www.elsevier.com / locate / bres
Research report
Effects of long-term infusion of anorexic concentrations of islet
amyloid polypeptide on neurotransmitters and neuropeptides in rat
brain
a ,
*
a b dUrban Arnelo
, Margery K. Herrington , Elvar Theodorsson , Thomas E. Adrian ,
d a c a d
¨
¨
Roger Reidelberger , Jorgen Larsson , Jan Marcusson , Lisa Strommer , Xianzhong Ding ,
aJohan Permert
a
Arvid Wretlind Laboratory for Metabolic Research, Department of Surgery, Karolinska Institutet at Huddinge University Hospital,
S-14186 Stockholm, Sweden
b
¨ ¨
Department of Clinical Chemistry, University Hospital, Linkoping University, S-58185, Linkoping, Sweden
c
¨ ¨
Department of Geriatric Medicine, University Hospital, Linkoping University, S-58185 Linkoping, Sweden
d
Department of Biomedical Sciences, Creighton University School of Medicine, Omaha, NE 68178, USA Accepted 3 October 2000
Abstract
Islet amyloid polypeptide (IAPP or amylin) potently reduces food intake in rats at or near physiological concentrations. Although the mechanisms of action of IAPP are not understood, the brain is a suggested site. Changes in hypothalamic and striatal neurotransmission have been reported following acute systemic administration of a pharmacological concentration of IAPP. In the current study, we evaluated the effects of chronic administration of low doses of IAPP on satiety-related neurotransmitters and neuropeptides in the hypothalamus, hippocampus, striatum, left cortex, and right cortex of the rat. Doses of 0, 5 and 25 pmol IAPP/ kg–min were administered subcutaneously for 2 or 5 days. Food intake was reduced by 27 and 44% (both P,0.001) for the 5 and 25 pmol / kg–min groups, respectively, in the 2-day experiment and was decreased by 14% (P,0.01) and 24% (P,0.001), respectively, in the 5-day experiment. Body weight was significantly decreased in a dose-dependent fashion. In the 2-day experiment, norepinephrine increased in the hypothalamus in the 5 pmol IAPP/ kg–min group, and neurotensin increased in the hippocampus in the 25 pmol / kg–min rats (both P,0.05). In the 5-day, 5 pmol / kg–min rats, 5-hydroxyindoleacetic acid (5-HIAA) increased in the hypothalmus and cholecystokinin (CCK) increased in the striatum (both P,0.05). In the 5-day, 25 pmol / kg–min group, neuropeptide Y (NPY) increased in the hypothalamus (P,0.01) and CCK increased in the hypothalmus and striatum (both P,0.05). The present study confirms that IAPP is a potent anorectic peptide at low doses and suggests that IAPP not only affects classical neurotransmitters in the brain but also alters concentrations of neuropeptides known to be involved in food intake. 2000 Elsevier Science B.V. All rights reserved.
Theme: Endocrine and autonomic regulation
Topic: Gastrointestinal and urogenital regulation
Keywords: Brain; Islet amyloid polypeptide; Monoamine; Neuropeptide; Satiety; Body weight
1. Introduction into the circulation together with insulin following food
ingestion [5,10]. Although IAPP has been demonstrated to Islet amyloid polypeptide (IAPP) is a 37-amino-acid exert several biological effects, including hypocalcemia peptide produced by the b-cells of the pancreatic islets [41], inhibition of gastric emptying [42], and impairment [40]. Under normal conditions, the peptide is co-released of glucose metabolism [21,36], a physiologic role for the peptide has not been established. More recently, IAPP has been shown to be a potent inhibitor of feeding in experi-*Corresponding author. Tel.: 146-8-5858-0000; fax: 1
46-8-5858-ments using a number of different models and modes of 3850.
E-mail address: [email protected] (U. Arnelo). treatment [2–6,9,11,12,22–24,26–28]. Because inhibition 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
has been seen with plasma levels of IAPP that are at or min, n510; 5 pmol / kg–min, n59; 25 pmol / kg–min, very close to physiological concentrations, it has been n59) and the other rats were infused for 5 days (IAPP suggested that IAPP may play a hormonal role in the dose: 0 pmol / kg–min, n59; 5 pmol / kg–min, n59; 25 control of food intake. pmol / kg–min, n59). After 2 or 5 days of treatment, the Although the mechanisms for an endocrine role of the animals were anesthetized again, and a blood sample was peptide are poorly understood, experimental data suggests taken from the abdominal aorta for measurement of blood that the central nervous system is a likely target. There is glucose, plasma IAPP, and plasma insulin. The animals evidence that IAPP can cross the blood brain barrier [7], were then decapitated with a guillotine and the brains suggesting that the IAPP in the brain enters from the removed and dissected on ice.
