49
DAFTAR PUSTAKA
1. Kemendag. Kemendag Mendorong Masyarakat Untuk Beralih Dari Minyak Goreng Curah Ke Minyak Goreng Kemasan. www.kemendag.go.id. Published 2013.
2. Ng C, Kamisah Y, Faizah O, et al. Involvement of Inflammation and Adverse Vascular Remodelling in the Blood Pressure Raising Effect of Repeatedly Heated Palm Oil in Rats. 2012.
3. Martin J, Joffre F, Vernevaut M. Biochemical and Molecular Action of Nutrients Cyclic Fatty Acid Monomers from Heated Oil Modify the Activities of Lipid Synthesizing and Oxidizing Enzymes in Rat Liver 1 , 2. 2000:1524-1530.
4. Adam S, Das S, Jaarin K. A Detailed Microscopic Study of The Changes in The Aorta of Experimental Model of Postmenopausal Rats Fed with Repeatedly Heated Palm Oil. Int J Exp Pathol. 2009;90(3):321-327.
5. Leong X, Aishah A, Nor AU, Das S, Jaarin K. Heated Palm Oil Causes Rise in Blood Pressure and Cardiac Changes in Heart Muscle in Experimental Rats. Acheives Med Res. 2008;39(6):567-572.
6. Goodman LS, Gilman A. Dasar Farmakologi Terapi. X. (Hardman JG, Limbird LE, Aisyah C, eds.). Jakarta: ECG; 2012.
7. Wilmana PF, Gunawan SG. Analgesik-Antipiretik Analgesik Anti-Inflamasi
Nonsteroid Dan Obat Gangguan Sendi Lainnya. Dalam: Farmakologi Dan Terapi. V. Jakarta: Balai Penerbit FKUI; 2007.
Faces of Glutathione, A Cellular Protagonist. Biochem Pharmacol. 2003;66 (8):1499-1503.
9. Ulilalbab A. Aktivitas Antioksidan Tablet Effervescent Rosella Ungu Sebagai Suplement Penghambat Laju Peroksidasi Melalui Pengujian In Vivo. PKM-P. 2010.
10. Qinna NA, Mallah EM, Arafat TA, Idkaidek NM. Effect Of Licorice and Grapefruit Juice on Paracetamol Pharmacokinetics in Human Saliva. Int J
Pharm Pharm Sci. 2012;4(4):158-162.
11. Barshop NJ, Capparelli E V, Sirlin CB, Jeffrey B, Lavine JE. Acetaminophen Pharmacokinetics in Children with Nonalcoholic Fatty Liver Disease. J Pediatr Gastroenterol Nutr. 2012;52(2):198-202.
12. Kumdi BV, Kolawole JA, Apeh E. The Effect of Yoyo Bitters on The Pharmacokinetics of Single Oral Dose Paracetamol Tablet in Human Volunteers. Int J Biol Chem Sci. 2011;5:717-723.
13. Pradana DA, Hayati F, Samudra AG, Setya A. Effect of Curcumin and Honey to Pharmacokinetics of Paracetamol in Male Wistar Rats. Eksakta. 2013;12(1):193.
14. Simaremare P, Andrie M, Bambang Wijianto. Effect of Durian Fruit Juice (Durio zibethinus Murr.) to Pharmacokinetic Profile of Paracetamol on Wistar Male Rats (Rattus norvegicus L.). Tradit Med J. 2013;18(3):178-186.
15. Ketaren S. Minyak Dan Lemak Pangan. Jakarta: Penerbit Universitas Indonesia; 2005.
16. Sartika RAD. Pengaruh Suhu dan Lama Proses Menggoreng (Deep Frying) Terhadap Pembentukan Asam Lemak Trans. Markara Sains. 2009;13:23-28.
17. Lestari P. Pemanfaatan Minyak Goreng Jelantah Pada Pembuatan Sabun Cuci Piring. Tesis. 2010.
18. Rukmini A. Regenerasi Minyak Goreng Bekas dengan Arang Sekam Menekan Kerusakan Organ Tubuh. Semin Nas Teknol 2007 (SNT 2007). 2007:1978-9777.
19. Mulyati S, Meilina H. Pemurnian Minyak Jelantah dengan Menggunakan Sari Mengkudu. 2007. http://222.124.186.229/gdl40/go.php?id=gdlnode-gdl-res-2007-srimulyati-1082&node-3517&start=6 .
20. Oktaviani ND. Hubungan Lamanya Pemanasan dengan Kerusakan Minyak Goreng Curah Ditinjau dari Bilangan Peroksida. J Biomedika. 2009;1:31-34.
