plants is unknown, glucosinolate breakdown products are proposed to act as allelo-chemicals and to play a role in plant defenses against herbivores, pests, and pathogens [3,4]. Fur-thermore, indolyl glucosinolates can be converted into indoleacetic acid and may thus contribute to active auxin levels in cruciferous plants [5]. As components of food for humans and feed for
* Corresponding author. Tel.: +1-530-7525549; fax: + 1-530-7529659.
E-mail address:[email protected] (S. Abel).
1Present address: Department of Molecular Biosciences, University
of California-Davis, School of Veterinary Medicine, One Shields Avenue, Davis, CA 95616, USA.
2Present address: Department of Anatomy and Embryology,
Uni-versity of Leiden, Medical Center, P.O. Box 9603, 2300 RC Leiden, The Netherlands.
livestock, the biological activities of glucosinolate hydrolysis products have generated considerable toxicological and pharmocological interest. De-pending on glucosinolate composition and on the prevalence of hydrolysis products, consumption of glucosinolates by mammals has been linked with goitrogenic effects (thiocyanates) or with a re-duced risk of developing cancer (isothiocyanates) in experimental animals [6 – 10].
Natural isothiocyanates derived from aromatic and aliphatic glucosinolates are effective chemo-protective agents that block chemical carcinogene-sis and prevent several types of cancer in rodent models [10]. Mechanistic studies have shown that isothiocyanates target mammalian Phase 1 and Phase 2 drug-metabolizing enzymes and their cod-ing genes, resultcod-ing in decreased carcinogen-DNA interactions and in increased carcinogen detoxifi-cation [8]. For example, the methionine-derived 4-methylsulfinylbutyl isothiocyanate (sul-foraphane) inhibits Phase 1 enzyme-mediated acti-vation of procarcinogens [11], induces Phase 2 detoxification enzymes such as quinone reductase (QR) and glutathione-S transferase in hepatoma cells [12,13], and blocks mammary tumor forma-tion in rats [14,15]. Sulforaphane is the most pow-erful natural inducer of chemoprotective enzymes thus far reported [12] and has become a metabolic target of breeding strategies to enhance the anti-carcinogenic potency of cruciferous vegetables [15,16].
The chemoprotective properties of natural isoth-iocyanates have renewed interest in glucosinolate biosynthesis. While significant progress has been made in understanding the biochemistry and enzy-mology of glucosinolate synthesis, very little is known about the structural and regulatory genes involved [2]. InArabidopsis thaliana(L.) Heynh., a member of the Cruciferae family and a premier reference species for plant biology [17], 23 differ-ent glucosinolates have been iddiffer-entified [18]. Inter-estingly, 4-methylsulfinylbutyl glucosinolate (glucoraphanin), precursor to sulforaphane, is the major leaf glucosinolate of ecotype Columbia [18,19]. Here, we show that a QR bioassay in murine hepatoma cells reliably reports gluco-raphanin content in A. thaliana. Furthermore, we have optimized the bioassay to allow for high-throughput analysis of leaf extracts. The bioassay for the major chemopreventive glucosinolate inA. thaliana, glucoraphanin, allows for rapid analysis
of a large number of samples in an effort to dissect glucosinolate biosynthesis by genetic and molecu-lar genetic approaches.
2. Materials and methods
2.1. Chemicals
NADP, FAD, 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2-methyl-1,4-naphthoquinone (menadione), bovine serum albumin (BSA), glucose 6-phosphate, crystal vio-let, baker’s yeast glucose-6-phosphate dehydroge-nase, and aryl sulfatase were obtained from Sigma Chemical Co. (St. Louis, MO); digitonin was ob-tained from Boehringer Mannheim (Indianapolis, IN); and acetonitrile, dimethyl sulfoxide, and dimethyl formamide were obtained from Fisher Scientific (Pittsburgh, PA). All the other chemicals were of commercial purity or of plant cell culture grade.