circulation. Brain administration appears to be more potent
than systemic administration for reducing food intake 2.2. Animals and housing conditions [3,31]. High-affinity binding sites for IAPP have been
demonstrated in scattered areas within the brain, including A total of 55 male Wistar rats (B&K Universal Ltd., parts of the hypothalamus [35]. IAPP-induced anorexia has Sollentuna, Sweden) were individually housed in plastic been observed following injections of pharmacological shoebox cages on aspen-chip bedding (Macrolon [3, doses, administered either intrahypothalamically in rats Scanbur, Køge, Denmark). Water and standard rodent [6,12] or intracerebroventricularly in rats [9,23,31] or mice pelleted chow (R36, Lactamin, Vadstena, Sweden) were [26]. In addition, intrahypothalamic injections of IAPP available ad libitum throughout the experiments. The have been shown to inhibit feeding induced by neuro- humidity of the air was maintained between 55 and 60%, peptide-Y (NPY) in rats [6]. and the temperature was kept at 22618C. The room had a Intrahypothalamic injections of IAPP have been reported 12-h light and dark cycle, with lights out at 1800. The to cause neurochemical alterations in the rat brain, with animals were weighed each day, and the daily chow intake increases in dopamine and serotonin neurotransmission was recorded after being corrected for spillage. The seen in the hypothalamus and corpus striatum [12]. Sys- experiment was approved by the Animal Research
Com-¨ temic administration of IAPP as a single bolus injection mittee for Southern Stockholm (Stockholms Sodra
¨ ¨ ¨
increased concentrations of serotonin in the hypothalamus Forsoksdjursetiska Namnd). Rats were handled according and decreased levels of the dopamine metabolite 3- to guidelines established by the National Board for Labora-methoxytyramine in the corpus striatum [11]. However, tory Animals (CFN) in Sweden.
changes in neuropeptides known to be involved in the
regulation of feeding have not been studied following 2.3. IAPP preparation and osmotic mini-pump infusion IAPP administration.
We have previously reported markedly elevated plasma The effects of IAPP were studied after 2 days of IAPP concentrations in patients with pancreatic cancer, a infusion, when peak inhibition of food intake was expected disease often associated with anorexia [30]. We have also and at 5 days, when the anorectic effects were expected to demonstrated that chronic IAPP infusion in rats, at a dose have diminished [2,3]. The Alzet (Alza Corp., Palo Alto, producing plasma levels similar to those of pancreatic CA) model 1003D mini-pump was used for the 2-day cancer patients, causes a reduction of food intake but has infusions and model 2001 was used for the 5-day infu-no effect on glucose metabolism [3–5]. However, these sions. Synthetic rat IAPP (Multiple Peptide Systems, San experiments did not investigate the mechanisms respon- Diego, CA) was dissolved in 75% DMSO / 25% saline sible for the anorectic effects of IAPP. (0.15 M). The osmotic mini-pumps were implanted 5 cm The aim of the present study was, therefore, to use doses behind the base of the skull and just to the right of the of IAPP that were anorectic but not diabetogenic to midline under aseptic surgical conditions, following induc-investigate the effects of chronically-elevated plasma tion of anesthesia with ketamine, 40 mg / kg IP (Ketalar 50 levels of IAPP on neurotransmitters and neuropeptides in mg / ml, Park-Davis, Barcelona, Spain). As soon as the
rat brain. animals awoke, they were placed in their regular cages and
had free access to food and water.
2. Materials and methods 2.4. Blood sampling and brain dissection
2.1. Study design After 2 or 5 days of treatment, the animals were
re-anesthetized with pentobarbital (Pentobarbitalnatrium, ˚
measured using a biochemical analyzer (YSI Model 2700 2.8. Statistical analyses select, Yellow Springs Instruments, Yellow Springs, OH).
