Leaf age- and paraquat concentration-dependent effects on the
levels of enzymes protecting against photooxidative stress
Leonardo M. Casano *, Mercedes Martı´n, Jose´ M. Zapata, Bartolome´ Sabater
Departamento de Biologı´a Vegetal,Uni6ersidad de Alcala´,Alcala´ de Henares,28871Madrid,Spain
Received 30 April 1999; received in revised form 23 June 1999; accepted 12 July 1999
Abstract
Antioxidant protective enzymes are usually induced in leaves under conditions of increased active oxygen generation, such as high light intensity, low CO2 fixation rate or in the presence of paraquat, which transports electrons from photosynthetic
machinery to oxygen to form O2−. However, at high photooxidative stress, even protective enzymes can be destroyed and leaf
cells become dead. The protective role of several chloroplastic activities was evaluated at increasing photooxidative stress in barley leaves of different ages. We investigated the effects of different paraquat concentrations (combined with low and high light intensities) in expanding and aged-senescent leaves on the activity of plastid peroxidase and on the activity and protein levels of plastid superoxide dismutase (SOD), glutathione reductase (GR) and NADH dehydrogenase of the complex including polypep-tides encoded by plastid ndh genes. The chloroplastic GR was the most sensitive to inactivation when photooxidative stress increased. SOD was preferentially induced in young-expanding leaves while NADH dehydrogenase and peroxidase were preferentially induced in adult-senescent leaves. The results suggest a limited role of GR in the protection against photooxidative stress and a close relation between the actions of Ndh complex and peroxidase. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Barley; Chloroplasts; Glutathione reductase;Hordeum6ulgare;ndhgenes; Peroxidase; Superoxide dismutase
www.elsevier.com/locate/plantsci
1. Introduction
Photooxidative stress arises in photosynthetic tissues when the rate of the production of reducing power is higher than the rate of its re-oxidation, mainly by CO2 reduction; e.g. in the light at low
temperature or with low CO2 supply when
drought stress closes stomata [1 – 5]. In these con-ditions, high concentrations of the reduced forms of photosynthetic electron carriers favour one-electron transfer reaction to oxygen producing superoxide anion radical (O2−) from which
derives other active oxygen species, as hydrogen peroxide (H2O2) and hydroxyl radical (OH).
Ac-tive oxygen species have deleterious effects in many cellular functions since they react with
un-saturated fatty acid components of membrane lipids, proteins and nucleic acids. To overcome in part these problems, living cells have developed a complex enzymic machinery which destroys O2−
and H2O2. Superoxide dismutases (SOD) [2,3,6],
peroxidases [4,5,7], chloroplast Ndh complex [8,9] (the complex with NADH dehydrogenase activity which includes polypeptides encoded by plastid
ndhgenes [10,11]) and glutathione reductases (GR) [12,13], among other enzymic activities, have been proposed to be involved in reactions which scav-enge O2− and/or H2O2.
Usually, enzymes involved in O2− and H2O2
scavenging in chloroplasts increase their levels when light intensity quickly increases or in the presence of toxic compounds as paraquat [14] which captures electrons from photosynthetic elec-tron transport to form O2− from oxygen. The
increase of protective enzymes under
photooxida-* Corresponding author. Tel.: +34-1-885-4911; fax: + 34-1-885-5066.
E-mail address:[email protected] (L.M. Casano)
tive stress is currently accepted as indicative of their role in the scavenging of O2− and/or H2O2.
However, sometimes, strong enough photooxida-tive stress masks the induction of protecphotooxida-tive en-zymes because high concentrations of active oxygen species quickly degrade the same enzymes which scavenge them. One possibility is that differ-ent scavenging systems are functional at differdiffer-ent stress intensities and at different developmental stages of the leaves. Thus, to deduce the involve-ment of a given enzyme in protection against photooxidative stress a range of stress intensity should be assayed in leaves at different develop-mental stages.
In this work we have subjected young-expand-ing primary leaves (7-day-old) and aged-senescent primary leaves (14-day-old) of barley to different photooxidative stress intensities produced by two light intensities and a range of paraquat concen-trations. We have investigated the effects of these treatments on the levels of chloroplastic SOD, hydroquinol peroxidase, GR and Ndh complex activities and on the levels of SOD, GR and Ndh complex protein, to determine the involvement of these activities in the protection of chloroplasts against photooxidative stress and the inactivation of the same activities by far strong photooxidative stress.
2. Materials and methods
2.1. Plant materials
Barley (Hordeum 6ulgare L cv. Hassan) was
grown on vermiculite in a controlled growth chamber at 23°C under a 16 h photoperiod of 100
mmol photon m−2s−1white light as described [3].
In the present work, we have used primary leaves of 7- and 14-day-old plants as young expanding and aged-senescent leaves respectively. Ten sub-apical leaf segments (3 cm length) were cut after 4 – 5 h from the beginning of photoperiod, and incubated at 23°C during 20 h with 10 ml of different concentrations of paraquat in growing light (GL) (100 mmol photon m−2s−1) or relative
photooxidative light (PhL) (300mmol photon m−2
s−1). In this experimental system, wounding
pro-vokes a transient increase of some antioxidant enzymes between 2 and 6 h after incubation. Thereafter, they stabilise at a level which depends on the incubation conditions [3].