21. Lee J, Lee S, Lee H, Park K, Choe E. Spinach (Spinacia oleracea) as A Natural Food Grade Antioxidant in Deep Fat Fried Products. J Agric Food
Chem. 2002;50:5664-5669.
22. Herawati, Akhlus S. Kinerja (Bht) sebagai Antioksidan Minyak Sawit pada Perlindungan terhadap Oksidasi Oksigen Singlet. Akta Kim. 2006;2:1-8. 23. Azeredo HMC, Faria JAF, Silva. Minimization of Proxide Formation Rate
in Soybean Oil by Antioxidant Combinations. Food Res Int. 2004;37:689-694.
Lemak Bebas (FFA), Bilangan Peroksida, dan Warna Gelap Minyak Goreng Bekas. Semin Nas Tek Kim Indones – SNTKI 2009. 2009:1-14. 25. Ghidurus M, Turtoi M, Boskou G, Niculita P, Stan V. Nutritional and
Health Aspects Related to Frying. Rom Biotechnol Lett. 2010;15(6).
26. Castillo’n PG, Artalejo FR, Fornés NS, et al. Intake of Fried Foods is Associated with Obesity in The Cohort of Spanish Adults from The European Prospective Investigation into Cancer and Nutrition. Am J Clin
Nutr. 2007;86:198-205.
27. Koch A, KÖnig B, Spielmann J, Leitner A, Stang GL, Eder K. Thermally Oxidized Oil Increases the Expression of Insulin-Induced Genes and Inhibits Activation of Sterol Regulatory Element-Binding Protein-2 in Rat Liver. J Nutr Biochem Mol Genet Mech. 2007;137:2018-2023.
28. Toussaint K, Yang XC, Zielinski MA, et al. What Do We (Not) Know About How Paracetamol (Acetaminophen) Works? J Clin Pharm Ther. 2010:617-638. doi:10.1111/j.1365-2710.2009.01143.x.
29. Liudmila LM, Sangkuhl K, Caroline FT, Garret AF, Russ BA, Teri EK. PharmGKB Summary: Pathways of Acetaminophen Metabolism at The Therapeutic Versus Toxic Doses. Pharmacogenet Genomics. 2015.
30. Katzung BG. Farmakologi: Dasar Dan Klinik Buku 2. I. Jakarta: Salemba Medika; 2002.
31. Ubaldo C, Hall NS, Le B. Postmarketing Review of Intravenous Acetaminophen Dosing Based on Food and Drug Administration Prescribing Guidelines. Pharmacother Publ. 2014;34:34-39.
32. Parkinson A. Biotransformation of Xenobiotics. V. (Klaassen C, ed.). New York: McGraw-Hill; 1996.
33. Gibson G. Introduction to Drug Metabolism. II. London: Blackie Academics and Professional; 1994.
34. Mitchell R, McGill, Jaeschke H. Metabolism And Disposition of Acetaminophen: Recent Advances in Relation to Hepatotoxicity and Diagnosis. Pharm Res. 2013;30(9):2174-2187.
35. Short Communication. Involvement of Human Cytochrome P450 2D6 in The Bioactivation of Acetaminophen. 2000;28(12):1397-1400.
36. Winarsi H. Antioksidan Alami Dan Radikal Bebas: Potensi Dan
Aplikasinya Dalam Kesehatan. V. Yogyakarta: Penerbit Kanisius; 2011.
37. Tjay TH, Rahardja K. Obat-Obat Penting : Khasiat, Penggunaan, Dan
Efek-Efek Sampingnya. VI. Jakarta: Penerbit PT. Elex Media Komputindo;
2002.
38. Katzung BG. Basic and Clinical Pharmacology. XII. New York: McGraw-Hill; 2011.
39. WHO. Research Guideliness For Evaluating The Safety and Efficacy of
Herbal Medicines. Manila: Regional Office for The Western Pasific; 2003.
40. Leong X-F, Mustafa MR, Das S, Jaarin K. Association of Elevated Blood Pressure and Impaired Vasorelaxation in Experimental Sprague-Dawley Rats Fed with Heated Vegetable Oil. Lipids Health Dis. 2010;9(66):1-10. 41. Farmakope Indonesia. Daftar Dosis Lazim Dan Dosis Maksimum. III.
42. Laurence DR, Bacharach AL. Evaluation of Drug Activities: Pharmacometrics, Ed. by D.R. Laurence and A.L. Bacharach. London:
Academic Press; 1964.
