www.elsevier.com/locate/ibmb
Dynamic regulation of prothoracic gland ecdysteroidogenesis:
Manduca sexta
recombinant prothoracicotropic hormone and brain
extracts have identical effects
L.I. Gilbert
a,*, R. Rybczynski
a, Q. Song
b, A. Mizoguchi
c, R. Morreale
d, W.A. Smith
d,
H. Matubayashi
e, M. Shionoya
e, S. Nagata
e, H. Kataoka
eaDepartment of Biology, Campus Box #3280, Coker Hall, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3280, USA bDepartment of Entomology, University of Missouri, Columbia, MO 65211, USA
cDivision of Biological Science, Graduate School of Science, Nagoya University, Chikusa-ku, Nagoya 464-8602, Japan dDepartment of Biology, Mugar Hall, Northeastern University, Boston, MA 02115, USA
eDepartment of Biotechnology, Graduate School of Agricultural and Life Science, The University of Tokyo, Yayoi, Bunkyo-ku, Tokyo 113,
Japan
Received 5 January 2000; received in revised form 15 March 2000; accepted 29 March 2000
Abstract
Multiple assays were conducted in order to determine if the recently available recombinant prothoracicotropic hormone (rPTTH) from Manduca sextais identical, or similar, to the natural hormone and if results from its use in a variety of assays confirm, or are inconsistent with, previous studies over the past 20 years on PTTH action using brain extract. Brain extracts and rPTTH showed similar, if not identical, effects on the cell biology ofManducaprothoracic gland cells with the following results: increased levels of cAMP (adenosine 39:59cyclic monophosphate) synthesis; requirement for extracellular Ca2+in in vitro studies; ecdysteroidogenesis
stimulation in vitro; stimulation of general and specific protein synthesis; immunocytochemical identification of the two lateral cells in each brain hemisphere as the source of PTTH (the prothoracicotropes); the ability of antibodies to rPTTH to inhibit ecdysteroido-genesis stimulation in vitro; and the multiple phosphorylation of the ribosomal protein S6. The data revealed that brain extract and rPTTH show equivalent effects in all of the assays, indicating that this rPTTH is the natural PTTH of Manducaand that the data generated with brain extracts over the past two decades are indeed relevant. 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Ecdysteroidogenesis, control of; S6 phosphorylation; Cyclic AMP, stimulation of; Molting, control of; Brain hormone
1. Introduction
Research on the mechanism(s) by which protho-racicotropic hormone (PTTH) stimulates ecdysteroidog-enesis began 27 years ago when Vedeckis and his col-leagues demonstrated a correlation between prothoracic gland cAMP titer and ecdysteroid biosynthesis in lepi-dopteran prothoracic glands (Vedeckis et al. 1974, 1976). A critical event for these and further studies was the development of the ecdysteroid radioimmunoassay (Borst and O’Connor, 1972). The research of Gibbs and
* Corresponding author. Tel.:+1-919-966-2055; fax: + 1-919-962-1344.
E-mail address:[email protected] (L.I. Gilbert).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 8 3 - 7
Riddiford (1977) showed that brain extracts could affect ecdysteroidogenesis in vivo, and that lateral regions of the brain possessed the PTTH activity. The second criti-cal event was the development of an in vitro assay that took advantage of the finding that each of the two
pro-thoracic glands in Manduca sexta synthesized and
secreted ecdysteroid at the same rate. Thus, a
quantitat-ive activation ratio (Ar) could be calculated and the
mechanism of PTTH action could be investigated in a rigorous manner (Bollenbacher et al., 1979). This led to the identification of the site of PTTH synthesis as two lateral cells in each brain hemisphere (Agui et al., 1979). The in vitro assay was, therefore, the basis for studies investigating the control of ecdysteroidogenesis.
included a requirement for extracellular Ca2+(Smith et al., 1985), increased activity of an adenylate cyclase
(Smith et al., 1984) now known to be a Ca2+-calmodulin
activated adenylate cyclase (Meller et al. 1988, 1990), and the subsequent activation of the cAMP protein kin-ase (PKA) (Smith et al., 1986; see review by Gilbert et al., 1988). Further studies revealed that the final step in the cascade ended with the multiple phosphorylation of the ribosomal protein S6 mediated by PKA and S6 kin-ase (Song and Gilbert, 1995; see review by Henrich et al., 1999).
The fourth critical event was the availability of
Mand-uca recombinant PTTH (rPTTH) produced by the
Kataoka laboratory (M. Shionoya, H. Matsubayashi, S. Nagata, H. Kuniyoshi, M. Asahina, L.M. Riddiford and H. Kataoka, in preparation). This was of great impor-tance for obvious reasons. In addition to being a probe for the study of the PTTH receptor, the utilization of rPTTH in the in vitro system could establish whether it was PTTH or one or more other molecules in brain extracts that elicited the transductory cascade and increased ecdysteroidogenesis in the prothoracic gland. This was important in order to establish, or cast doubt on, the conclusion that PTTH is the active component in the crude and semi-pure brain extracts utilized in the studies summarized above. Indeed, the use of crude extracts was viewed quite negatively by at least one granting agency and even led to negative reviews of
manuscripts. Thus, the availability of Manduca rPTTH
was vital to corroborate (or not) 20 years of research on PTTH action as well as for future studies. For these reasons we decided to compare the action of rPTTH and brain extracts using a variety of critical parameters, and present the data here.
2. Materials and methods
2.1. Animals
Since the actual experiments described herein using live Manduca larvae were conducted in two different laboratories (Gilbert, Smith), there were slight variations
in the raising of theManducalarvae. In all cases animals
were timed precisely and the slight insect-rearing vari-ations had no effect on the experimental data, so that one can make relative comparisons using animals from both laboratories.
In the Gilbert and Smith laboratories,Manduca were
reared on an artificial diet at 25°C, .60% relative
humidity and under a photoperiod of 16 h light/8 h dark with 2400 h Arbitrary Zeitgeber Time (lights off) set at 2200 h Eastern Standard Time. A synchronous popu-lation of animals was selected by routinely staging on days 0 of the third larval instar, fifth larval instar, and at pupation.