2.2. Preparation of leaf crude extracts
For zymographic and Western blot assays, ac-tivities and proteins were assayed in whole leaf extracts obtained as follows: ten 3-cm leaf seg-ments were homogenised with a mortar and pestle in 2 ml of 50 mM potassium phosphate pH 7.0/1 mM L-ascorbic acid/1 mM EDTA/5% (w/v)
polyvinylpirrolidone, and centrifuged at 500×g
for 10 min. Except for extracts for the assay of GR and SOD, Triton X-100 was added to super-natant to make a final 2% (w/v) solution and gently stirred for 30 min. The suspension was centrifuged at 20 000×g for 30 min. Supernatants contained 0.7 – 1.3 mg protein per ml.
2.3. Isolation of chloroplasts
Enzymes corresponding to the specific chloro-plastic activities investigated in crude extracts were identified by comparison with those of chloroplas-tic fractions and by parallel zymograms and West-ern blots after native PAGE (see 2.4).
Intact chloroplasts were isolated as described [8]. Leaf segments were homogenised in an Omni-mixer (Sorvall) with six volumes of freshly pre-pared isotonic buffer E (50 mM potassium phosphate/1 mM L-ascorbic acid/1 mM EDTA/
0.33 M sorbitol, pH 7.0) supplemented with 5% (w/v) polyvinylpyrrolidone. The homogenate was filtered through eight layers of muslin and cen-trifuged at 200×g for 5 min. The supernatant was centrifuged at 2000×g for 10 min. The pellet of chloroplasts was washed with buffer E (10 ml per 1 g original leaves) to obtain a preparation of chloroplasts free from soluble or mitochondrial fractions [7 – 9]. To obtain high percentages (\
80%) of intact chloroplasts, all steps from leaf segments to pellet of washed chloroplasts were performed at 0 – 5°C in no more than 45 min. The chloroplast pellet was resuspended for osmotic shock in buffer E without sorbitol (buffer H) at 0 – 4°C with gentle shaking during 6 min and then centrifuged for 15 min at 4500×g. The superna-tant was used to assay SOD and GR. The thy-lakoid pellet was resuspended to approximately 2 mg protein per ml in buffer H and used for solubilisation of thylakoid-bound Ndh complex or peroxidase.
gently stirred for 15 min. After centrifugation at 10 000×g for 20 min, the Ndh complex was solu-bilised from the pellet, which was resuspended in 20 mM Tris – HCl pH 7.0/5 mM EDTA, by adding 10% (w/v) Triton X-100 to make a final concentra-tion of 2% (5 mg detergent per mg protein) and gently stirred for 30 min. The suspension was centrifuged at 105 000×g for 45 min. The super-natant (around 4 mg protein per ml, ca. 40% of original thylakoid proteins) was the NADH dehy-drogenase solubilised fraction.
Peroxidase was solubilised from the thylakoid membranous fraction by adding 10% (w/v) Triton X-100 to make a final 1.5% solution and gently stirred for 45 min. The suspension was centrifuged at 20 000×gfor 30 min. The supernatant (around 0.9 mg protein per ml) contained the peroxidase solubilised from thylakoid.
All the isolation procedures (Sections 2.2 and 2.3) were performed at 4°C.
2.4. Gel electrophoresis, immunoassays and
zymograms
Native PAGE was carried out at 5°C (usually with 20 – 100mg protein samples) in a linear
gradi-ent gel of 3 – 10% polyacrylamide (2.5% bis-acry-lamide) in the same way as SDS-PAGE with the exception that gels contained 0.1% (w/v) Triton X-100 (for NADH dehydrogenase and peroxidaxe zymograms) instead of SDS [15] or neither SDS nor Triton X-100 (for SOD and GR zymograms). For immunoblot analyses, after SDS-PAGE, proteins were transferred to PVDF membranes (Millipore). Immunocomplexes with antibodies prepared against the NDH-F polypeptide encoded by the ndhF gene [9], Cu/Zn SOD (chloroplastic) [16], or chloroplastic GR [13] were detected with the peroxidase Western blotting analysis system (Boehringer).
For zymograms, NADH dehydrogenase activity of Ndh complex was detected by incubation of gel slices for 20 – 30 min at 30°C in darkness in 50 mM potassium phosphate (pH 8.0)/1 mM Na2– EDTA/
0.2 mM NADH and 0.5 mg per ml nitro blue tetrazolium. In controls without NADH no stain developed. Staining for peroxidase was performed by following standard methods with 4-methoxy-a
-naphthol [7] as substrate. SOD was detected in gel by the photochemical nitro blue tetrazolium stain-ing method [17] and Cu/Zn SOD identified as
before [16]. GR was detected by incubating with NADPH, GSSG and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide [12]. Diaphorase bands were discriminated by performing the stain-ing procedure without GSSG. Bands were scanned with a UVP Easy Digital Image analyser to com-paratively quantify activity values which were ex-pressed as percentages of the reference activity (that of freshly detached primary leaves of 7- or 14-day-old plants).