After the rats were decapitated, the brains were rapidly The effects of IAPP infusion on change in body weight removed and dissected on ice. The hypothalamus, hip- were evaluated by one-way analysis of variance (ANOVA). pocampus, striatum, left cortex, and right cortex were snap Planned comparisons of mean body weight change at the 5 frozen in liquid nitrogen. Both plasma and brain tissues and 25 pmol / kg–min doses with the mean levels for the were stored frozen at 2808C until subsequent extraction controls were evaluated by direct contrasts of means using and radioimmunoassay or detection of biogenic amines by the statistical program SYSTAT. Effects of IAPP on daily high-pressure liquid chromatography (HPLC). food intakes were determined by separate repeated mea-sures ANOVA with IAPP dose as the between-group factor and day of infusion as the within-group factor. Again, 2.5. Extraction and radioimmunoassay of IAPP and
planned comparisons were evaluated by direct contrasts of insulin
means using the statistical program SYSTAT. Effects of IAPP on other parameters were analyzed similarly using a The extraction and radioimmunoassay procedure
previ-2 factor ANOVA, with the duration of infusion and dose of ously described in detail [30] was modified to include
IAPP as the two factors. Planned contrasts were used to acidification of plasma samples with acetic acid (final
determine the effect of low and high doses of IAPP acetic acid concentration 0.25 M) and centrifugation to
compared with the 0 dose at for the 2 or 5 day infusion, remove precipitated proteins before extraction of IAPP and
using the statistical program SYSTAT. insulin from the supernatant on Sep-Pak, C-18
reverse-For each analysis, the difference was considered signifi-phase cartridges (Waters, Milford, MA.). The eluates were
cant if P,0.05. Values are expressed as means6S.E.M. lyophilized and resuspended with 800ml assay buffer (10
mM KH PO , 60 mM Na HPO2 4 2 4,10 mM EDTA, 7.6 mM sodium azide and 0.3% bovine serum albumin) for
3. Results
radioimmunoassay [30] Insulin concentrations were mea-sured using a rat insulin radioimmunoassay kit from Linco
3.1. Effects of IAPP on food intake and body weight Research, Inc. (St. Charles, MO). Recovery after
extrac-tion was measured for both peptides, and the results were
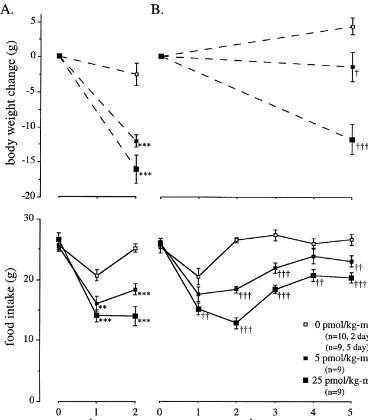
The lower panels in Fig. 1 show the effects of IAPP on corrected accordingly.
daily food intake during the 2-day (Fig. 1A), and 5-day (Fig. 1B) treatment periods. On the day before insertion of 2.6. Extraction of brain tissue samples and measurement the osmotic mini-pumps, daily food intake was similar in of biogenic amines by HPLC all groups. IAPP significantly decreased food intake
dose-dependently during the 2-day and the 5-day treatment The frozen tissue samples were minced, boiled for 10 periods. The 24 h food intake during the second day in the min in ten volumes of 1 M acetic acid. N-methyl-5- 2-day treated animals was reduced by 27% in the 5 hydroxytryptamine (NM-5HT) and 3,4-dihydroxybenzyl- pmol / kg–min group, from a control value of 25.262.3 g amine (DHBA), the internal standards for serotonin and to 18.462.6 g (P,0.001) and by 44% to 14.064.7 g in the catecholamines, respectively, were added. The samples 25 pmol / kg–min group (P,0.001). Likewise, food intake were then homogenized, centrifuged and the supernatants during the fifth day in the rats receiving IAPP for 5 days lyophilized and stored at2208C until subsequent analysis. was reduced by 14% in the 5 pmol / kg–min group, from a The samples were reconstituted in 1 ml phosphate buffer control value of 26.662.7 g to 23.062.6 g (P,0.01), and (0.05 M, pH 7.4). 0.6 ml was mixed with 4.4 ml 10 mM by 24% to 20.362.4 g (P,0.001) in the 25 pmol / kg–min HCl and further purified on a cation-exchange column group.
before being analyzed for norepinephrine (NE) and dopa- At the time of osmotic mini-pump insertion, there were mine (DA) on a HPLC system, using a reverse-phase no significant differences in body weight among the groups column under isocratic conditions and an electrochemical receiving treatment for 2 days (35863, 36162, and detector, as previously described [37]. 35863 g for the groups receiving 0, 5, and 25 pmol / kg–
Fig. 1. Effects of 2-day (A), and 5-day (B) infusions of islet amyloid polypeptide (0, 5, and 25 pmol / kg–min) on daily food intake (bottom) and body weight change (top) in ad libitum fed rats. Day0 denotes the day before insertion of the osmotic mini-pump. Values are means6S.E.M. **5P,0.01, ***5P,0.001 vs. 0 pmol / kg–min in the 2-day group; †5P,0.05, ††5P,0.01, †††5P,0.001 vs. 0 in the 5-day group.