2.2. Plant material
Arabidopsis thaliana (L.) Heynh. (ecotype Co-lumbia) and mutant line TU1 derived from eco-type Columbia [19] were obtained from the Biological Resource Center at Ohio State Univer-sity. Organically grown green onions (Allium sa -ti6a) and apples (Malus malus) were obtained
from a local market and were stored at −20°C after purchase. When Arabidopsis plants were grown to maturity, seeds were planted in vermi-culite, and modified Hoagland’s nutrient media was subirrigated every 3 days [20]. For plant growth under sterile conditions, seeds were surface sterilized for 10 min in 30% (v/v) bleach contain-ing 0.1% (v/v) Triton X-100 and were placed on solid medium containing 8 g l−1 agar, 5 g l−1 sucrose and 2.15 g l−1 (0.5×) Murashige-Skoog salts [21], pH 5.6. All the Arabidopsis plants were grown in a growth chamber at 22°C under illumi-nation with fluorescent and incandescent light at an intensity of 60 mE m−2 s−1 for 16 h daily.
2.3. Preparation of plant extracts
Prochaska et al. [22]. The plant material was ho-mogenized with 2 vol. of deionized cold water in a Waring blender for 2 min at 4°C. The suspension was lyophilized, and 400 mg of the dried powder were extracted with 14 ml acetonitrile for 24 h at 4°C. The extract was filtered and evaporated to dryness in a rotating evaporator (B40°C). The residue was dissolved in 100-ml acetonitrile and was directly used for induction of Hepa 1c1c7 murine hepatoma cells. Aqueous extracts (water extracts and phosphate buffer extracts) from the Arabidopsis plants grown under greenhouse and sterile conditions were prepared from rosette and primary leaves, respectively. Using a polypropy-lene mini-pestle, the leaf material (40 mg fresh weight) was homogenized in an 1.5-ml Eppendorf tube with either 50 ml deionized water or 50 ml extraction buffer (5 mM K2HPO4– KH2PO4, 1 mM EDTA, pH 7.6). The pestle was rinsed with 150-ml water or extraction buffer, and the ho-mogenate was vortexed for 2 h at room tempera-ture. For organic extracts using triple solvent solution [15], leaves (200 mg fresh weight) were homogenized with 1 ml triple solvent (prepared by mixing of equal volumes of dimethyl sulfoxide, dimethyl formamide, and acetonitrile) and ex-tracted at −50°C for 1 h. All extracts were cleared by repeated centrifugation (10 min at 16 000×g), and the supernatant was directly used for induction of Hepa 1c1c7 murine hepatoma cells. Plant material was extracted on at least three occasions and analyzed separately.
2.4. Induction of cultured Hepa 1c1c7 murine hepatoma cells
The Hepa 1c1c7 murine hepatoma cell line was kindly provided by Paul Talalay, The Johns Hop-kins University School of Medicine. Mutant Hepa 1c1c7 cells, BPrc1 and TAOc1BPrc1, were ob-tained from Michael Denison, University of Cali-fornia, Davis. All cell lines were propagated in
a-minimal essential medium supplemented with 10% fetal calf serum (FCS) [15] in a humidified incubator in 5% CO2 at 37°C as previously de-scribed [22,23]. To monitor inducer potency of plant extracts, Hepa 1c1c7 murine hepatoma cells were grown in 96-well microtiter plates. Typically, 10 000 Hepa 1c1c7 cells were seeded into each well, grown for 24 h, and then induced for 24 h by exposure to fresh culture medium containing serial
dilutions of the plant extract to be assayed (aceto-nitrile, water, phosphate buffer, or triple solvent extracts). Usually, 20 ml of the extract was diluted to 4 ml with cell culture medium, and two-fold serial dilutions were prepared in the microtiter plate using an octapipet, one column of wells receiving the same amount of plant extract. The final volume in each well was 200 ml, and the concentration of the extract solvent was 0.5%. For the assay of triple solvent extracts, ascorbic acid (0.5 mM final concentration) and 0.003 units of myrosinase (Sigma) were added to each well to achieve complete glucosinolate hydrolysis.