2.5. Enzyme assays
As reference for absolute rates of some activities (see legends for Figs. 3 and 5), the activity NADH:FeCN oxidoreductase specific of Ndh complex was assayed at 30°C by measuring the reduction of FeCN at 420 nm (extinction coeffi-cient: 1.03 mM−1 cm−1) and the oxidation of
NADH at 340 nm (extinction coefficient: 6.22 mM−1cm−1) in a Beckman DU-650
spectropho-tometer. The reaction mixture, with a final volume of 1.0 ml, included 50 mM potassium phosphate (pH 7.5)/1 mM Na2– EDTA/0.2 mM NADH/1
mM FeCN and variable enzyme preparations. The rate was determined from linear absorbance de-crease between 45 and 240 s. Control values, ob-tained without protein, were subtracted. No detectable transformation of one substrate was observed in controls without the other substrate. The spectrophotometric assay of peroxidase was performed at 30°C in a 1.0 ml assay volume containing 0.5 mM hydroquinone (HQ)/0.1 mM H2O2/50 mM potassium phosphate buffer pH 7.0.
After mixing, the enzymatic reaction was initiated by adding 10 ml of enzyme. The oxidation of HQ
was recorded as the increase in absorbance at 250 nm (extinction coefficient: 19 mM−1cm−1) over a
time period of 2 min. The absorbance increases were always linear with respect to time. Appropri-ate controls were subtracted [7].
Specific activities are expressed as mmol of
NADH or HQ consumed per min per mg protein.
2.6. Other determinations
All reported results were reproduced at least three times. When appropriate, standard devia-tions are indicated by bars in figures.
3. Results
3.1. Effects of photooxidati6e stress on the le6els
of proteins, chlorophylls and carotenoids
Leaves of 7- and 14-day-old plants were used to know the influence of leaf age on the
photooxida-tive effects of paraquat and high light intensity. Until leaf detachment, barley plants were growing in photoperiodic regime under the low intensity light (100 mmol m−2 s−1). Fig. 1 shows the
changes of soluble proteins, chlorophylls and carotenoids of barley leaf segments after incuba-tion during 20 h under low light intensity (growing light, GL, 100 mmol m−2s−1) and photooxidative
light (PhL, 300 mmol m−2 s−1) and 0, 50, 100 or
300 nM paraquat.
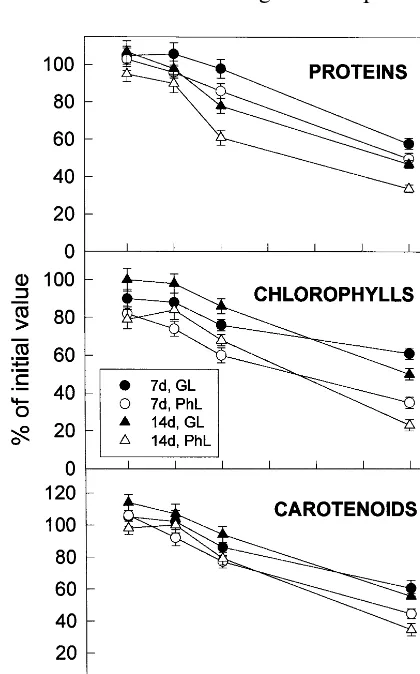
Paraquat produced a progressive yellowing of leaf segments which was dependent, at least, of its concentration and on the light intensity. At a concentration of 100 nM, and even more at 300 nM, paraquat produced a clear photooxidative damage when measured by soluble protein, chlorophyll or carotenoids decrease. The decreases of proteins, chlorophylls and carotenoids were more pronounced when the paraquat incubations were carried out under high (PhL, empty symbols) than under low (GL, full symbols) light intensities. Paraquat at 50 nM did not have any apparent effects in comparison with incubation in the ab-sence of paraquat. In addition, the photooxidative effect of paraquat stimulating the decrease of proteins was more pronounced in 14-day-old aged leaves than in 7-day-old expanding leaves. Al-though chlorophylls diminished slightly more in expanding than in aged leaves at concentrations up to 100 nM paraquat, they decreased more in aged than in expanding leaves at 300 nM paraquat. The paraquat-stimulated carotenoid de-crease, while more pronounced at high (PhL) than at low (GL) light intensity, was rather similar in expanding and in aged leaves.
Apparently, under the conditions of assays, only concentrations of paraquat higher than 50 nM increased the production of active oxygen species large enough to stimulate the degradation of proteins, chlorophylls and carotenoids with re-spect to incubation in the absence of paraquat.
3.2. Effects of photooxidati6e stress on chloroplast
SOD acti6ity and protein
SOD has been thoroughly investigated as an activity typically induced under photooxidative stress, presumably as a response to scavenge O2−.
Thus, it is a good tool to know how much strong photooxidative conditions are required to surpass the induction of protective response by the delete-rious effects of active oxygen species.