43. Rusdiana T, Sjuib F, Asyarie S. Interaksi Farmakokinetik Kombinasi Obat Parasetamol dan Fenilpropanolamin Hidroklorida sebagai Komponen Obat Flu. Skripsi. 2009:2-4.
44. Depkes RI. Farmakope Indonesia. IV. Jakarta: Departemen Kesehatan Republik Indonesia; 1995.
45. Sarwono J. Pintar Menulis Karya Ilmiah Kunci Sukses Dalam Menulis
Ilmiah. Yogyakarta: CV. Andi OFFSET; 2010.
46. Hakim L. Farmakokinetik. Yogyakarta: Bursa Ilmu; 2010.
47. Slattery JT, Wilson JM, BS TFK, Nelson SD. Dose-dependent pharmacokinetics of acetaminophen: Evidence of glutathione depletion in humans. Clin Pharmacol Ther. 1987;41(4):413-418.
48. Forrest JAH, Clements JA, Prescott LF. Clinical Pharmacokinetics of Paracetamol. Springer Int Publ. 1982;7(2):93-107.
Lampiran 3. Surat ijin penelitian Laboratorium Farmakologi dan Terapi FK UGM
Lampiran 5. Surat keterangan bebas Laboratorium Farmakologi dan Terapi FK UGM
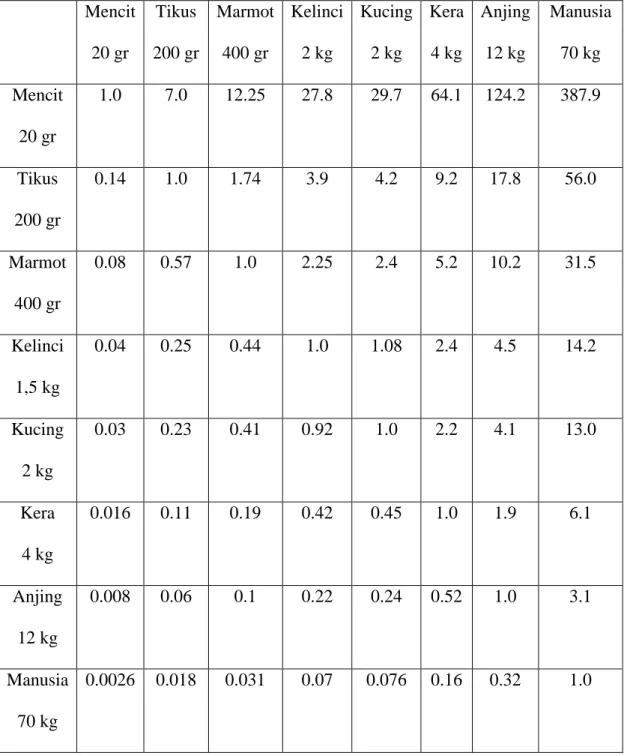
Lampiran 6. Tabel konversi perhitungan dosis
TABEL KONVERSI PERHITUNGAN DOSIS LAURENCE & BACHARACH42 Mencit 20 gr Tikus 200 gr Marmot 400 gr Kelinci 2 kg Kucing 2 kg Kera 4 kg Anjing 12 kg Manusia 70 kg Mencit 20 gr 1.0 7.0 12.25 27.8 29.7 64.1 124.2 387.9 Tikus 200 gr 0.14 1.0 1.74 3.9 4.2 9.2 17.8 56.0 Marmot 400 gr 0.08 0.57 1.0 2.25 2.4 5.2 10.2 31.5 Kelinci 1,5 kg 0.04 0.25 0.44 1.0 1.08 2.4 4.5 14.2 Kucing 2 kg 0.03 0.23 0.41 0.92 1.0 2.2 4.1 13.0 Kera 4 kg 0.016 0.11 0.19 0.42 0.45 1.0 1.9 6.1 Anjing 12 kg 0.008 0.06 0.1 0.22 0.24 0.52 1.0 3.1 Manusia 70 kg 0.0026 0.018 0.031 0.07 0.076 0.16 0.32 1.0
Lampiran 7. Berat badan tikus dan rerata sisa pakan perlakuan
1. Berat badan tikus
No. Kelompok Berat Dosis
1 K1 233 gram 1,45 mL 2 K2 189 gram 1,18 mL 3 K3 249 gram 1,55 mL 4 K4 217 gram 1,36 mL 5 K5 176 gram 1,1 mL 6 P1 255 gram 1,59 mL 7 P2 211 gram 1,31 mL 8 P3 274 gram 1,71 mL 9 P4 300 gram 1,87 mL 10 P5 257 gram 1,60 mL
2. Rerata sisa pakan perlakuan
No. Kelompok Rerata sisa pakan
1 P1 2,83 gram
2 P2 3,38 gram
3 P3 2,36 gram
4 P4 1,87 gram
Lampiran 8. Absorbansi parasetamol pada darah tikus
1. Absorbansi parasetamol pada darah kelompok kontrol
Menit ke- Kelompok K1 K2 K3 K4 K5 Rerata 0 0 0 0 0 0 0 3 0,499 0,523 0,497 0,613 0,539 0,534 5 0,808 0,836 0,833 0,857 0,854 0,838 10 0,927 0,927 0,957 0,732 0,937 0,896 20 0,975 1,141 0,923 1,133 1,169 1,068 30 1,112 1,001 1,124 1,008 1,014 1,052 40 1,078 0,943 0,914 0,940 0,953 0,966 60 0,892 0,895 0,783 0,926 0,920 0,883 90 0,830 0,843 0,829 0,840 0,863 0,841 120 0,735 0,749 0,869 0,770 0,765 0,778 180 0,651 0,671 0,671 0,766 0,699 0,692 240 0,499 0,496 0,466 0,512 0,526 0,500 300 0,402 0,426 0,419 0,469 0,466 0,436 360 0,190 0,219 0,189 0,172 0,226 0,199