2.5. Assay of quinone reductase acti6ity
After Hepa 1c1c7 cells were exposed to plant test extracts in culture medium for 24 h, QR activity was assayed in cell lysates, using mena-dione, MTT, and a NADPH-generating system [23]. The cell culture medium was decanted and 50
ml of lysing solution (0.8% digitonin, 2 mM EDTA, pH 7.8) was added to each well. To facili-tate cell lysis, microtiter plates were incubated for 10 min at 37°C and were subsequently agitated on an orbital shaker (100 rpm) for 10 min at room temperature. For measurement of QR activity, 200
acid reagent [15]. The potency of QR induction by plant extracts is expressed as unit g−1fresh or dry weight. One unit of QR inducer activity is defined as the amount of plant material required to double the specific QR activity in a microtiter well con-taining 200-ml cell culture medium.
2.6. HPLC analysis of desulfoglucosinolates
Glucosinolates were extracted from A. thaliana leaves (50 mg fresh weight) by boiling in water (1 ml) [19]. After washing the leaves with water (1 ml), the combined extract was applied to a DEAE-Sephadex A-25 (40 mg) column (pyridine acetate form). The glucosinolates were converted into their desulfo analogs by overnight treatment with 100 ml 0.1% (1.4 U) aryl sulfatase, and the desul-foglucosinolates were eluted with 1 ml water [18]. HPLC of desulfo-glucosinolates was carried out using a Shimadzu VP Liquid Chromatograph. Samples (100 ml) were separated at ambient tem-perature on a Waters Spherisorb C18 column (150×4.6 mm i.d., 5-mm particle size), using the following methanol gradient in water at a flow rate of 1.0 ml min−1: 3% (5 min), 3 – 21% (6 – 20 min). Desulfoglucosinolates were detected at 226 and 280 nm. Sinigrin (allyl glucosinolate) was used as a standard. Farnham et al. [24] reported that the relative integrated absorbance areas for
equimolar concentrations of glucoraphanin and sinigrin at 226 nm are identical.
3. Results
3.1. Induction of quinone reductase in murine hepatoma cells by Arabidopsis leaf extracts
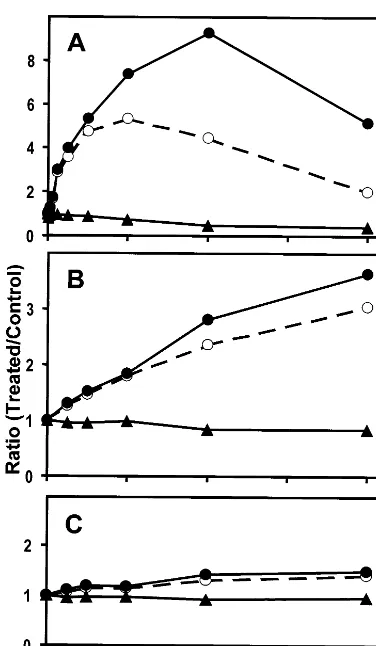
Methylsulfinylalkyl isothiocyanates are potent and specific inducers of mammalian Phase 2 detoxification enzymes such as QR or glutathione-S transferase [7]. Since methylsulfinylalkyl glucosi-nolates are synthesized in A. thaliana and glucoraphanin is the most abundant leaf glucosi-nolate of ecotype Columbia [17,18], we tested the potency of Columbia leaf extracts in inducing QR activity in cultured Hepa 1c1c7 murine hepatoma cells. As indicated by the colorimetric QR bioassay, rosette leaves of A. thaliana contain readily detectable levels of Phase 2 enzyme induc-ers (Figs. 1 and 2A). We used green onion and apple extracts as controls for the bioassay, which were reported to contain high and negligible levels of QR inducers, respectively [22]. As shown in Fig. 2, at the highest concentration tested (acetonitrile extract derived from 8 mg dry weight ml−1 cell culture medium), green onion extract induced murine specific QR activity 3.5-fold, whereas the effect of apple extract was marginal (less than 20% induction). The potency of green onions is calcu-lated at 2250 U g−1 dry weight, while apple extracts are considered inactive (Fig. 2B and C). Interestingly, the potency of acetonitrile extract of mature leaves of A. thaliana, calculated at 38 500 U g−1 dry weight, exceeded that of green onion extract by more than one order of magnitude (Fig. 2A). Less than 0.15 mg dry weight ml−1 medium were required forA. thaliana extract to double the specific QR activity of Hepa 1c1c7 cells, as op-posed to 2.3 mg dry weight ml−1medium of green onion extract. Maximal, about 9-fold, induction of specific QR activity by A. thaliana extract was achieved at a concentration of 4 mg dry weight ml−1medium. Examination of detectable cytotox-icity showed that A. thaliana extract at 2 mg dry weight ml−1 medium reduced cell density by 20%. However, the cytotoxic effect was negligible at and below 1 mg dry weight ml−1medium (Fig. 1BFig. 2A).