Fig. 1. Effects of leaf age, photooxidative light and paraquat concentration on the levels of proteins, chlorophylls and carotenoid of leaf segments. Expanding 7-day-old and aged-senescent 14-day-old leaves of barley were incubated at 23°C during 20 h with the indicated concentrations of paraquat (PT) in growing light (GL) (100 mmol photon m−2 s−1) or
relative photooxidative light (PhL) (300 mmol photon m−2
s−1). Remaining soluble proteins, chlorophylls and
carotenoids were expressed as percentages of the values in freshly detached leaves: 12.3 mg protein · g FW−1, 975 mg
chlorophyll · g FW−1 and 337 mg carotenoid · g FW−1 for
7-day-old plants and 11.1 mg protein · g FW−1, 896 mg
chlorophyll · g FW−1 and 302 mg carotenoid · g FW−1 for
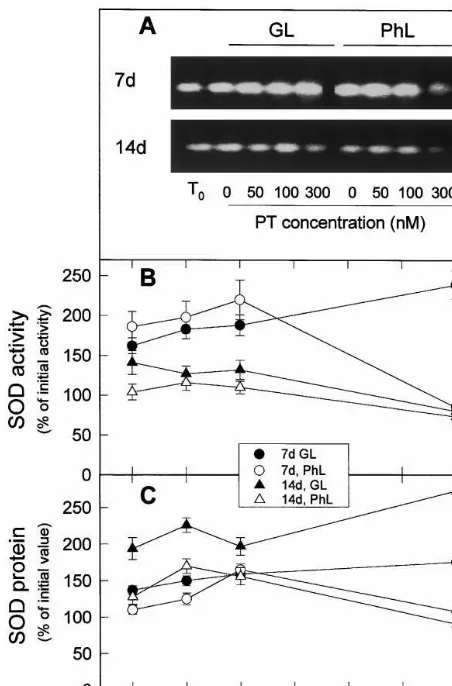
Fig. 2. Effects of leaf age, photooxidative light and paraquat concentration on the levels of Cu/Zn SOD activity and protein. (A) Typical zymogram of plastid Cu/Zn SOD includ-ing activities of freshly detached leaves (T0) and of leaves
incubated at 23°C during 20 h with the indicated concentra-tions of paraquat (PT) in growing light (GL) or relative photooxidative light (PhL). Twenty mg protein of leaf crude extracts were loaded per lane. (B) Specific activities of Cu/Zn SOD deduced from experiments as those of A. (C) Cu/Zn SOD protein level relative to total soluble protein in leaves treated as in A and deduced from Western blots (not repre-sented) with antibody against Cu/Zn SOD. Twentymg protein were loaded per lane. In B and C, respectively, activities and protein were expressed as percentages of the values in freshly detached 7-day-old leaves or 14-day-old plants (for compari-son seeT0of A). Values are means of at least three different
experiments.
aged 14-day-old leaves (Fig. 2(A), numerical data not represented) and increased further after 20 h incubation of leaves under GL (60%) or PhL (80%) (Fig. 2(A) and (B)). When photooxidative stress was increased by rising paraquat concentra-tion from 0 to 300 nM, SOD activity increased almost linearly in expanding leaves under GL. At high light intensity (PhL), SOD specific activity of expanding leaves increased more than at GL up to 100 nM paraquat concentration. A further in-crease of paraquat concentration (300 nM) deter-mined a decrease of SOD activity, presumably because the inductive effect of photooxidative stress on SOD was surpassed by the inactivation and degradation of SOD produced by the high concentrations of active oxygen species.
The overall pattern of SOD protein levels in expanding leaves receiving different light and paraquat treatments (Fig. 2(C)) was similar to that of SOD activity, although increases of activity in respect to zero time were around twice of the corresponding increases of protein, which sug-gested that photooxidative stress, produced by PhL, paraquat or both factors, stimulated the synthesis of new SOD protein and favoured the activation (or inhibited the inactivation) of SOD protein.
When 14-day-old leaves were cut and incubated under GL, SOD activity increased some 40% in respect to zero time (Fig. 2(A)). The presence of paraquat up to 100 nM did not cause a further increase of SOD activity (Fig. 2(B)). In addition, PhL and paraquat did not increase SOD activity in these leaves. These results agree with previous findings [3] describing the lost of sensitivity to SOD activity induction in senescent leaves. De-tachment and incubation under GL and PhL in-creased SOD protein (Fig. 2(C)) more than SOD activity (Fig. 2(B)); but, similarly to the effect on activity, PhL incubation decreased SOD protein levels in respect to GL incubation. It is possible that most of new synthesised SOD protein was inactive in aged leaves. Apparently reactive oxygen species inactivated SOD to a form still reacting with polyclonal antibodies.
These results suggest that although photooxida-tive stress induced the synthesis of SOD protein, at high photooxidative stress (PhL and 300 nM paraquat for expanding leaves and lower paraquat concentrations for senescent leaves) SOD was in-activated at a higher rate than it was induced. Chloroplastic SOD activity and protein was
esti-mated from zymograms and Western blots per-formed as described elsewhere [16].