P,0.001). The dose of 25 pmol / kg–min caused an even tween the study groups in the 2-day experiment. However, greater weight loss of 16.062.0 g (P,0.001). In the 5-day in the 5-day study, the IAPP-treated rats had lower plasma experiment, the weight loss of 1.362.1 g in the 5 pmol / insulin levels than the controls.
kg–min group and 11.762.1 g in the 25 pmol / kg–min
group were significantly different from the 4.461.2 g 3.3. Effects of IAPP on neurotransmitters and weight gain of the control group (P,0.05 and P,0.001, neuropeptides
respectively).
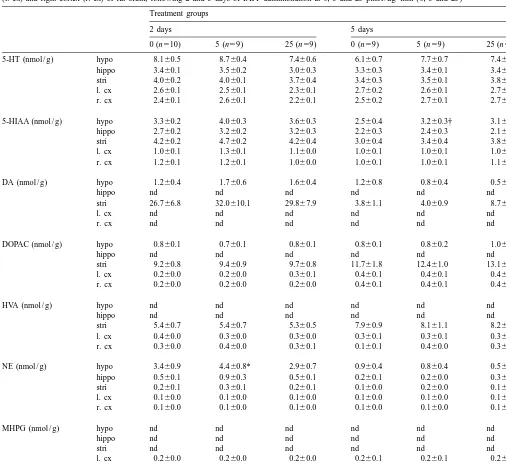
As shown in Table 2, IAPP increased NE in the 3.2. Effects of IAPP on blood glucose, plasma IAPP, hypothalamus in the 2-day, 5 pmol / kg–min group and and insulin levels 5-HIAA in the hypothalamus in the 5-day, 5 pmol / kg–min group compared to the control groups at the respective Table 1 shows that plasma IAPP concentrations were time points (both P,0.05).
hypo-Table 1
Concentrations of blood glucose, plasma IAPP, and plasma insulin, following 2 and 5 days of IAPP administration at 0, 5 and 25 pmol / kg-min (0, 5, and
a
25)
Treatment groups
2 days 5 days
0 (n510) 5 (n59) 25 (n59) 0 (n59) 5 (n59) 25 (n59) Blood glucose (mmol / l) 10.960.2 10.260.4 9.960.5 10.160.3 9.760.4 9.860.5 Plasma IAPP (pmol / l) 7.262.0 64.465.0* 198.0634.5*** 12.863.1 48.263.5 160.5617.5††† Plasma insulin (pmol / l) 209.2633.4 183.3626.5 175.4626.9 291.3635.9 163.0615.5†† 147.0621.3†††
a
Values are means6S.E.M. *5P,0.05, ***5P,0.001vs. 0 in the 2-day group; ††5P,0.01, †††5P,0.001vs. 0 in the 5-day group.
Table 2
Concentrations of serotonin (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), and 3-methoxy-4-hydroxyphenylglycol (MHPG) in the hypothalamus (hypo), hippocampus (hippo), striatum (stri), left cortex
a
(l. cx) and right cortex (r. cx) of rat brain, following 2 and 5 days of IAPP administration at 0, 5 and 25 pmol / kg–min (0, 5 and 25) Treatment groups
2 days 5 days
0 (n510) 5 (n59) 25 (n59) 0 (n59) 5 (n59) 25 (n59) 5-HT (nmol / g) hypo 8.160.5 8.760.4 7.460.6 6.160.7 7.760.7 7.460.2 hippo 3.460.1 3.560.2 3.060.3 3.360.3 3.460.1 3.460.2 stri 4.060.2 4.060.1 3.760.4 3.460.3 3.560.1 3.860.2 l. cx 2.660.1 2.560.1 2.360.1 2.760.2 2.660.1 2.760.1 r. cx 2.460.1 2.660.1 2.260.1 2.560.2 2.760.1 2.760.1 5-HIAA (nmol / g) hypo 3.360.2 4.060.3 3.660.3 2.560.4 3.260.3† 3.160.3 hippo 2.760.2 3.260.2 3.260.3 2.260.3 2.460.3 2.160.2 stri 4.260.2 4.760.2 4.260.4 3.060.4 3.460.4 3.860.6 l. cx 1.060.1 1.360.1 1.160.0 1.060.1 1.060.1 1.060.1 r. cx 1.260.1 1.260.1 1.060.0 1.060.1 1.060.1 1.160.1 DA (nmol / g) hypo 1.260.4 1.760.6 1.660.4 1.260.8 0.860.4 0.560.2
hippo nd nd nd nd nd nd
stri 26.766.8 32.0610.1 29.867.9 3.861.1 4.060.9 8.760.6
l. cx nd nd nd nd nd nd
r. cx nd nd nd nd nd nd
DOPAC (nmol / g) hypo 0.860.1 0.760.1 0.860.1 0.860.1 0.860.2 1.060.1
hippo nd nd nd nd nd nd
stri 9.260.8 9.460.9 9.760.8 11.761.8 12.461.0 13.161.0 l. cx 0.260.0 0.260.0 0.360.1 0.460.1 0.460.1 0.460.1 r. cx 0.260.0 0.260.0 0.260.0 0.460.1 0.460.1 0.460.1
HVA (nmol / g) hypo nd nd nd nd nd nd
hippo nd nd nd nd nd nd