Fig. 2. Induction of QR activity in murine Hepa1c1c7 cells by acetonitrile extract ofA.thalianaleaves (A), green onion (B), and apple (C). Shown are, expressed as ratios of treated cells to control cells, total QR activities (open circles), cell densities (triangles), and specific QR activities (closed circles). Repre-sentative data of one experiment out of three independent plant extractions are shown.
thaliana. The acetonitrile extraction method is lengthy and requires more leaf material than a developing seedling can provide. We tested three procedures to rapidly extract isothiocyanates or glucosinolates from rosette leaves of A. thaliana plants. During tissue homogenization, myrosinase-catalyzed hydrolysis of glucosinolates to isothio-cyanates is favored at neutral pH, whereas nitrile formation dominates at acidic conditions and re-quires ferrous ions [3]. We compared QR inducer potencies of buffered (5 mM K2HPO4/KH2PO4, 1 mM EDTA, pH 7.6) and non-buffered (H2O) leaf extracts. As shown in Table 1A, the QR inducer potency of phosphate buffer extracts was as about twice as high as the potency of water extracts, which suggests preferential formation of isothio-cyanates during leaf extraction with phosphate buffer.
Tissue homogenization at low temperature with equal volumes of dimethyl sulfoxide, dimethyl for-mamide, and acetonitrile (triple solvent) inacti-vates myrosinases and quantitatively extracts glucosinolates [15]. Using triple solvent extraction and a myrosinase-supplemented QR assay, Fahey et al. [15] showed that nearly all the inducer activity of cruciferous plants is derived from glu-cosinolates. When applied to A. thaliana leaves, the QR inducer potency of triple solvent extracts was comparable to the potency of phosphate buffer extracts (see Table 1A), indicating that glu-cosinolates are readily hydrolyzed by endogenous myrosinase activities during tissue homogenization in aqueous buffers. Leaf extraction with phos-phate buffer was the method of choice in subse-quent experiments because of its simplicity and effectiveness in extracting QR inducers. The plant-to-plant variation in QR inducer potencies among different samples of Columbia leaf extract was between 5 and 10% (see Table 1).