3.3. Effects of photooxidati6e stress on chloroplast
NADH dehydrogenase complex (Ndh) acti6ity and
protein
Zymograms comparison of crude extracts and thylakoid solubilised complex and Western blot
assays (results not shown) allowed identification of NADH dehydrogenase of plastid Ndh complex as a well defined second low migration band in crude extract which corresponded to the first low migrat-ing band in thylakoids.
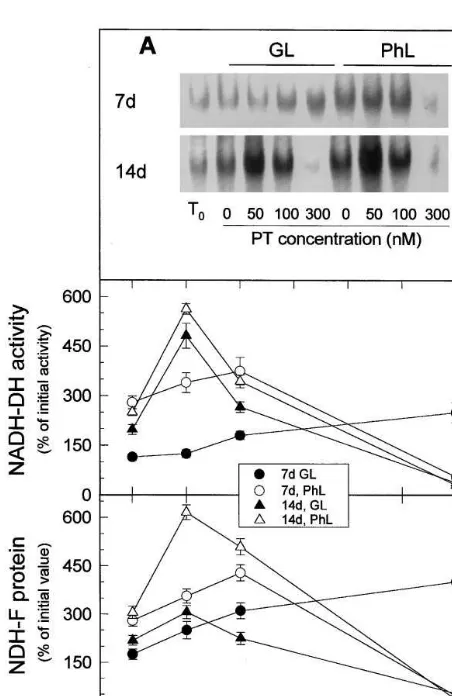
Zero-time Ndh specific activity was about 80% higher in aged 14-day-old leaves than in expanding 7-day-old leaves (Fig. 3(A), numerical data not represented). The effects of increasing photooxida-tive stress (produced by low and high irradiances and different paraquat concentrations) on Ndh activity and protein (detected with antibody against NDH-F) (Fig. 3) were rather similar to the effects on SOD activity: at low – moderate pho-tooxidative stress (in respect to the previous grow-ing conditions) activity and protein increased while at high photooxidative stress activity and protein decreased. There were however some dif-ferences. While the induction of SOD activity was mainly observed in expanding leaves (compare , with , in Fig. 2), the induction of Ndh activity and protein was more pronounced in aged leaves and required milder photooxidative condi-tions in aged than in expanding leaves (Fig. 3). The induction of Ndh activity and protein and their inactivation by excessive photooxidative stress, in general, required lower concentration of paraquat at high (PhL) (open symbols) than at low (GL) (filled symbols) light intensity, indicating that high light intensities and paraquat acted by similar compensatory effects: by increasing the concentration of reactive oxygen species. The re-sults also supported a role for Ndh complex on the protection against reactive oxygen species.
3.4. Effects of photooxidati6e stress on thylakoid
peroxidase
Thylakoid peroxidase oxidised several quinones [7], among them plastoquinone, and has been pro-posed to scavenge H2O2 by oxidising the
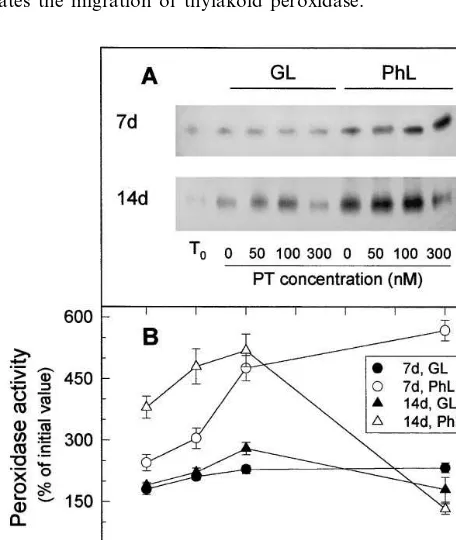
plas-toquinone reduced by the action of Ndh complex. The activity of thylakoid peroxidase may be deter-mined in crude extract by zymographic analysis because its low migration allows us to distinguish it from other peroxidases (Fig. 4).
Zero-time thylakoid peroxidase specific activity was about 200% higher in aged 14-day-old leaves than in expanding 7-day-old leaves (Fig. 5(A), numerical data not represented). Thylakoid perox-idase increased in expanding leaves when
photoox-Fig. 3. Effects of leaf age, photooxidative light and paraquat concentration on the levels of Ndh activity and NDH-F protein. (A) Typical zymogram of plastid NADH dehydroge-nase activity of the Ndh complex including activities of freshly detached leaves (T0) and of leaves incubated at 23°C
during 20 h with the indicated concentrations of paraquat (PT) in growing light (GL) or relative photooxidative light (PhL). One-hundred mg protein of leaf crude extracts were loaded per lane. (B) Specific activities of Ndh complex de-duced from experiments as those of A. (C) Level of NDH-F protein of Ndh complex relative to total soluble protein in leaves treated as in A and deduced from Western blots with antibody against NDH-F. Twenty mg protein of leaf crude extracts were loaded per lane. In B and C, respectively, activities and protein were expressed as percentages of the values in freshly detached 7-day-old leaves (4 nmol NADH oxidised min−1mg protein−1) or 14-day-old plants (7 nmol
NADH oxidised min−1 mg protein−1) (for comparison see
T0of A). Values are means of at least three different
Fig. 4. Comparative isoenzyme pattern of whole leaf extract and thylakoid peroxidases after staining with 4-methoxy-a-naphtol. Fortymg proteins was loaded per lane. Arrow indi-cates the migration of thylakoid peroxidase.
idative stress was raised by increasing paraquat concentrations (Fig. 5). The inductive effect of paraquat was particularly pronounced at high (PhL) relative light intensity and, although no clear effect of inhibition by excess of paraquat was observed under the conditions assayed, at least, the inductive effect was almost saturated between 100 and 300 nM paraquat.