2.2. Immunocytochemistry
Rabbit antiserum against recombinant Manduca
PTTH (rPTTH) was generated by conventional methods with rPTTH as an immunogen. The antiserum obtained was mixed with the same volume of saturated ammonium sulfate solution, and after centrifugation the precipitate was dissolved in 0.1 M Tris–HCl buffer, pH 8.0. The IgG (antibody) in the solution was purified using a protein-A-conjugated column (HiTrap Protein A, Pharmacia).
Whole mount immunocytochemistry was performed by a modification of the method of O’Brien et al. (1988). Heads of day 0 pupae and day 3 fifth-instar larvae were fixed in aqueous Bouin’s solution for 2 h, followed by thorough washing with 50 mM of phosphate-buffered saline (PBS; pH 7.4) containing 0.1% sodium azide. The brain and retrocerebral complexes (brain, corpora car-diaca, corpora allata: Br–CC–CA) were dissected in the same buffer and the neural sheath was partially removed.
The Br–CC–CA were stored at 4°C in PBS containing
2% Triton X-100 and 0.1% sodium azide (PBS–Triton)
for 1 day and then incubated overnight at 4°C with
anti-rPTTH rabbit antibody in PBS–Triton (2µg/ml). The
specimens were washed three times with PBS containing 0.05% Tween-20 (PBS–Tween) followed by a 2 h incu-bation at room temperature with horseradish peroxidase
(HRP)-conjugated sheep anti-rabbit IgG antibody
(Boehringer Mannheim) and diluted 1:200 with PBS– Triton without sodium azide. The specimens were washed three times with PBS–Tween and then incubated for 10 min with 50 mM Tris-buffered saline, pH 7.6,
containing 1.3 mM 3,39-diaminobenzadine
tetrahydroch-loride and 0.02% H2O2. Following three washes in PBS
and one in distilled water, the tissues were dehydrated through graded ethanol and cleared with methyl salicy-late.
Sections of Br–CC–CA were immunostained by mod-ifying the method described previously (Mizoguchi et al., 1987). Heads of day 3 fifth-instar larvae were fixed in aqueous Bouin’s solution for 4 h followed by washing with 70% ethanol. The Br–CC–CA were dissected out in 70% ethanol and dehydrated through graded ethanol,
embedded in paraffin, and sectioned at 7µm. Specimens
were incubated first with anti-rPTTH rabbit antibody
1µg/ml for 3 h and then with 1:500 diluted
HRP-conju-gated second antibody for 1.5 h. As a substrate for HRP, 3-amidino-9-ethylcorbazole was used. The immunos-tained specimens were countersimmunos-tained with Mayer’s hematoxylin solution. All incubations were performed at room temperature.
2.3. Measurement of cAMP
pre-viously (Smith et al., 1984). To measure cAMP, glands were transferred to standing droplets of Grace’s medium (0.03 ml), with or without rPTTH or brain extract, for 5 min at room temperature. In all cases, matched pairs of glands from the same animal were used to compare experimental and control treatments. After 5 min, glands were flash-frozen on dry ice. Cyclic AMP was extracted and measured with the use of cAMP Enzyme Immunoas-say Kit (BIOMOL Res. Lab., Plymouth Meeting, PA). Briefly, cyclic AMP was extracted by addition of 0.21 ml of 0.1 M HCl, followed by vigorous vortexing. Standards and samples (0.1 ml) were acetylated for increased sensitivity, and the cAMP content determined according to kit instructions.
Standard and phosphate-free Grace’s insect culture media were obtained from Gibco (Grand Island, NY).
Carrier-free [32P]O
4 (10 mCi/ml) was from Amersham.
Brain extract containing only big PTTH was prepared as described previously (Song and Gilbert, 1995).
2.4. Stimulation of ecdysteroidogenesis
For dose–response experiments, the prothoracic glands from day 6 last-instar (V6) larvae were dissected under lepidopteran saline at room temperature. Individ-ual glands were placed in cell culture wells (16 mm
diameter, 2 ml maximum volume) containing 50µl of
Grace’s insect tissue culture medium for at least 30 min at room temperature. The medium was then carefully
removed and 50µl of fresh medium containing the
indi-cated doses of rPTTH or brain extract was added to each well. After incubation for 1 h, the medium was collected for ecdysteroid quantification by radioimmunoassay (RIA) (Warren and Gilbert 1986, 1988).
For the time-course study, prothoracic glands from V3
and V6 larvae were incubated with 0.25 ng/gland of
rPTTH or 0.25 brain equivalent/gland of brain extract (the predetermined doses from the dose–response study described above) for the indicated period of time. The medium was then collected for ecdysteroid quantification by RIA. For developmental comparisons, prothoracic glands (P0) were also dissected and preincubated as above. The preincubation medium was replaced with
either 25µl of Grace’s medium or with 25µl of Grace’s
medium containing either 0.1 ng or 1.0 ng rPTTH. After
a 90 min incubation, 20µl of the medium was stored
and analyzed as described above.
2.5. Effect of anti-rPTTH monoclonal antibodies on Manduca brain extract PTTH activity
Monoclonal 3H3 antibody (5µg), which was
gener-ated against the rPTTH and purified using a protein A
column (BioRad MAPS II kit), was incubated with 25µl
(2.5 brain equivalents) of P0brain extract (Bollenbacher
et al. 1979, 1984) for 1 h at room temperature, with
gentle shaking. During this period, five pairs of P0
pro-thoracic glands were dissected out and preincubated for
<30 min in 50µl of Grace’s medium. After the 1 h
incu-bation of brain extract with antibody, this mix was
diluted with 100µl of Grace’s medium. The
preincu-bation medium was then removed from the glands and
replaced with either 25µl of Grace’s medium or with
25µl of the diluted antibody/brain extract (25µl<0.5
brain equivalent). This incubation was continued for
90 min, at which point 20µl of the incubation mix was
removed and frozen for eventual RIA analysis (Warren and Gilbert 1986, 1988) using the SHO 3 antibody (courtesy of Dr Sho Sakurai; see Kiriishi et al., 1990). The monoclonal 3H3 antibody as well as a polyclonal
antibody againstManducarPTTH (both developed by A.