3.3. Test of mutant Hepa 1c1c7 cells defecti6e in Phase 1 enzyme expression
Phase 2 detoxification enzymes are transcrip-tionally activated by two classes of inducers (i) monofunctional inducers that directly and specifi-cally activate Phase 2 genes; and (ii) bifunctional inducers that sequentially activate Phase 1 genes and, after subsequent chemical modification by Phase 1 enzymes, genes encoding Phase 2 enyzmes [8]. Induction of QR in wild type Hepa 1c1c7
Table 1
Potency of QR induction ofA.thaliana (ecotype Columbia) leaf extracts, inducer potencies are given per gram fresh weight as the mean (9S.D.) (n=3)
Extraction solvent Murine cell line Inducer potency
(A)Rosette lea6es of4-week-old soil-grown mature plants
6250 (550) Hepa 1c1c7
H2O
Hepa 1c1c7 11 150 (850) Phosphate buffer
Hepa 1c1c7
Triple solvent 12 500 (900)
(B)Primary lea6es of2-week-old agar-grown seedlings
Hepa 1c1c7
Phosphate buffer 16 650 (1150) 12 500 (1050) BPrc1
Phosphate buffer
TAOc1BPrc1 14 300 (1100) Phosphate buffer
3.2. Comparison of extraction methods
murine hepatoma cells is responsive to both mono- and bifunctional inducers. The mutant cell lines BPrc1 and TAOc1BPrc1, both derived from Hepa 1c1c7 cells, are defective in the expression of the Phase 1 enzyme, cytochrome P1-450 [25]. Thus, QR induction in response to bifunctional inducers, but not monofunctional inducers, is impaired in both mutant cell lines [25].
To assess the contribution of bifunctional induc-ers to the total QR inducer potency of leaf extract and thus the ability to preferentially detect mono-functional inducers such as sulforaphane, we com-pared QR induction in wild type and mutant Hepa 1c1c7 cells. As shown in Table 1B, the QR inducer potency of leaf extract obtained from 2-week-old Columbia seedlings grown on agar plates was similar in TAOc1BPrc1 cells and in BPrc1 cells. However, the QR inducer potency of the same extract was 15 and 30% higher in wild type Hepa 1c1c7 cells than in TAOc1BPrc1 and BPrc1 cells, respectively. The difference in inducer potency of the same sample between wild type and mutant murine hepatoma cells indicates presence of bi-functional inducers in Columbia leaf extract, which amount to about 20% of the total QR inducer potency (Table 1B). In order to minimize
detection of bifunctional quinone reductase induc-ers, the TAOc1BPrc1 cell line was used in subse-quent experiments.
3.4. Quinone reductase inducer potencies of mutant and wild type Arabidopsis strains
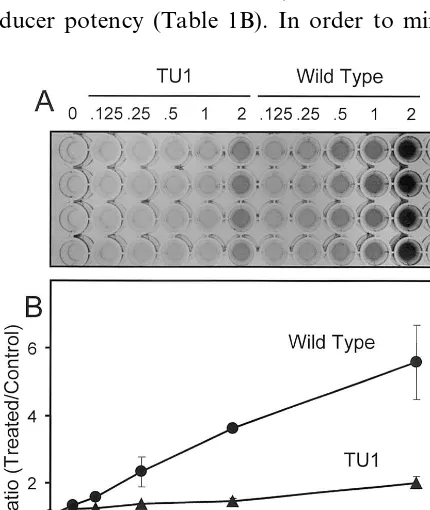
Finally, we tested the feasibility of the QR assay to identify plants with altered glucosinolate profi-les. We compared QR inducer potencies of wild type leaf extracts with extracts of mutant line TU1 (ecotype Columbia). The TU1 line was identified in a mutant screen by HPLC for plants with altered glucosinolate metabolism [19]. The single recessive mutation (gsm1-1) affects synthesis of glucoraphanin, which was reported to accumulate in TU1 leaves to less than 2% of wild type [19]. Reexamination of glucosinolate profiles of both accessions by HPLC analysis showed that the leaves of Columbia wild type plants contained glucoraphanin at a concentration of 400 nmol g−1 fresh weight, whereas levels of glucoraphanin in leaves of TU1 plants were measured at 7 nmol g−1 fresh weight (data not shown). Importantly, the difference in glucoraphanin content between wild type and TU1 leaf extracts correlated with a large difference in QR inducer potencies in TAOc1BPrc1 cells, which was readily visualized by the colorimetric QR assay (Fig. 3). The QR in-ducer potency of TU1 plants (2500 U g−1 fresh weight) was less than 20% of wild type plants (14 300 U g−1fresh weight) and likely reflects the reduced concentration of glucoraphanin in TU1 leaves.