stri 5.460.7 5.460.7 5.360.5 7.960.9 8.161.1 8.260.9 l. cx 0.460.0 0.360.0 0.360.0 0.360.1 0.360.1 0.360.1 r. cx 0.360.0 0.460.0 0.360.1 0.160.1 0.460.0 0.360.1 NE (nmol / g) hypo 3.460.9 4.460.8* 2.960.7 0.960.4 0.860.4 0.560.0 hippo 0.560.1 0.960.3 0.560.1 0.260.1 0.260.0 0.360.1 stri 0.260.1 0.360.1 0.260.1 0.160.0 0.260.0 0.160.0 l. cx 0.160.0 0.160.0 0.160.0 0.160.0 0.160.0 0.160.0 r. cx 0.160.0 0.160.0 0.160.0 0.160.0 0.160.0 0.160.0
MHPG (nmol / g) hypo nd nd nd nd nd nd
hippo nd nd nd nd nd nd
stri nd nd nd nd nd nd
l. cx 0.260.0 0.260.0 0.260.0 0.260.1 0.260.1 0.260.1 r. cx 0.260.0 0.260.0 0.360.1 0.260.1 0.260.1 0.260.1
a
Table 3
Concentrations of neuropeptide-Y (NPY), neurotensin (NT), and cholecystokinin (CCK) in the hypothalamus (hypo), hippocampus (hippo), striatum (stri),
a
left cortex (l. cx) and right cortex (r. cx) of rat brain, following 2 and 5 days of IAPP administration at 0, 5 and 25 pmol / kg–min (0, 5 and 25) Treatment groups
2 days 5 days
0 (n510) 5 (n59) 25 (n59) 0 (n59) 5 (n59) 25 (n59) NPY (pmol / g) hypo 91.267.1 104.866.5 101.865.5 74.865.8 90.467.1 105.869.6††
hippo 31.061.4 32.461.9 32.161.8 24.562.6 25.862.7 27.163.9 stri 37.762.7 36.662.5 41.062.2 33.864.0 35.061.4 35.964.9 l. cx 24.761.2 22.961.7 28.561.8 24.161.3 23.262.9 25.063.5 r. cx 27.261.7 26.861.8 28.561.4 21.861.8 23.362.2 20.360.8 NT (pmol / g) hypo 80.264.2 79.066.2 77.762.8 63.166.3 70.067.2 74.664.5 hippo 8.860.5 10.060.4 10.360.5* 7.460.9 7.260.6 7.360.7 stri 5.460.4 5.060.3 6.960.5 4.060.3 4.560.5 5.260.6 l. cx 2.060.2 1.960.1 1.860.2 1.860.1 1.960.2 1.860.2 r. cx 1.960.1 1.860.1 1.860.2 1.960.1 1.960.2 1.860.2 CCK (pmol / g) hypo 93.066.0 90.066.2 88.366.2 68.567.6 68.866.1 87.964.2†
hippo 80.769.3 95.065.5 103.664.6 88.9611.2 101.967.4 103.669.6 stri 84.7612.3 106.3610.1 120.8614.2 64.9612.6 105.266.9† 108.2614.1† l. cx 88.8614.2 74.767.0 87.669.8 80.4613.8 81.068.6 88.1610.8 r. cx 73.0611.4 78.667.6 86.368.4 73.767.5 75.8611.2 96.9616.2
a
Values are means6S.E.M. *5P,0.05 vs. 0 in the 2-day group. †5P,0.05, ††5P,0.001 vs. 0 in the 5-day group.
thalamus and striatum (both P,0.05) and NPY in the would stimulate the release of more insulin and IAPP into hypothalamus (P,0.01). The 5-day, 5 pmol / kg–min the circulation.
group also had an increase in CCK in the striatum (P, Insulin levels were reduced after 5 days of IAPP 0.05). In the 2-day, 25 pmol / kg–min group, NT was infusion but not at 2 days. This is likely to be a result of increased in the hippocampus compared to the 2-day the prolonged reduction in food intake, since loss of body control group (P,0.05). weight is associated with lower insulin levels [15]. Alter-natively, the reduction in insulin concentrations could reflect a direct inhibition of food-stimulated insulin secre-tion by IAPP. Although the effect of IAPP on b-cell
4. Discussion function is controversial, a number of studies have
re-ported inhibition of insulin secretion by IAPP [19]. In a IAPP is a potent anorectic agent, but the mechanism for previous experiment with low doses of IAPP, we measured inhibition of food intake is not clear. We have previously insulin levels in fasted rather than ad libitum-fed animals shown that chronic IAPP infusion in rats, at doses produc- and saw no differences in insulin between the IAPP-treated ing plasma levels similar to those of pancreatic cancer and control groups [4].