Mizoguchi) recognizeManducaPTTH in Western blots.
2.6. Effect of rPTTH on general and specific protein synthesis
Early V4 prothoracic glands (larvae between 8.0 and
9.0 g live weight) were dissected and preincubated for
30 min in 50µl of low-methionine Grace’s medium
(10% of the normal methionine content of Grace’s; Ryb-czynski and Gilbert, 1994). The preincubation medium
was removed and replaced with 25µl of low-methionine
Grace’s medium containing 10µCi of 35S-methionine
(ICN: 1175 Ci/mmol) with or without 0.25 ng of rPTTH (about 0.325 nM). After incubation periods of 30, 60 or 90 min, the prothoracic glands were removed,
flash-frozen and stored at280°C. The analysis of newly
syn-thesized, radiolabeled proteins was performed by determining the TCA (trichloroacetic acid)-precipitable radioactivity per gland and by SDS (sodium dodecyl sulfate)–polyacrylamide gel electrophoresis (PAGE) of gland proteins, as described by Rybczynski and Gil-bert (1994).
2.7. Stimulation of protein phosphorylation
To investigate the protein phosphorylation patterns
elicited by rPTTH, prothoracic glands from V3 and V6
larvae and P0 new pupae were prepared as described
above. Four glands as a group were placed in a culture
plate well containing 150µl of phosphate-free Grace’s
medium for at least 30 min. After removal of the
medium, 150µl of fresh phosphate-free medium, to
which carrier-free [32P]O4 (20µCi/150µl) was added,
was placed into each well and preincubated for 1 h to label the endogenous adenosine triphosphate (ATP) pool (Song and Gilbert, 1995). The glands were then
incu-bated for the indicated period of time in 150µl of fresh
microtube containing 40µl of 3X SDS gel sample
buffer, heated to 100°C for 5 min and subjected to SDS–
PAGE and autoradiography.
2.8. Two-dimensional PAGE (2D-PAGE) analysis of multiple S6 phosphorylation
2D-PAGE, as described previously (Song and Gilbert, 1995), was used to analyze the multiple-site phosphoryl-ation of S6 stimulated by rPTTH. For purificphosphoryl-ation of the 80S ribosomal proteins for 2D-PAGE analysis, 40 glands at a time were prelabeled for 1 h in 0.8 ml of fresh phos-phate-free Grace’s medium to which carrier-free
[32P]O4(100µCi/0.8 ml) was added, followed by rPTTH
challenge (0.25 ng/gland) for 1 h. A total of 120 labeled glands were collected for ribosomal protein purification as described previously (Song and Gilbert, 1995). The purified 80S ribosomal proteins were subjected to 2D-PAGE and autoradiography.
3. Results and discussion
The Results and discussion sections have been com-bined to facilitate comparison of the responses of the
Manducaprothoracic glands to brain extracts containing PTTH and rPTTH. The physiological interpretation of these data is not fully reiterated here since it has been discussed in depth in the original publications cited that utilized brain extract or semi-purified brain extract. Thus, for each series of experiments, we determine if the brain extract and rPTTH elicit identical, similar or different responses using a variety of critical and repro-ducible assays.
3.1. In vitro assay of rPTTH activity
To investigate if rPTTH can stimulate ecdysteroidog-enesis in the prothoracic glands in vitro as does brain
extract, individual glands from V6 larvae were prepared
and incubated for 1 h in 50µl of Grace’s medium
con-taining the indicated doses of rPTTH or brain extract. The medium was then collected at the end of the incu-bation period and the ecdysteroid content quantified by
RIA. The data revealed anED50(effective dose yielding
a 50% positive effect) for V6 glands stimulated by
rPTTH of approximately 0.125 ng/gland (Fig. 1). At a dose of 0.25 ng/gland, rPTTH elicited a maximum five-fold increase in ecdysteroid production by the stimulated glands when compared with control glands, a result simi-lar to that of glands stimulated by brain extract (0.25 brain equivalent/gland) (Fig. 1). It should be noted that at the dose of 1 brain equivalent/gland (which is four times the dose necessary for maximum stimulation), brain extract showed an inhibitory effect on
ecdystero-idogenesis in the V6gland (Fig. 1) while rPTTH showed
Fig. 1. Ecdysteroidogenesis by V6prothoracic glands in response to
varying doses of rPTTH or brain extract. The data represent the mean±standard error of the mean (SEM); N=10 glands/dose. The amount of ecdysteroids produced by matched-pair control glands has been subtracted from the data.
no inhibitory effect on ecdysteroidogenesis at a similar dose (i.e., four times that necessary for maximum stimulation).
A time-course study, using the predetermined dose of rPTTH (0.25 ng/gland) and brain extract (0.25 brain
equivalent/gland), was then conducted with V6 glands.
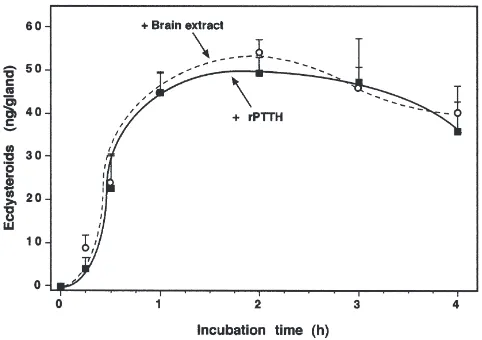
rPTTH and brain extract showed a similar stimulatory effect (Fig. 2). A significant increase in ecdysteroid syn-thesis was observed 15 min after stimulation by both rPTTH and brain extract. The rate of ecdysteroidogen-esis increased rapidly at 30 min and 1 h. The medium reached a peak accumulation at 2 h, before decreasing slightly at 3 h.