2. Absorbansi parasetamol pada darah kelompok perlakuan
Menit ke- Kelompok P1 P2 P3 P4 P5 Rerata 0 0 0 0 0 0 0 3 0,849 0,835 0,816 0,829 0,837 0,833 5 1,059 1,037 1,069 1,041 1,193 1,080 10 1,156 1,193 1,289 1,166 1,041 1,169 20 1,344 1,293 1,139 1,331 1,337 1,289 30 1,318 1,314 1,315 1,326 1,307 1,316 40 1,286 1,277 1,277 1,270 1,294 1,281 60 1,257 1,239 1,270 1,255 1,276 1,259 90 1,156 1,145 1,162 0,934 1,143 1,108 120 1,052 1,022 1,011 1,072 1,030 1,037 180 0,906 0,931 0,881 0,934 1,109 0,952 240 0,859 0,812 0,842 1,118 0,859 0,898 300 0,730 0,763 0,731 0,738 0,765 0,745 360 0,462 0,455 0,425 0,435 0,452 0,446
Lampiran 9. Kadar parasetamol pada darah tikus
1. Kadar parasetamol pada darah kelompok kontrol
Menit ke- Kadar K1 K2 K3 K4 K5 Rerata 0 0 0 0 0 0 0 3 16,871 17,663 16,805 20,634 18,191 18,033 5 27,069 27,993 27,894 28,686 28,587 28,046 10 30,980 30,997 31,987 24,561 31,327 29,970 20 32,581 38,059 30,865 37,795 38,983 35,657 30 37,102 33,439 37,498 33,670 33,868 35,116 40 35,980 31,525 30,568 31,426 31,855 32,271 60 29,842 29,941 26,244 30,964 30,766 29,551 90 27,795 28,224 27,762 28,125 28,884 28,158 120 24,660 25,122 29,083 25,815 25,650 26,066 180 21,888 22,548 22,548 25,683 23,472 23,228 240 16,871 16,772 15,782 17,300 17,762 16,898 300 13,670 14,462 14,231 15,881 15,782 14,805 360 6,673 7,630 6,640 6,079 7,861 6,977
2. Kadar parasetamol pada darah kelompok perlakuan
Menit ke- Kadar P1 P2 P3 P4 P5 Rerata 0 0 0 0 0 0 0 3 28,422 27,960 27,333 27,762 28,026 27,901 5 35,353 34,627 35,683 34,759 39,776 36,040 10 38,554 39,776 42,944 38,884 34,759 38,983 20 44,759 43,076 37,993 44,330 44,528 42,937 30 43,901 43,769 43,802 44,165 43,538 43,835 40 42,845 42,548 42,548 42,317 43,109 42,673 60 41,888 41,294 42,317 41,822 42,515 41,967 90 38,554 38,191 38,752 31,228 38,125 36,970 120 35,122 34,132 33,769 35,782 34,396 34,640 180 30,304 31,129 29,479 31,228 37,003 31,828 240 28,752 27,201 28,191 37,300 28,752 30,040 300 24,495 25,584 24,528 24,759 25,650 25,003 360 15,650 15,419 14,429 14,759 15,320 15,116
Lampiran 10. Ln kadar paraseamol pada darah tikus
1. Ln kadar parasetamol pada darah kelompok kontrol
Menit ke- K1 K2 K3 K4 K5 Rerata 0 0 0 0 0 0 0 3 2,826 2,871 2,822 3,027 2,901 2,889 5 3,298 3,332 3,328 3,356 3,353 3,334 10 3,433 3,434 3,465 3,201 3,444 3,396 20 3,484 3,639 3,430 3,632 3,663 3,570 30 3,614 3,510 3,624 3,517 3,522 3,557 40 3,583 3,451 3,420 3,448 3,461 3,472 60 3,396 3,399 3,267 3,433 3,426 3,384 90 3,325 3,340 3,324 3,337 3,363 3,338 120 3,205 3,224 3,370 3,251 3,245 3,259 180 3,086 3,116 3,116 3,246 3,156 3,144 240 2,826 2,820 2,759 2,851 2,877 2,826 300 2,615 2,672 2,655 2,765 2,759 2,693 360 1,898 2,032 1,893 1,805 2,062 1,938