patients, causes a reduction of food intake but has no effect In the present experiment, 5-HIAA, a metabolite of on glucose metabolism [3–5]. The current study investi- 5-HT, was modestly increased in the hypothalamus in the gated the effects of chronic, systemic administration of 5-day, 5 pmol / kg–min treated group (P,0.05). This is these low doses of IAPP on neurotransmitters and neuro- consistent with data from previously published experi-peptides in the rat brain. Circulating levels of glucose, ments reporting increases in 5-HT in the hypothalamus of insulin and IAPP were also measured. Significant inhibi- rats after IV administration of 25 nmol / kg IAPP [11] and tion of food intake was seen throughout the experimental increases in 5-HIAA in the striatum and hypothalamus of periods, demonstrating that the peptide was still exerting a food-restricted rats that lost weight [13].
[11]. There were differences in content between days 2 and reflecting compensatory responses to chronically reduced 5 in all groups of animals; DA content in the striatum and food intake or loss of body weight. Further studies are NE content in the hypothalamus were higher at 2 days than needed to identify localized changes in transmitter con-at 5 days. The higher values con-at 2 days may reflect effects centrations and to clarify the pathways through which of anesthesia or surgical stress [20,29]. IAPP causes anorexia and loss of body weight.
Significant increases in CCK were seen in the striatum and hypothalamus of rats receiving IAPP infusions for 5 days. CCK is a prominent neuropeptide that is produced in
Acknowledgements
these regions [33,34]. These results suggest that IAPP-induced anorexia may be mediated in part by the release
The authors acknowledge Dr. Feng Wang for valuable and action of endogenous CCK in or near these brain sites.
support in preparation of the manuscript, and Lise-Lotte This idea is further supported by studies showing that
Jahrl for skillful technical assistance. This work was exogenous CCK can inhibit food intake when administered
supported by grants from the Swedish Cancer Society at low doses directly into several sites within the
medial-¨
(3450-B95-03XCC and 2870-B96-06XAC), Forenade Liv basal hypothalamus [8,14]. Testing of this hypothesis
Mutual Group Life Insurance Company (311801), the would require injecting CCK into the hypothalamus or
Swedish Society for Medical Research (970023), and the striatum, determining whether food intake is inhibited, and
Nebraska Cancer and Smoking-Related Disease Program then seeing if a dose of CCK receptor antagonist that
(LB595). blocks the effect of the CCK injections reverses the
anorexic effects of peripheral administration of IAPP. Future studies to identify the neurons involved in the
IAPP-induced changes in CCK content would be valuable. References
NPY, a polypeptide that potently stimulates food intake
[17], was increased in the hypothalamus in the 5-day, 25 [1] A. Alwark, B. Ahren, Phentolamine reverses NPY-induced inhibi-tion of insulin secreinhibi-tion in isolated rat islets, Eur. J. Pharmacol. 135 pmol / kg–min group (P,0.01). This increase in NPY
(1987) 307–311. shows that IAPP, unlike leptin [25], does not produce
[2] U. Arnelo, J.E. Blevins, J. Larsson, J. Permert, P. Westermark, R.D. anorexia by inhibiting NPY. The increase in NPY is likely
Reidelberger, T.E. Adrian, Effects of acute and chronic infusion of to reflect a counter-regulatory mechanism that compensates islet amyloid polypeptide on food intake in rats, Scand. J. Gastroen-for the anorectic effects of IAPP. This indirect effect of terol. 31 (1996) 83–89.
[3] U. Arnelo, J. Permert, T.E. Adrian, J. Larsson, P. Westermark, R.D. IAPP could be confirmed by pair-feeding experiments.