To test the effect of rPTTH on ecdysteroidogenesis by glands from different developmental stages, glands from
Fig. 2. Time course of rPTTH- or brain-extract-stimulated ecdystero-idogenesis by V6 prothoracic glands. The data represent the
V3, V6and P0animals were prepared and challenged for 1 h with rPTTH (0.25 ng/gland). The data revealed that rPTTH induced an increase of approximately three times
basal ecdysteroid production by the glands from V3and
P0animals and a fourfold increase by V6glands (Fig. 3).
Thus, rPTTH and brain extract elicited similar, if not identical, stimulatory effects on the prothoracic gland in vitro in terms of dose response, time course and develop-mental studies. The maximum activation ratio for ecdys-teroid biosynthesis by rPTTH-stimulated glands versus control (non-stimulated) glands was approximately four to six times at all stages examined (Fig. 3), as obtained also with brain extract. The inhibitory effect on ecdys-teroidogenesis in glands stimulated by a high dose of brain extract (1 brain equivalent/gland, about four times that necessary for maximum stimulation) is in contrast to our analogous studies with rPTTH that show no inhi-bition at the equivalent dose. This suggests the presence of an inhibitory factor in the brain extract as was found for Bombyx mori by Hua et al. (1999), who suggested that such a factor is a PTTH antagonist.
Using the in vivo assay of Gibbs and Riddiford (1977), the rPTTH stimulation of ecdysteroidogenesis was also basically identical to brain extract stimulation (M. Shionoya, H. Matsubayashi, S. Nagata, H. Kuni-yoshi, M. Asahina, L.M. Riddiford and H. Kataoka, in preparation). Knowing that the effects of PTTH and brain extract on ecdysteroidogenesis both in vivo and in vitro are identical, we proceeded to examine the action of rPTTH on other parameters such as cAMP levels, general and specific protein synthesis, etc., since the original observations utilized crude and semi-pure prep-arations of PTTH.
Fig. 3. rPTTH-stimulated ecdysteroidogenesis by the prothoracic glands in vitro from V3, V6 and P0 animals. The data represent the
mean±SEM;N=10 glands/group.
3.2. Effects of PTTH on cAMP levels
Native forms of Manduca PTTH preparations
stimu-late cAMP synthesis prior to enhancing ecdysteroid syn-thesis and secretion (Smith et al., 1984). To determine whether rPTTH has a similar effect on glandular cAMP,
prothoracic glands were removed from V3 larvae or P0
pupae, and challenged with a maximally effective dose of rPTTH (1.6 nM) for 5 min. As shown in Fig. 4, rPTTH alone causes a significant increase in cAMP syn-thesis in larval prothoracic glands. In pupal glands, cAMP synthesis cannot be detected unless cAMP break-down is concomitantly inhibited with IBMX (isobutyl methyl xanthine) (Fig. 4, stippled bar). Even in the pres-ence of IBMX, cAMP synthesis is stimulated more strongly in larval than in pupal glands.
The stimulation of cAMP synthesis by rPTTH inM.
sexta prothoracic glands is similar, if not identical, to that previously observed for brain extract and semi-pure native PTTH (big or small) (Smith et al. 1984, 1986; Smith and Pasquarello, 1989; Watson et al., 1993). As would be expected for a relevant second messenger, cAMP levels increase within 5 min, with peak levels pre-ceding a detectable increase in ecdysteroid secretion (Smith et al., 1984). Cyclic AMP phosphodiesterase
lev-els inM. sextaprothoracic glands are elevated at the end
of the fifth larval stage and beginning of the pupal stage. Thus, in order to measure PTTH-stimulated changes in cAMP synthesis in early pupal glands, a phosphodiester-ase inhibitor (IBMX) must be added. Similarly, both
crude and recombinant B. mori PTTH stimulate cAMP
synthesis, and this effect is seen most readily in the pres-ence of IBMX (Gu et al. 1996, 1998; Dedos et al., 1999). Mean levels of cAMP synthesis stimulated by rPTTH
in larval glands (10 pmol) are less than the mean levels elicited by big PTTH (20–30 pmol) (Smith et al., 1984; Smith and Pasquarello, 1989). The native form of the hormone may stimulate cAMP production more effec-tively than the recombinant hormone as a result of yet unidentified post-translational modifications such as gly-cosylation. Alternatively, additional factors present in PTTH preparations used in earlier studies may have aug-mented cAMP synthesis independently of PTTH.
3.3. Effects of rPTTH in calcium-free saline
Ecdysteroid secretion stimulated by rPTTH clearly
depends upon the presence of external Ca2+. As seen in
Fig. 5, rPTTH does not stimulate ecdysteroid
synthesis/secretion in Ca2+-free saline, although basal
rates are unaffected. Overall rates of ecdysteroid syn-thesis are lower in saline than in Grace’s medium. How-ever, the results are otherwise identical to previous find-ings in which PTTH-stimulated ecdysteroid secretion was strongly dependent upon extracellular calcium (Smith et al., 1985; Girgenrath and Smith, 1996). See
also data onM. sextasmall PTTH (Hayes et al., 1995),
G. mellonella PTTH (Birkenbeil, 1996) and B. mori
recombinant PTTH (Gu et al., 1998; Dedos et al., 1999). An increase in intracellular calcium has been measured
in the prothoracic glands of Manduca in response to
crude PTTH (Birkenbeil, 1998). The prothoracic glands of Manduca possess a Ca2+-sensitive adenylyl cyclase, and external calcium is required in order for PTTH to stimulate cAMP synthesis (Smith et al., 1984; Meller et al., 1988). Calcium dependence of cAMP synthesis is
also seen for B. mori in response to the recombinant
form of PTTH for this species (Gu et al., 1998; Dedos et al., 1999).