2. Ln kadar parasetamol pada darah kelompok perlakuan
Menit ke- P1 P2 P3 P4 P5 Rerata 0 0 0 0 0 0 0,000 3 3,347 3,331 3,308 3,324 3,333 3,329 5 3,565 3,545 3,575 3,548 3,683 3,583 10 3,652 3,683 3,760 3,661 3,548 3,661 20 3,801 3,763 3,637 3,792 3,796 3,758 30 3,782 3,779 3,780 3,788 3,774 3,780 40 3,758 3,751 3,751 3,745 3,764 3,754 60 3,735 3,721 3,745 3,733 3,750 3,737 90 3,652 3,643 3,657 3,441 3,641 3,607 120 3,559 3,530 3,520 3,577 3,538 3,545 180 3,411 3,438 3,384 3,441 3,611 3,457 240 3,359 3,303 3,339 3,619 3,359 3,396 300 3,198 3,242 3,200 3,209 3,245 3,219 360 2,750 2,736 2,669 2,692 2,729 2,715
Lampiran 11. Parameter farmakokinetik
1. Parameter farmakokinetik kelompok kontrol
Parameter Farmakokinetik Kelompok K1 K2 K3 K4 K5 Rerata Kel 0,00445 0,00389 0,00416 0,00416 0,00374 0,00408 t1/2e 155,800 178,392 166,587 166,461 185,155 170,479 Ka 0,123 0,156 0,089 0,205 0,144 0,143 t1/2a 5,657 4,457 7,831 3,386 4,800 5,226 tmaks 28,086 24,335 36,252 19,428 25,972 26,815 Cmax 36,848 35,168 33,419 37,554 35,505 35,699 AUC0-inf 9386,096 9950,508 9341,139 9780,476 10454,477 9782,539 Vd 277,849 244,213 319,101 267,207 224,897 266,653 Cl 1,236 0,949 1,327 1,112 0,842 1,093
2. Parameter farmakokinetik kelompok perlakuan
Parameter Kelompok Farmakokinetik P1 P2 P3 P4 P5 Rerata Kel 0,00271 0,00272 0,00292 0,00248 0,00265 0,00269 t1/2e 255,720 254,779 237,329 278,806 261,509 257,629 Ka 0,152 0,107 0,084 0,182 0,126 0,130 t1/2a 4,547 6,448 8,278 3,818 5,495 5,717 tmaks 26,917 35,096 41,533 23,967 31,286 31,760 Cmaks 45,102 43,890 43,555 44,400 45,201 44,430 AUC0-inf 17902,400 17752,205 16839,270 18959,450 18531,310 17996,927 Vd 262,184 217,040 278,215 317,449 260,650 267,108 Cl 0,711 0,590 0,812 0,789 0,691 0,719
Lampiran 12. Hasil uji statistik
1. Jumlah sampel tiap kelompok
Case Processing Summary
Tikus
Cases
Valid Missing Total
N Percent N Percent N Percent
K Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0% t1/2 e Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0% Ka Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0% t1/2 a Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0% tmax Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0% Cp max Kontrol 5 100,0% 0 0,0% 5 100,0% Perlakuan 5 100,0% 0 0,0% 5 100,0%
AUC 0-inf Kontrol 5 100,0% 0 0,0% 5 100,0%
Perlakuan 5 100,0% 0 0,0% 5 100,0%
Vd Kontrol 5 100,0% 0 0,0% 5 100,0%
Perlakuan 5 100,0% 0 0,0% 5 100,0%
Perlakuan 5 100,0% 0 0,0% 5 100,0%
2. Hasil uji normalitas data pengaruh pemberian minyak jelantah terhadap profil farmakokinetik parasetamol pada darah
Tests of Normality
Tikus
Kolmogorov-Smirnova Shapiro-Wilk
Statistic df Sig. Statistic df Sig.