Reidelberger, Chronic infusion of islet amyloid polypeptide causes Fasting has been shown to increase NPY content [32] and
anorexia in rats, Am. J. Physiol. 271 (1996) R1654–R1659. mRNA [16]. The increase in NPY concentrations seen at 5
[4] U. Arnelo, J. Permert, J. Larsson, R. Reidelberger, C. Arnelo, T.E. days but not at 2 days in the 25 pmol / kg–min rats might Adrian, Chronic low dose islet amyloid polypeptide infusion reduces also be associated with the lower insulin levels in the food intake, but does not influence glucose metabolism, in unre-strained conscious rats: Studies using a novel aortic catheterization 5-day animals, since interactions between NPY and insulin
technique, Endocrinology 138 (1997) 4081–4085. have been reported [1]. As noted above for CCK,
identifi-[5] U. Arnelo, R.D. Reidelberger, T.E. Adrian, J. Larsson, J. Permert, cation of the neurons involved in the IAPP-induced
Sufficiency of postprandial plasma levels of islet amyloid poly-changes in NPY content would be interesting. peptide for suppression of feeding in rats, Am. J. Physiol. 275
The only change in neurotensin content seen was a very (1998) R1537–R1542.
small increase induced by the higher infusion dose of IAPP [6] A. Balasubramaniam, V. Renugopalakrishnan, M. Stein, J.E. Fischer, W.T. Chance, Syntheses, structures and anorectic effects of human after 2 days. This is unlikely to be of major biological
and rat amylin, Peptides 12 (1991) 919–924. significance.
[7] W.A. Banks, A.J. Kastin, Differential permeability of the blood– The regulation of food intake and body weight is a brain barrier to two pancreatic peptides: insulin and amylin, Peptides complex process that is likely to involve interactions 19 (1998) 883–889.
between a number of brain regions and nuclei. Recent [8] J.E. Blevins, B.G. Stanley, R.D. Reidelberger, Brain regions where cholecystokinin suppresses feeding in rats, Brain Res. 860 (2000) studies have suggested that the hindbrain is involved in the
1–10. satiety effects of IAPP [24,35]. The present study confirms
[9] S.M. Bouali, S.J. Wimalawansa, F.B. Jolicoeur, In vivo central that IAPP is a potent anorectic peptide at low doses in rats actions of rat amylin, Reg. Pept. 56 (1995) 167–174.
and suggests that IAPP not only affects classical neuro- [10] P.C. Butler, J. Chou, W.B. Carter, Y.N. Wang, B.H. Bu, D. Chang, transmitters in the brain but also alters concentrations of J.K. Chang, R. Rizza, Effects of meal ingestion on plasma amylin concentration in NIDDM and non-diabetic humans, Diabetes 39 neuropeptides known to be involved in food intake. The
(1990) 752–756. changes seen after 2 days of IAPP infusion, at the time of
[11] W.T. Chance, A. Balasubramaniam, A. Stallion, J.E. Fischer, peak inhibition of food intake were modest. In contrast, Anorexia following the systemic injection of amylin, Brain Res. 607 more marked changes in hypothalamic NPY and CCK (1993) 185–188.
J.E. Fischer, Anorexia following the intrahypothalamic administra- food intake in mice: Effects on motivation to eat and mechanism of tion of amylin, Brain Res. 539 (1991) 352–354. action, Pharmacol. Biochem. Behav. 56 (1997) 123–129. [13] W.T. Chance, T. Foley-Nelson, J.L. Nelson, J.E. Fischer, Neuro- [29] T. Ono, Effects of mesocortical dopaminergic lesions on
stress-transmitter alterations associated with feeding and satiety, Brain induced changes in monoamine metabolism in the discrete brain Res. 416 (1987) 228–234. regions of rats, Yakubutsu Seishin Kodo 12 (1992) 93–103. [14] J.N. Crawley, R.L. Corwin, Biological actions of cholecystokinin, [30] J. Permert, J. Larsson, G.T. Westermark, M.K. Herrington, L.
Peptides 15 (1994) 731–755. Christmanson, P.M. Pour, P. Westermark, T.E. Adrian, Islet amyloid [15] D.V. Godin, S.A. Wohaieb, Nutritional deficiency, starvation, and polypeptide in patients with pancreatic cancer patients and diabetes,
tissue antioxidant status, Free Radic. Biol. Med. 5 (1988) 165–176. N. Engl. J. Med. 330 (1994) 313–318.
[16] T.M. Hahn, J.F. Breininger, D.G. Baskin, M.W. Schwartz, Coexpres- [31] P.A. Rushing, M.M. Hagan, R.J. Seeley, T.A. Lutz, S.C. Woods, sion of Agrp and NPY in fasting-activated hypothalamic neurons, Amylin: a novel action in the brain to reduce body weight, Nat. Neurosci. 1 (1998) 271–272. Endocrinology 141 (2000) 850–853.
[17] M.R. Jain, T.L. Horvat, P.S. Kalra, S.P. Kalra, Evidence that NPY [32] A. Sahu, P.S. Kalra, S.P. Kalra, Food deprivation and ingestion Y1 receptors are involved in stimulation of feeding by orexins induce reciprocal changes in neuropeptide Y concentrations in the (hypocretins) in sated rats, Regul. Pept. 87 (2000) 19–24. paraventricular nucleus, Peptides 9 (1988) 83–86.