4. Discussion
In this communication we describe the utilization of an in vitro bioassay for QR activity in mammalian cells to rapidly monitor content of chemopreventive glucosinolates in leaf extracts of A. thaliana. The bioassay for QR inducer potency in murine hepatoma cells was initially developed to identify synthetic and natural compounds that specifically induce mammalian Phase 2 detoxifica-tion enzymes involved in carcinogen metabolism [23]. Since then, the QR bioassay has been successfully employed to identify Phase 2 enzyme inducers in broccoli (sulforaphane), red wine
Fig. 3. Induction of QR activity in murine TAOc1BPrc1 cells
by phosphate buffer extracts of wild type (circles) and TU1 (triangles) A. thaliana leaves. (A) colorimetric QR assay (C, reagent blank), (B) quantitative data (error bars denote S.D.,
(resveratol), and tomatillos (withantholides) [15,22,26,27]. The bioassay has also been used to evaluate the potency of various purified phyto-chemicals and synthetic compounds [12,28,29]. In-terestingly, these studies have revealed that the isothiocyanate, sulforaphane, derived from its glu-cosinolate precursor, glucoraphanin, is an ex-tremely potent inducer of QR in murine hepatoma cells and that sulforaphane is far more active than any previously identified naturally occurring Phase 2 enzyme inducer [12,29].
Aliphatic glucosinolates such as glucoraphanin are widely distributed in the Cruciferae [1 – 4]. Since glucoraphanin is the major leaf glucosinolate ofA. thaliana(ecotype Columbia) [18,19], we have adapted and evaluated the QR bioassay as a ver-satile tool to rapidly assess Arabidopsis leaf ex-tracts for glucosinolate content. A previous systematic survey conducted by Prochaska et al. [22] of extracts of commonly consumed vegetables and fruits for QR inducer activity identified green onion and broccoli to be particularly rich sources for Phase 2 enzyme inducers. Here, we show that Columbia leaf extracts contained high activities of QR inducers, which exceeded those measured for green onions under our conditions. The use of mutant murine hepatoma cell lines defective in Phase 1 enzyme expression [22,25] allowed for an estimate of the contribution of monofunctional Phase 2 enzyme inducers to total QR inducer potency. Based on our data obtained for cell line TAOc1BPrc1, approximately 85% of the total Phase 2 enzyme inducer potency of Columbia leaf extracts was derived from monofunctional induc-ers, such as sulforaphane. Indeed, as indicated by a direct comparison of glucoraphanin content and QR inducer potency betweenArabidopsiswild type plants and the mutant TU1 line [19], more than 80% of the total QR inducer potency ofArabidop -sisleaf extracts can be attributed to methylsulfiny-lalkyl isothiocyanates, which are largely represented by sulforaphane. The TU1 phenotype is caused by a single nuclear mutation, which affects glucosinolate biosynthesis and leads to a reduction of aliphatic glucosinolate levels in leaf tissues, in particular of glucoraphanin [19]. Our QR bioassay and HPLC analysis data are in agreement with results of two recent studies in Brassica, which have demonstrated a highly posi-tive correlation between QR inducer potencies and HPLC-determined glucoraphanin concentrations
of broccoli extracts [16,29]. Thus, QR inducer potency of leaf extracts can serve as a suitable primary indicator for glucoraphanin content, and perhaps total glucosinolate abundance, in A. thaliana.