Fig. 5. Effects of rPTTH on ecdysteroid secretion in calcium-free medium. Individual prothoracic glands were incubated for 90 min in calcium-free saline (black bar), calcium-free saline containing 1.6 nM rPTTH (white bar), saline containing 1 mM calcium (slashed bar), or saline containing 1 mM calcium and 1.6 nM rPTTH (stippled bar). The medium was then assayed for ecdysone by RIA. Each group represents the mean±SEM of six glands.
3.4. Effects of rPTTH on general and specific protein synthesis
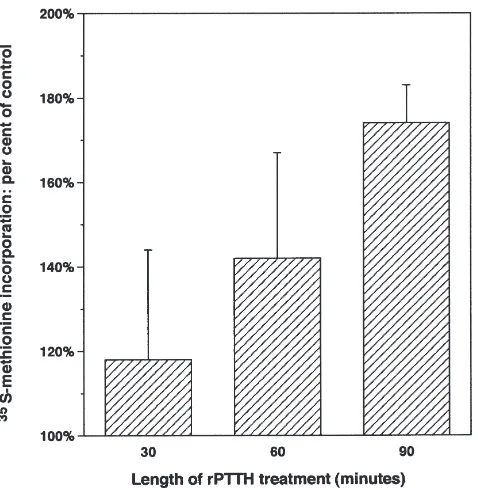
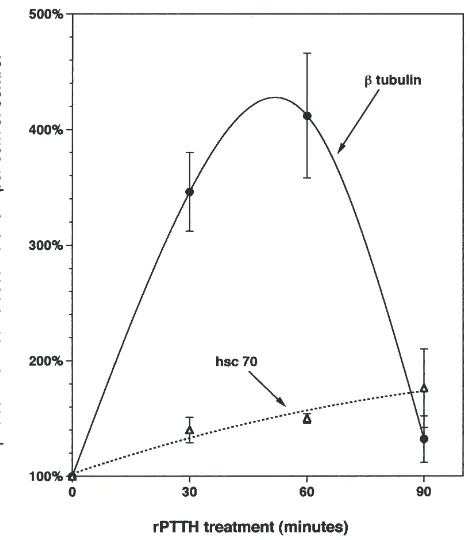
The data revealed that rPTTH stimulated a progressive increase in protein synthesis as measured by TCA-pre-cipitable radioactivity (Fig. 6). In addition, SDS–PAGE indicated that rPTTH induced a temporally varying pat-tern of changes in synthesis and/or turnover of specific
proteins includingβ-tubulin and heat shock cognate 70
(HSC) protein (Figs. 7 and 8). Thus, the rPTTH stimu-lates rapid increases in general and specific protein thesis in the prothoracic gland. Analysis of newly syn-thesized, radiolabeled proteins via SDS–PAGE and autoradiography indicates that the pattern of increases in specific translation and/or protein accumulation matches that observed using brain extract and dibutyryl cAMP at several time points during the fifth instar and early pupal–adult development (Rybczynski and Gilbert, 1994; Rybczynski and Gilbert, 1995a,b; see also Gilbert
et al., 1997). Protein synthesis using Ca2+ionophore has
not been explored sufficiently at multiple developmental time points to make a comparison, although the data from early fifth instar prothoracic glands match the rPTTH observations. Previous work indicated that PTTH-containing brain extracts stimulated the overall rate of translation in prothoracic glands in a way that was independent of ecdysteroid synthesis, i.e., that PTTH could function as a “growth factor” (Rybczynski and Gilbert, 1994). Thus, the results obtained with rPTTH confirm that this cytokine-like activity resides in the PTTH molecule and not in another, unidentified
pro-Fig. 6. Time course of rPTTH-stimulated protein synthesis, as meas-ured by TCA-precipitated, 35S-methionine-labeled proteins (see
Fig. 7. The stimulation of the synthesis of specific prothoracic gland proteins by rPTTH and visualization by SDS–PAGE of 35
S-methionine-labeled proteins. Newly synthesized prothoracic gland proteins were S-methionine-labeled with 35S-methionine in the presence or absence of rPTTH during in
vitro incubations, as described previously (Rybczynski and Gilbert, 1994). The amount of protein loaded per gel lane was adjusted to equalize the amount of35S-methionine per lane, as determined in TCA precipitations.
Fig. 8. Time course of rPTTH-stimulatedβ-tubulin and HSC 70 syn-thesis. Following SDS–PAGE and autoradiography, the intensity of the band representing radiolabeledβ-tubulin and HSC 70 was quantified via scanning as described by Rybczynski and Gilbert (1994). The amount of protein loaded per gel lane was adjusted to equalize the amount of 35S-methionine per lane, as determined in TCA
precipi-tations.
tein found in the brain extracts. These data are consistent with the hypothesis that rPTTH has multiple effects on the cell biology of the prothoracic glands in addition to stimulating ecdysteroidogenesis as does brain extract (Rybczynski and Gilbert, 1994). Additional functions could include control of the growth and maintenance of prothoracic gland cells or modulation of the feedback control of ecdysteroid synthesis.
3.5. Effect of monoclonal antibody to rPTTH on PTTH stimulation of ecdysteroidogenesis by the prothoracic gland
When monoclonal antibody 3H3 was added to the brain extract–prothoracic gland in vitro assay system, the
activation ratio for paired glands averaged 0.85±0.16,
indicating that the 3H3 antibody inhibited the normal ecdysteroidogenic activity present in the brain extract
(expected Ar for 0.5 brain equivalents: 4.0–6.0+). The
molar ratio of antibody to PTTH was <400, based on
the molecular weights of IgG (150,000) and PTTH
(<30,000 as a native homodimer; see Rybczynski et al.,
1996) and assuming 1 ng PTTH per brain equivalent. These data suggest strongly that the natural PTTH binds to an antibody generated against rPTTH and therefore that the rPTTH is equivalent to the single natural PTTH of Manduca in these extracts.