[18] C.S. Joekel, M.K. Herrington, J.A. Vanderhoof, T.E. Adrian, Post- [33] S.N. Schiffmann, J.J. Vanderhaeghen, Distribution of cells con-natal development of circulating cholecystokinin and secretin, taining mRNA encoding cholecystokinin in the rat central nervous pancreatic growth, and exocrine function in guinea pigs, Int. J. system, J. Comp. Neurol. 304 (1991) 219–233.
Pancreatol. 13 (1993) 1–13. [34] K.B. Seroogy, K. Dangaran, S. Lim, J.W. Haycock, J.H. Fallon, [19] E. Karlsson, IAPP as a regulator of glucose homeostasis and Ventral mesencephalic neurons containing both cholecystokinin- and pancreatic hormone secretion, Int. J. Mol. Med. 3 (1999) 577–584. tyrosine hydroxylase-like immunoreactivities project to forbrain [20] F.G. DeKeyser, R.R. Leker, J Weidenfeld, Activation of the regions, J. Comp. Neurol. 279 (1989) 397–414.
adrenocortical axis by surgical stress: involvement of central norepi- [35] P.M. Sexton, G. Paxinos, M.A. Kenney, P.J. Wookey, K. Beaumont, nephrine and interleukin-1, Neuroimmunomodulation 7 (2000) 182– In vitro autoradiographic localization of amylin binding sites in rat
188. brain, Neuroscience 62 (1994) 553–567.
[21] B. Leighton, G.J.S. Cooper, Pancreatic amylin and calcitonin gene- [36] R. Sowa, T. Sanke, J. Hirayama, H. Tabata, H. Furuta, S. Nishi-related peptide cause resistance to insulin in skeletal muscle in vitro, mura, K. Nanjo, Islet amyloid polypeptide amide causes peripheral Nature 335 (1988) 632–635. insulin resistance in vivo in dogs, Diabetologia 33 (1990) 118–120.
´
[22] T.A. Lutz, T.R. Pieber, B. Walzer, E. Del Prete, E. Scharrer, [37] C. Stenfors, P. Bjellerup, A.A. Mathe, E. Theodorsson, Concurrent Different influence of CGRP (8-37), an amylin and CGRP antago- analysis of neuropeptides and biogenic amines in brain tissue of rats nist, on the anorectic effects of cholecystokinin and bombesin in treated with electroconvulsive stimuli, Brain Res. 698 (1995) 39– diabetic and normal rats, Peptides 18 (1997) 643–649. 45.
´
[23] T.A. Lutz, R. Rossi, J. Althaus, E. Del Prete, E. Scharrer, Amylin [38] E. Theodorsson-Norheim, A. Hemsen, J.M. Lundberg, Radioim-reduces food intake more potently than calcitonin gene-related munoassay for neuropeptide Y (NPY): chromatographic characteri-peptide (CGRP) when injected into the lateral brain ventricle in rats, zation of immunoreactivity in plasma and tissue extracts, Scand. J. Peptides 19 (1998) 1533–1540. Clin. Lab. Invest. 45 (1985) 355–365.
[24] T.A. Lutz, M. Senn, J. Althaus, E. Del Prete, F. Ehrensperger, E. [39] E. Theodorsson-Norheim, S. Rosell, Characterization of human Scharrer, Lesion of the area postrema / nucleus of the solitary tract plasma neurotensin-like immunoreactivity after fat ingestion, Regul. (AP/ NTS) attenuates the anorectic effects of amylin and calcitonin Pept. 6 (1983) 207–218.
gene-related peptide (CGRP) in rats, Peptides 19 (1998) 309–317. [40] P. Westermark, C. Wernstedt, E. Wilander, K. Sletten, A novel [25] J.G. Mercer, K.M. Moar, D.V. Rayner, P. Trayhurn, N. Hoggard, peptide in the calcitonin gene related family as an amyloid fibril Regulation of leptin receptor and NPY gene expression in hypo- protein in the endocrine pancreas, Biochem. Biophys. Res. Com-thalamus of leptin-treated obese (ob / ob) and cold-exposed lean mun. 140 (1986) 827–831.
mice, FEBS Lett. 402 (1997) 185–188. [41] S.J. Wimalawansa, R.D. Gunasekera, H.K. Datta, Hypocalcemic [26] J.E. Morley, J.F. Flood, Amylin decreases food intake in mice, actions of amylin amide in humans, J. Bone Miner. Res. 7 (1992)
Peptides 12 (1991) 865–869. 1113–1116.