Relatively small quantities of Arabidopsis leaf tissues (less than 50 mg) are required to rapidly extract in a simple one-step procedure glucosino-late-derived isothiocyanates in amounts sufficient for the QR bioassay. Both the extraction proce-dure of the plant material and the microtiter plate format of the bioassay are amenable to automa-tion to allow for high-throughput analysis of leaf sample extracts. Although separation and identifi-cation of glucosinolates by HPLC analysis has proven a straightforward analytical tool [18,30], sample preparation and cost of analysis can be limiting factors in large-scale screening procedures. The robustness of the colorimetric QR assay and its potential for parallel sample analysis provide a viable alternative to rapidly assess in a primary assay chemopreventive glucosinolate content of a large number of samples, which can be followed up by HPLC analysis of a smaller sample set of interest. We are currently using this dual strategy to identify genes involved in the biosynthesis of chemopreventive glucosinolates in a molecular ge-netic approach in A. thaliana [19,31].
Acknowledgements
H.B. Gross and T. Dalebout contributed equally to this work. The authors thank Dr Paul Talalay and Dr Jed Fahey (The Johns Hopkins University School of Medicine) for Hepa 1c1c7 cells and for valuable advice, Dr Michael Denison (University of California, Davis) for mutant Hepa 1c1c7 cells, BPrc1 and TAOc1BPrc1, and Kristina Abel for advice on cell culture. This work was supported by grants from the United States De-partment of Agriculture (Fund for Rural America) and from the University of California, Davis (Fac-ulty Research Grant) to S. Abel.
References
[2] B.H. Halkier, L. Du, The biosynthesis of glucosinolates, Trends Plant Sci. 2 (1997) 425 – 431.
[3] J.E. Poulton, B.L. Møller, Glucosinolates, Methods Plant Biochem. 9 (1993) 209 – 237.
[4] G.R. Fenwick, R.K. Heaney, W.J. Mullin, Glucosino-lates and their breakdown products in food and food plants, Crit. Rev. Food Sci. Nutr. 18 (1983) 123 – 201. [5] J. Normanly, B. Bartel, Redundancy as a way of life —
IAA metabolism, Curr. Opn. Plant Biol. 2 (1999) 207 – 213.
[6] W.M.F. Jongen, Glucosinolates inBrassica: occurrence and significance as cancer-modulating agents, Proc. Nutr. Soc. 55 (1996) 433 – 446.
[7] Y. Zhang, P. Talalay, Anticarcinogenic activities of or-ganic isothiocyanates: chemistry and mechanisms, Can-cer Res. 54 (1994) 1976s – 1981s.
[8] P. Talalay, Y. Zhang, Chemoprotection against cancer by isothiocyanates and glucosinolates, Biochem. Soc. Trans. 24 (1996) 806 – 810.
[9] S. Hecht, Chemoprevention of cancer by isothio-cyanates, modifiers of carcinogen metabolism, J. Nutr. 129 (1999) 768s – 774s.
[10] L.W. Wattenberg, Inhibition of carcinogenesis by minor dietary constituents, Cancer Res. 52 (1992) 2085s – 2091s. [11] S. Barcelo, K. Mace, A.M.A. Pfeifer, J.K. Chipman, Production of DNA strand breaks byN -nitrosodimethy-lamine and 2-amino-3-methylimidazo[4,5-f]quinoline in THLE cells expressing human CYP isoenzymes and inhibition by sulforaphane, Mutation Res. 402 (1998) 111 – 120.
[12] T. Prestera, W.D. Holtzclaw, Y. Zhang, P. Talalay, Chemical and molecular regulation of enzymes that detoxify carcinogens, Proc. Natl. Acad. Sci. USA 90 (1993) 2965 – 2969.
[13] K. Maheo, F. Morel, S. Langouet, H. Kramer, E. Le Ferrec, B. Ketterer, A. Guillouzo, Inhibition of cy-tochromes P-450 and induction of glutathione S -trans-ferases by sulforaphane in primary human and rat hepatocytes, Cancer Res. 57 (1997) 3649 – 3652. [14] Y. Zhang, T.W. Kensler, C.G. Cho, G.H. Posner, P.
Talalay, Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates, Proc. Natl. Acad. Sci. USA 91 (1994) 3147 – 3150. [15] J.W. Fahey, Y. Zhang, P. Talalay, Broccoli sprouts: an
exceptionally rich source of inducers of enzymes that protect against chemical carcinogens, Proc. Natl. Acad. Sci. USA 94 (1997) 10367 – 10372.