In contrast, previous work with a monoclonal antibody
against Bombyx PTTH revealed binding to Manduca
PTTH in such extracts but not in a way that prevented
evidence that in P1 brains, there is only one significant, active PTTH species; i.e., the anti-PTTH antibody was able to completely block brain-extract-elicited ecdys-teroidogenesis.
Thus far, the effects of rPTTH were identical to those elicited by brain extract, but we wished to identify the cells synthesizing this rPTTH by immunocytochemistry.
3.6. Immunocytochemistry
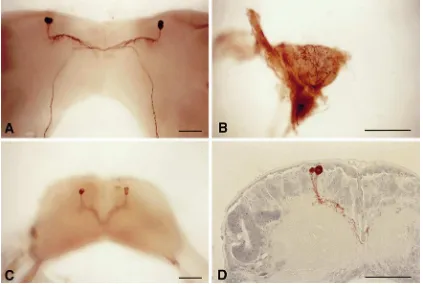
Fig. 9 shows the results of immunocytochemical analysis using rPTTH polyclonal antibodies. These data are basically identical to those obtained using mono-clonal antibodies (mAbs) generated against partially
pur-ifiedManduca PTTH (O’Brien et al., 1988) and against
a synthetic Bombyx PTTH fragment on Manduca Br–
CC–CA complexes (Dai et al., 1994). That is, the anti-bodies stained only two pairs of lateral neurosecretory cells of the brain (the prothoracicotropes) in both stages examined, and the pathway of the axons was the same as shown in the previous studies in which other PTTH antibodies were used. The axons originating from the lateral neurosecretory cells are fasciculated, traverse the midline of the brain to enter the contralateral lobe, pro-ceed posteriorly toward the retrocerebral nerve, and reach the corpora allata (neurohemal organs) where they form arborized endings. Between the soma and the
mid-Fig. 9. Immunocytochemical detection of rPTTH in the brain and corpus allatum. (a) Whole mount of the pupal P0 Manduca brain showing
staining for PTTH in cell bodies and axons of prothoracicotropes. (b) Whole mount of arborized axons in the P0corpus allatum (PTTH neurohemal
organ). (c) Whole mount of cell bodies and axons of prothoracicotropes in the V3larval brain. (d) Section of V3cell bodies and dendrites (arborized
axons) of prothoracicotropes located at the periphery of the neuropile. Scale bars=100µm.
line of the brain, many collaterals branching from the axons were observed (see whole mount stain) and they were located along the periphery of the neurophile (see section stain). In contrast to another report using a differ-ent mAb (Westbrook et al., 1993), no other cells were stained with our antibody.
Thus, the PTTH peptide was localized to only the lat-eral cells identified by Agui et al. (1979) as the protho-racicotropes and in the corpus allatum identified pre-viously as the neurohemal organ for PTTH (Agui et al., 1980).
Whole mount in situ hybridization of day 1, pupal
brains with a 1 kbManducaPTTH cDNA probe labeled
with digoxigenin showed that the PTTH RNA was present only in the two lateral neurosecretory cells in each protocerebrum (M. Shionoya, H. Matsubayashi, S. Nagata, H. Kuniyoshi, M. Asahina, L.M. Riddiford and H. Kataoka, in preparation). These cells are likely the same cells identified with the rPTTH antibody and are in the same position as the pairs of cells found to be biologically active in stimulating ecdysteroid biosynth-esis by the prothoracic glands (Agui et al., 1979).
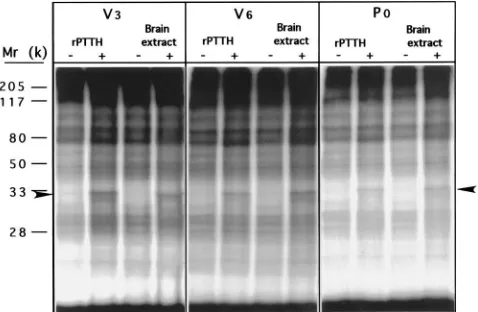
3.7. rPTTH-stimulated phosphorylation of S6
Fig. 10. Time course of rPTTH-stimulated (a) or brain-extract-stimulated (b) S6 phosphorylation in the prothoracic glands of V6Manducalarvae.
The phosphoproteins were separated by 12.5% SDS–PAGE and subjected to autoradiography. The arrowheads indicate the position of the phos-phorylated S6 protein.
the prothoracic gland of Manduca is the multiple-site
phosphorylation of S6, an important regulatory protein of the 40S subunit of the ribosome (Song and Gilbert 1994, 1995). To investigate the effect of rPTTH on
pro-tein phosphorylation, prothoracic glands from V6 larvae
were prelabeled with [32P]O4for 1 h, followed by rPTTH
stimulation for 1 h, and the phosphoproteins were then subjected to SDS–PAGE and autoradiography. Fig. 10 shows that similar protein phosphorylation patterns are observed with both rPTTH- and brain-extract-stimulated glands. S6 phosphorylation was observed 15 min after glands were challenged by rPTTH and by brain extract (Fig. 11), and reached the maximum level at 1 h in both cases. Dephosphorylation of S6 in rPTTH-stimulated glands began at 2 h after stimulation and S6 phosphoryl-ation returned to its basal level at 3 h after stimulphosphoryl-ation (Fig. 10). However, S6 dephosphorylation in
brain-Fig. 11. rPTTH- or brain-extract-stimulated S6 phosphorylation in the prothoracic glands of V3, V6and P0animals. The phosphoproteins
were separated by 15% SDS–PAGE and subjected to autoradiography. The arrowheads indicate the position of phosphorylated S6 proteins.