As for Ndh activity (Fig. 3), the increase of peroxidase activity (Fig. 5) in response to pho-tooxidative stress was more pronounced in aged than in expanding leaves. Very strong photooxida-tive stress (300 nM paraquat) inactivated peroxi-dase which reached the maximun at around 100 nM paraquat with both GL and PhL in aged leaves.
3.5. Effects of photooxidati6e stress on glutathione
reductase (GR) acti6ity and protein
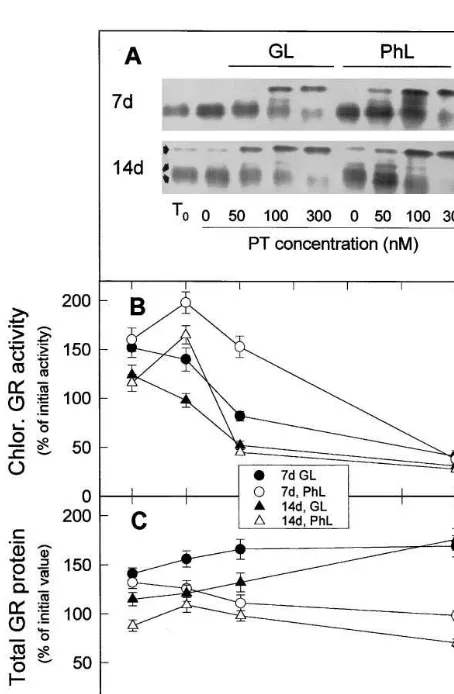
After non-denaturing electrophoresis and zy-mography, three main GR activities bands were detected (Fig. 6(A)): one low migrating extra-chloroplastic and a couple of fast migrating chloroplastic (results not shown). The three activi-ties reacted with our polyclonal antibodies [13] and could not be distinguished by Western blot after SDS-PAGE. Thus, in Fig. 6 we represent the effect of different photooxidative treatments on chloroplast GR activities in B and on total GR protein in C.
In both type of leaves, chloroplastic GRs in-creased after 20 h incubation without paraquat in GL and PhL, in respect to zero time. The presence of 50 nM paraquat further increased the activity of leaves incubated under PhL. Paraquat concen-trations higher than 50 nM in PhL and any paraquat concentration under GL decreased the chloroplastic activities (Fig. 6(B)) but, almost in a correlative way, they increased the extrachloro-plastic GR activity (Fig. 6(A)). Significantly, paraquat concentration had little effect on the level of total GR protein (Fig. 6(C)).
Although zero time chloroplastic GR specific activities in aged 14-day-old leaves was about 44% higher than in expanding 7-day-old leaves (Fig. 6(A), numerical data not represented), the in-creases of chloroplastic GR activity at 0 (under GL and PhL) or 50 nM paraquat (under PhL) (Fig. 6(B)) were more pronounced in expanding than in aged leaves.
Fig. 5. Effects of leaf age, photooxidative light and paraquat concentration on the levels of thylakoid peroxidase activity. (A) Typical zymogram of thylakoid peroxidase including activities of freshly detached leaves (T0) and of leaves
incu-bated at 23°C during 20 h with the indicated concentrations of paraquat (PT) in growing light (GL) or relative photooxi-dative light (PhL). Fiftymg protein of leaf crude extracts were loaded per lane. (B) Specific thylakoid peroxidase activities deduced from at least three different experiments as those of A. In B, activities were expressed as percentages of the values in freshly detached 7-day-old leaves (25 nmol HQ oxidised min−1 mg protein−1) or 14-day-old plants (75 nmol HQ
4. Discussion
In contrast to chlorophyll, carotenoid and most of the soluble protein, which decreased when the leaf was subjected to progressively stronger pho-tooxidative stress, the specific activities of chloro-plastic SOD, Ndh complex, peroxidase and GR
increased under most of the moderate photooxida-tive conditions applied to expanding and aged leaves. By far, chloroplast Ndh complex and thy-lakoid peroxidase showed the higher increases (in both activity and protein) in response to detach-ment and increased photooxidative conditions.