[16] K. Faulkner, R. Mithen, G. Williamson, Selective in-crease of the potential anticarcinogen 4-methylsulfinyl-butyl glucosinolate in broccoli, Carcinogenesis 19 (1998) 605 – 609.
[17] D.W. Meinke, J.M. Cherry, C. Dean, S.D. Rounsley, M. Koorneef, Arabidopsis thaliana: a model plant for genome analysis, Science 282 (1998) 662 – 682.
[18] L.R. Hogge, D.W. Reed, E.W. Underhill, G.W. Haughn, HPLC separation of glucosinolates from leaves and seeds ofArabidopsis thalianaand their identification
using thermospray liquid chromatography/mass spec-trometry, J. Chromatogr. Sci. 26 (1988) 551 – 556. [19] G.W. Haughn, L. Davin, M. Giblin, E.W. Underhill,
Biochemical genetics of secondary metabolites in Ara
-bidopsis thaliana. The glucosinolates, Plant Physiol. 97 (1991) 217 – 226.
[20] S. Abel, M.D. Nguyen, A. Theologis, The PS-IAA4/ 5-like family of early auxin-inducible mRNAs in Ara
-bidopsis thaliana, J. Mol. Biol. 251 (1995) 533 – 549. [21] T. Murashige, F. Skoog, A revised medium for rapid
growth and bioassays with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 473 – 497.
[22] H.J. Prochaska, A.B. Santamaria, P. Talalay, Rapid detection of inducers of enzymes that protect against carcinogens, Proc. Natl. Acad. Sci. USA 89 (1992) 2394 – 2398.
[23] H.J. Prochaska, A.B. Santamaria, Direct measurement of NAD(P)H:Quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers, Anal. Biochem. 169 (1988) 328 – 336. [24] M.W. Farnham, K.K. Stephenson, J.W.Fahey, Capacity
of broccoli to induce a mammalian chemoprotective enzyme varies among inbred lines, J. Am. Soc. Hort. Sci. 125, in press.
[25] M.J. De Long, A.B. Santamaria, P. Talalay, Role of cytochrome P1-450 in the induction of
NAD(P)H:quinone reductase in a murine hepatoma cell line and its mutants, Carcinogenesis 8 (1987) 1549 – 1553. [26] M. Jang, L. Cai, G.O. Udeani, K.V. Slowing, C.F. Thomas, C.W. Beecher, H.H. Fong, N.R. Farnsworth, A.D. Kinghorn, R.G. Mehta, R.C. Moon, J.M. Pezzuto, Cancer chemopreventive activity of resveratol, a natural product derived from grapes, Science 275 (1997) 218 – 220.
[27] E.J. Kennelly, C. Gerhauser, L.L. Song, Induction of quinone reductase by withantholides isolated from
Physalis philadelphica (Tomatillos), J. Agric. Food Chem. 45 (1997) 3771 – 3777.
[28] A. Dinkova-Kostova, P. Talalay, Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxication enzymes, Carcinogenesis 20 (1999) 911 – 914.
[29] G.H. Posner, C.G. Cho, J.V. Green, Y. Zhang, P. Talalay, Design and synthesis of bifunctional isothio-cyanate analogs of sulforaphane: correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes, J. Med. Chem. 37 (1994) 170 – 176.
[30] T. Prestera, J.W. Fahey, W.D. Holtzclaw, C. Abeygu-nawardana, J.L. Kachinski, P. Talalay, Comprehensive chromatographic and spectroscopic methods for the sep-aration and identification of intact glucosinolates, Anal. Biochem. 239 (1996) 168 – 179.
[31] C. Alonso-Blanco, M. Koorneef, Naturally occurring variation in Arabidopsis: an underexploited resource of plant genetics, Trends Plant Sci. 5 (2000) 22 – 29.