extract-stimulated glands did not start until 3 h after stimulation and the rate of S6 phosphorylation returned to its basal level by 4 h. The rapid dephosphorylation of S6 in rPTTH-stimulated glands may be due to the lability of pure rPTTH in Grace’s medium. It should also be noted that the phosphorylation of a yet uncharacterized 25 kDa protein was enhanced at 2 h, reaching a maximum level at 3 h for rPTTH-stimulated glands and at 4 h for brain extract-stimulated glands (Fig. 11). S6 phosphorylation patterns resulting from stimulation by
rPTTH in glands from V3, V6and P0animals were
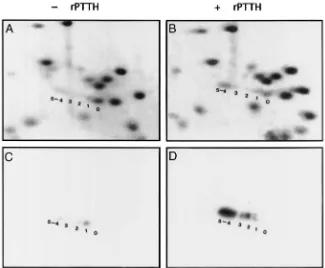
simi-lar to those from brain extract stimulation (Fig. 11). 2D-PAGE analysis revealed that all five phosphoryl-ation sites of S6 characterized previously were occupied in rPTTH-stimulated glands as shown in a Commassie-blue-stained gel map [Fig. 12(b)] and confirmed by a corresponding autoradiograph [Fig. 12(d)]. Most of the
phosphorylated S6 was in the P4–5phosphorylation state,
a result similar to that found with brain-extract-stimu-lated glands (Song and Gilbert, 1995). In contrast, only a trace amount of S6 phosphorylation was detected in control glands as evidenced in both Commassie-blue staining [Fig. 12(a)] and the corresponding autoradio-graph [Fig. 12(c)]. The S6 in control samples was
radiol-abeled predominately in the P1 state, indicating that a
basal level of S6 phosphorylation exists. Thus, all the data concerning the phosphorylation of S6 are consistent with the premise that the rPTTH is indeed the natural
PTTH in the Manduca sexta brain, and that it is the
Fig. 12. 2D-PAGE mini-gel maps revealing the degree of S6 phosphorylation in ribosomal proteins from control (a, c) and rPTTH-stimulated (b, d) prothoracic glands. Panels (a) and (b) show Coomassie-blue staining. (c) and (d) are autoradiographs corresponding to (a) and (b), respectively. The numbers indicate the phosphorylation states of the S6 protein.
4. Conclusions
We conclude from these data that the rPTTH from the Kataoka Laboratory is identical or homologous to the
natural PTTH of Manduca sexta, and that previous
research data derived from the use of crude or semi-purified brain extracts did indeed reflect the effects of PTTH.
5. Addendum
The Gilbert laboratory (Q. Song, R. Rybczynski, L.I. Gilbert) generated the data seen in Figs. 1–3, 6–8, 10– 12. The Smith laboratory generated the data in Figs. 4 and 5, while A. Mizoguchi was responsible for Fig. 9. Drs Matsubayashi, M. Shionoya, S. Nagata and H. Kataoka were responsible for the cloning and expression of the rPTTH, as well as preparation of the antigen for the production of rPTTH antibodies.
Acknowledgements
L.I.G. thanks Pat Cabarga for clerical assistance. The research from the Gilbert laboratory was supported by grants from NSF (IBN 9603710) and NIH (DK-30018).
References
Agui, N., Granger, N., Bollenbacher, W., Gilbert, L.I., 1979. Cellular localization of the insect prothoracicotropic hormone:in vitroassay of a single neurosecretory cell. Proc. Natl. Acad. Sci., USA 76, 5694–5698.
Agui, N., Bollenbacher, W., Granger, N., Gilbert, L.I., 1980. Corpus allatum is release site for the insect prothoracicotropic hormone. Nature 285, 669–670.
Birkenbeil, H., 1996. Involvement of calcium in prothoracicotropic stimulation of ecdysone synthesis in Galleria mellonella. Arch. Insect Biochem. Physiol. 33, 39–52.
Birkenbeil, H., 1998. Intracellular calcium in prothoracic glands of
Manduca sexta. J. Insect Physiol. 44, 279–286.
Bollenbacher, W.E., Agui, N., Granger, N., Gilbert, L.I., 1979.In vitro
activation of insect prothoracic glands by the prothoracicotropic hormone. Proc. Natl. Acad. Sci., USA 76, 5148–5152.
Bollenbacher, W.E., Katahira, E., O’Brien, M., Gilbert, L.I., 1984. Insect prothoracicotropic hormone: evidence for two molecular forms. Science 224, 1243–1245.
Borst, D.W., O’Connor, J.D., 1972. Arthropod molting hormone: radioimmune assay. Science 178, 418–419.
Dai, J.-D., Mizoguchi, A., Gilbert, L.I., 1994. Immunoreactivity of neurosecretory granules in the brain–retrocerebral complex of Man-duca sexta to heterologous antibodies against Bombyx protho-racicotropic hormone and bombyxin. Invert. Reprod. Devel. 26, 187–196.
Dedos, S., Fugo, H., Nagata, S., Takamiya, M., Kataoka, H., 1999. Differences between recombinant PTTH and crude brain extracts in cAMP-mediated ecdysteroid secretion from the prothoracic glands of the silkworm, Bombyx mori. J. Insect Physiol. 45, 415–422.
Man-duca sexta: localization by a larval assay. J. Exp. Biol. 66, 255– 266.
Gilbert, L.I., Combest, W., Smith, W., Meller, V., Rountree, D., 1988. Peptides, second messengers and insect molting. BioEssays 8, 153–157.
Gilbert, L.I., Song, Q., Rybczynski, R., 1997. Control of ecdystero-idogenesis: activation and inhibition of prothoracic gland activity. Invert. Neurosci. 3, 205–216.
Girgenrath, S., Smith, W.A., 1996. Investigation of presumptive mobil-ization pathways for calcium in the steroidogenic action of big pro-thoracicotropic hormone. Insect Biochem. Mol. Biol. 26, 455–463. Gu, S.H., Chow, Y.S., Lin, F.J., Wu, J.L., Ho, R.J., 1996. A deficiency in prothoracicotropic hormone transduction pathway during the early last larval instar ofBombyx mori. Mol. Cell. Endocrinol. 120, 99–105.
Gu, S.H., Chow, Y.S., O’Reilly, D.R., 1998. Role of calcium in the stimulation of ecdysteroidogenesis by recombinant protho-racicotropic hormone in the prothoracic glands of the silkworm,
Bombyx mori. Insect Biochem. Mol. Biol. 28, 861–867.