It must be noticed that mild photooxidative stress due to application of relative high light intensity and/or moderate concentrations of paraquat increased the four activities assayed, but strong photooxidative stress produced by high concentration (100 – 300 nM) of paraquat de-creased the activities, possibly by active oxygen-in-duced inactivation [16]. Presumably, the production of reactive oxygen species increases as light intensity or paraquat concentration increases. As a consequence, the concentration of paraquat for the maximum increase of activities was usually lower under high (PhL) than under low (GL) light intensities. Such concentration of paraquat was different in expanding and aged leaves, confirming that the response against photooxidative stress depends on the ontogenic state of the leaf [3,21]. The most effective concentration of paraquat also varied from one to other enzyme. In some cases, as SOD (Fig. 2) and GR (Fig. 6), significant differences among the responses of activity and protein suggest that the levels of specific forms of the respective protein (active, inactive, plastidial, cytosolic,…) are also affected by photooxidative stress. Clearly, to deduce from paraquat assays the involvement of one enzyme in the protection against photooxidative stress, a range of paraquat concentrations must be assayed to identify a possi-ble range of positive response of enzyme levels. Taking into account that significant declines in total soluble proteins (Fig. 1) required at least 100 nM paraquat (Fig. 1), the total leaf levels of SOD, Ndh complex, peroxidase and GR increased (as well as the specific activities) when the leaves were treated with 50 nM paraquat.
The chloroplastic GR activities were the most sensitive to inactivation by high photooxidative stress. In most cases, treatments with 100 nM paraquat decreased these activities more than the amount of total soluble proteins decreasing their specific activity below the 100% of the zero time value (Fig. 6(B)). The decrease of the plastid GR was compensated by an increase in the extra-chloroplastic (probably cytosolic) activity (Fig. 6(A)). In pea, only one gene has been found for
Fig. 6. Effects of leaf age, photooxidative light and paraquat concentration on the levels of GR activity and protein. (A) Typical zymogram showing plastid (fast migrating) and extra-chloroplastic (low migrating) GR activities including activities of freshly detached leaves (T0) and of leaves incubated at
23°C during 20 h with the indicated concentrations of paraquat (PT) in growing light (GL) or relative photooxida-tive light (PhL). Twentymg protein of leaf crude extracts were loaded per lane. (B) Specific activities of plastid GR activity deduced from experiments as those of A. (C) Total GR protein level relative to total soluble protein in leaves treated as in A and deduced from Western blots (not represented) with antibody against the protein. Twenty mg protein were loaded per lane. In B and C, respectively, activities and protein were expressed as percentages of the values in freshly detached 7-day-old leaves or 14-day-old plants (for compari-son seeT0of A). Values are means of at least three different
chloroplastic, mitochondrial and cytosolic GR [22]. If this is also true for barley, the results of Fig. 6 suggest that photooxidative stress could favour an extrachloroplastic over a chloroplastic fate for the gene product. In addition, the ob-served change in isoform pattern (Fig. 6(A)) led to an almost constant total GR protein relative to soluble leaf protein (Fig. 6(C)). However, since soluble proteins were diminished by 100 and 300 nM paraquat (Fig. 1), total leaf GR was consider-ably decreased these treatments.
GR has been proposed [4] to be involved in a way that scavenges H2O2 with ascorbate which is
oxidised to monodehydroascorbate and dehy-droascorbate. Ascorbate is regenerated with NAD(P)H by the successive actions of dehy-droascorbate:glutathione oxidoreductase and GR. However, the ascorbate – glutathione cycle does not seem to be the main scavenging system of active oxygens in chloroplasts under photooxida-tive stress, as suggested by the high instability of the chloroplastic ascorbate peroxidase [23,24] and the decrease of chloroplastic GR (Fig. 6) with comparatively low photooxidative stress. Glu-tathione reductase may also be involved in other processes such as the regulation of the reduced (−SH) state of protein. Cu/Zn SOD, Ndh com-plex and thylakoid peroxidase seem to be involved in the protection against photooxidative stress as they increase in a range of photooxidative condi-tions (Figs. 2, 3 and 5). The results clearly support a route for scavenging reactive oxygen species according to the sequence of reactions catalysed by the NADH dehydrogenase activity of the Ndh complex, peroxidase (acting with reduced plas-toquinone, PQH2, as substrate), SOD and the
non-enzymic one-electron transfer (Mehler reac-tion) from reduced ironsulphur protein (of the photosynthetic electron transport chain) to O2. Of
course, H2O2 could be formed in the cell by other
reactions than that catalysed by SOD.
The responses of Ndh complex and peroxidase to photooxidative stress are similar and, being preferentially induced in aged leaves, they contrast with the response of SOD which is preferentially induced in expanding leaves. The ontogenic stage of aged-senescent leaves could provide some clues to explain this difference. It is possible that going to death, scavenging superoxide anion O2−is not a
priority for senescent leaves, thus they do not increase SOD activity. In contrast, the scavenging
of H2O2 with the combined action of Ndh
com-plex and thylakoid peroxidase, may be important to avoid the induction by H2O2 of genes involved
in defensive responses [25], preventing in turn a useless consumption of metabolic energy in leaves going to death.
Acknowledgements
This work has been supported by the Spanish DGICYT (Grant No. PB96-0675).
References
[1] K. Asada, M. Takahashi, Production and scavenging of active oxygen in photosynthesis, in: D.J. Kyle, C.B. Osmond, C.J. Artzen (Eds.), Photoinhibition, Elsevier, North-Holland, 1987, pp. 227 – 287.
[2] C. Bowler, M. Van Montagu, D. Inze´, Superoxide dis-mutase and stress tolerance, Ann. Rev. Plant Physiol. Plant Mol. Biol. 43 (1992) 83 – 116.