Hayes, G.C., Muehleisen, D.P., Bollenbacher, W.E., Watson, R.D., 1995. Stimulation of ecdysteroidogenesis by small protho-racicotropic hormone: role of calcium. Mol. Cell. Endocrinol. 115, 105–112.
Henrich, V., Rybczynski, R., Gilbert, L.I., 1999. Peptide hormones, steroid hormones and puffs: mechanisms and models in insect development. In:. Litwack, G. (Ed.), Vitamins and Hormones, vol. 55. Academic Press, San Diego, CA, pp. 73–125.
Hua, Y.J., Tanaka, Y., Nakamura, K., Sakakibara, M., Nagata, S., Kataoka, H., 1999. Identification of a prothoracicostatic peptide in the larval brain of the silkworm,Bombyx mori. J. Biol. Chem. 274, 31169–31173.
Kiriishi, S., Rountree, D.B., Sakurai, S., Gilbert, L.I., 1990. Prothoracic gland synthesis of dehydroecdysone and its hemolymph 3-reductase mediated conversion to ecdysone in representative insects. Experientia 46, 716–721.
Meller, V.H., Combest, W.L., Smith, W.A., Gilbert, L.I., 1988. A cal-modulin-sensitive adenylate cyclase in the prothoracic glands of the tobacco hornworm,Manduca sexta. Mol. Cell. Endocrinol. 59, 67–76.
Meller, V.H., Sakurai, S., Gilbert, L.I., 1990. Developmental regulation of calmodulin-dependent adenylate cyclase activity in an insect endocrine gland. Cell Regulation 1, 771–780.
Mizoguchi, A., Ishizaki, H., Nagasawa, H., Kataoka, H., Isogai, A., Tamura, S., Suzuki, A., Fujino, M., Kitada, C., 1987. A monoclonal antibody against a synthetic fragment of 4K-prothoracicotropic hor-mone of the silkmoth,Bombyx mori: characterization and immu-nohistochemistry. Mol. Cell. Endocrinol. 51, 227–235.
O’Brien, M.A., Katahira, E.J., Flanagan, T.R., Arnold, L.W., Haugh-ton, G., Bollenbacher, W.E., 1988. A monoclonal antibody to the insect prothoracicotropic hormone. J. Neurosci. 8, 3247–3257. Rybczynski, R., Gilbert, L.I., 1994. Changes in general and specific
protein synthesis that accompany ecdysteroid synthesis in
stimu-lated prothoracic glands ofManduca sexta. Insect Biochem. Mol. Biol. 24, 175–189.
Rybczynski, R., Gilbert, L.I., 1995a. Prothoracicotropic hormone elic-its a rapid, developmentally-specific, synthesis of βtubulin in an insect endocrine gland. Dev. Biol. 169, 15–28.
Rybczynski, R., Gilbert, L.I., 1995b. Prothoracicotropic hormone-stimulated expression of a hsp 70 cognate protein in the insect prothoracic gland. Mol. Cell Endocrinol. 115, 73–85.
Rybczynski, R., Mizoguchi, A., Gilbert, L.I., 1996.Bombyxand Mand-uca prothoracicotropic hormones: an immunologic test for relatedness. Gen. Comp. Endocrinol. 102, 247–254.
Smith, W.A., Pasquarello, T.J., 1989. Developmental changes in phos-phodiesterase activity and hormonal response in the prothoracic glands ofManduca sexta. Mol. Cell. Endocrinol. 63, 239–246. Smith, W., Bollenbacher, W.E., Gilbert, L.I., 1984. The role of cyclic
AMP in the regulation of ecdysone synthesis. Mol. Cell. Endocri-nol. 37, 285–294.
Smith, W., Bollenbacher, W.E., Gilbert, L.I., 1985. Calcium–cyclic AMP interactions in prothoracicotropic hormone stimulation of ecdysone synthesis. Mol. Cell. Endocrinol. 39, 71–78.
Smith, W., Combest, W., Gilbert, L.I., 1986. Involvement of cyclic AMP-dependent protein kinase in prothoracicotropic hormone-stimulated ecdysone synthesis. Mol. Cell Endocrinol. 47, 25–33. Song, Q., Gilbert, L.I., 1994. S6 phosphorylation results from
protho-racicotropic hormone stimulation of insect prothoracic glands: a role for S6 kinase. Devel. Genetics 15, 332–338.
Song, Q., Gilbert, L.I., 1995. Multiple phosphorylation of ribosomal protein S6 and specific protein synthesis are required for protho-racicotropic hormone-stimulated ecdysteroid biosynthesis in the prothoracic glands ofManduca sexta. Insect Biochem. Mol. Biol. 25, 591–602.
Vedeckis, W., Bollenbacher, W., Gilbert, L.I., 1974. Cyclic AMP as a possible mediator of prothoracic gland activation. Zool. Jb. Phy-siol. Bd. 78, 440–448.
Vedeckis, W., Bollenbacher, W., Gilbert, L.I., 1976. Insect prothoracic glands: a role for cyclic AMP in the stimulation of α-ecdysone secretion. Mol. Cell. Endocrinol. 5, 81–88.
Warren, J., Gilbert, L.I., 1986. Ecdysone metabolism and distribution during the pupal–adult development of Manduca sexta. Insect Biochem. 16, 65–82.
Warren, J., Gilbert, L.I., 1988. Radioimmunoassay: ecdysteroids. In: Gilbert, L.I., Miller, T.A. (Eds.), Immunological Techniques in Insect Biology, Springer Series in Experimental Entomology. Springer-Verlag, Heidelberg, pp. 181–214.
Watson, R.D., Yeh, W.E., Muehleisen, D.P., Watson, C.J., Bollen-bacher, W.E., 1993. Stimulation of ecdysteroidogenesis by small prothoracicotropic hormone: role of cyclic AMP. Mol. Cell. Endoc-rinol. 92, 221–228.