[3] L.M. Casano, M. Martı´n, B. Sabater, Sensitivity of superoxide dismutase transcript levels and activities to oxidative stress is lower in mature-senescent than in young barley leaves, Plant Physiol. 106 (1994) 1033 – 1039.
[4] C.H. Foyer, M. Lelandais, K.J. Kunert, Photooxidative stress in plants, Physiol. Plant. 92 (1994) 696 – 717. [5] G.J. Kelly, E. Latzko, The carbon metabolisms of
un-stressed and un-stressed plants, Prog. Bot. 58 (1997) 187 – 220.
[6] A.S. Gupta, R.P. Webb, A.S. Holaday, R.D. Allen, Overexpression of superoxide dismutase protects plants from oxidative stress, Plant Physiol. 103 (1993) 1067 – 1073.
[7] J.M. Zapata, B. Sabater, M. Martı´n, Identification of a thylakoid peroxidase of barley which oxidizes hy-droquinone, Phytochemistry 48 (1998) 1119 – 1123. [8] M. Martı´n, L.M. Casano, B. Sabater, Identification of
the product ofndhAgene as a thylakoid protein synthe-sized in response to photooxidative treatment, Plant Cell Physiol. 37 (1996) 293 – 298.
[9] R. Catala´, B. Sabater, A. Gue´ra, Expression of the plastid ndhF gene product in photosynthetic and non-photosynthetic tissues of developing barley seedlings, Plant Cell Physiol. 38 (1997) 1382 – 1388.
[10] J. Cuello, M.J. Quiles, M.E. Albacete, B. Sabater, Prop-erties of a large complex with NADH dehydrogenase activity from barley thylakoids, Plant Cell Physiol. 36 (1995) 265 – 271.
[12] N.R. Madamanchi, J.M. Anderson, R.G. Alscher, C.L. Cramer, J.L. Hess, Purification of multiple forms of glutathione reductase from pea (Pisum sati6um L.)
seedling and enzyme levels in ozone-fumigated pea leaves, Plant Physiol. 100 (1992) 138 – 145.
[13] H.R. Lascano, L.D. Go´mez, L.M. Casano, V.S. Trippi, Changes in glutathione reductase activity and protein content in wheat leaves and chloroplasts exposed to photooxidative stress, Plant Physiol. Biochem. 36 (1998) 321 – 329.
[14] G.L. Matters, J.G. Scandalios, Effect of free radical-gen-erating herbicide paraquat on the expression of the su-peroxide dismutase (SOD) genes in maize, Biochem. Biophys. Acta 882 (1986) 29 – 38.
[15] D.R. Kuonen, P.J. Roberts, I.R. Cottingham, Purifica-tion and analysis of mitochondrial membrane proteins on non-denaturing gradient of polyacrylamide gel, Anal. Biochem. 153 (1986) 221 – 226.
[16] L.M. Casano, L.D. Go´mez, H.R. Lascano, C.A. Gonza´-lez, V.S. Trippi, Active oxygen-induced inactivation and degradation of CuZn – SOD in wheat chloroplasts ex-posed to photooxidative stress, Plant Cell Physiol. 38 (1997) 433 – 440.
[17] C.O. Beauchamp, I. Fridovich, Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels, Anal. Biochem. 44 (1971) 276 – 287.
[18] M.M. Bradford, Superoxide dismutase. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye
binding, Anal. Biochem. 72 (1976) 248 – 254.
[19] F.R. Wathley, D.I. Arnon, Photosythetic phosphorila-tion in plants, Methods Enzymol. 6 (1963) 308 – 313. [20] H.K. Lichtenthaler, Chlorophylls and carotenoids:
pig-ments of photosynthetic membranes, Methods Enzymol. 148 (1987) 350 – 382.
[21] J. Kurepa, D. He´rouart, M. Van Montagu, D. Inze´, Differential expression of CuZn- and Fe-superoxide dis-mutase genes of tobacco during development, oxidative stress and hormonal treatments, Plant Cell Physiol. 38 (1997) 463 – 470.
[22] G. Creissen, H. Reynnolds, Y Xue, P. Mullineaux, Simultaneous targeting of pea glutathione reductase and of a bacterial fusion protein to chloroplasts and mito-chondria in transgenic tobacco, Plant J. 8 (1995) 167 – 175.
[23] I. Iturbe-Ormaetxe, P.R. Escudero, C. Arrese-Igor, M. Becana, Oxidative damage in pea plants exposed to water deficit or paraquat, Plant Physiol. 116 (1998) 173 – 181.
[24] T. Shikanai, T. Takeda, H. Yamauchi, S. Sano, K.-I. Tomizawa, A. Yokota, S. Shigeoka, Inhibition of ascor-bate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts, FEBS Lett. 428 (1998) 47 – 51.
[25] S. Karpinski, H. Reynolds, B. Karpinska, G. Wingsle, G. Creissen, P. Mullineaux, Systemic signaling and acclima-tion in response to excess excitaacclima-tion energy in Arabidop-sis, Science 284 (1999) 654 – 657.