K Kontrol ,215 5 ,200* ,955 5 ,776 Perlakuan ,242 5 ,200* ,960 5 ,806 t1/2 e Kontrol ,233 5 ,200* ,955 5 ,775 Perlakuan ,224 5 ,200* ,960 5 ,810 Ka Kontrol ,186 5 ,200* ,987 5 ,968 Perlakuan ,143 5 ,200* ,988 5 ,974 t1/2 a Kontrol ,201 5 ,200* ,945 5 ,705 Perlakuan ,151 5 ,200* ,967 5 ,856 tmax Kontrol ,218 5 ,200* ,961 5 ,812 Perlakuan ,158 5 ,200* ,975 5 ,907 Cp max Kontrol ,170 5 ,200* ,968 5 ,860 Perlakuan ,223 5 ,200* ,913 5 ,489
AUC 0-inf Kontrol ,208 5 ,200* ,923 5 ,550
Perlakuan ,181 5 ,200* ,973 5 ,896
Vd Kontrol ,177 5 ,200* ,976 5 ,915
Cl Kontrol ,165 5 ,200* ,963 5 ,827
Perlakuan ,188 5 ,200* ,945 5 ,702
*. This is a lower bound of the true significance. a. Lilliefors Significance Correction
3. Data statistik tiap kelompok tikus
Group Statistics
Tikus N Mean Std. Deviation Std. Error Mean
K Kontrol 5 ,00407972780 ,000274126637 ,000122593159 Perlakuan 5 ,00269712000 ,000155985487 ,000069758830 t1/2 e Kontrol 5 170,479096609 829920 11,4539973104 24269 5,12238332004 1685 Perlakuan 5 257,628618431 438200 14,8945071830 27645 6,66102611052 1743 Ka Kontrol 5 ,14310600 ,042864090 ,019169404 Perlakuan 5 ,13025000 ,038147003 ,017059858 t1/2 a Kontrol 5 5,22606919674 9445 1,66790022788 1934 ,745907657846 279 Perlakuan 5 5,71698286521 6155 1,74044517644 2508 ,778350745127 413 tmax Kontrol 5 26,8146739056 70844 6,16647524914 2414 2,75773156773 0478 Perlakuan 5 31,7597965211 85734 6,91135484236 3327 3,09085184882 9348
Cp max Kontrol 5 35,6986385271 20620 1,60294832713 1813 ,716860284777 261 Perlakuan 5 44,4295873633 12020 ,725415300988 269 ,324415584985 648
AUC 0-inf Kontrol
5 9782,53915586 973400 455,993310553 765640 203,926407936 678430 Perlakuan 5 17996,9273378 1966300 809,476730020 997500 362,008998906 239000 Vd Kontrol 5 266,653455326 880360 35,7895563981 15520 16,0055761981 49765 Perlakuan 5 267,107723967 029640 36,1606095854 29930 16,1715162281 70365 Cl Kontrol 5 1,09323841562 9088 ,199821197738 269 ,089362756297 639 Perlakuan 5 ,718606093145 465 ,088105330237 085 ,039401901518 038
4. Hasil uji homogenitas varians
Independent Samples Test
Levene's Test for Equality of Variances t-test for Equality of Means F Sig. t df K Equal variances assumed 2,026 ,192 9,80 2 8
Equal variances not assumed 9,80 2 6,34 5 t1/2 e Equal variances assumed ,039 ,849 -10,3 71 8 Equal variances not assumed -10,3 71 7,50 5 Ka Equal variances assumed ,002 ,963 ,501 8 Equal variances not assumed ,501 7,89 4 t1/2 a Equal variances assumed ,029 ,869 -,455 8 Equal variances not assumed -,455 7,98 6 tmax Equal variances assumed ,162 ,698 -1,19 4 8 Equal variances not assumed -1,19 4 7,89 8 Cp max Equal variances assumed 2,230 ,174 -11,0 96 8
Equal variances not assumed -11,0 96 5,57 2 AUC 0-inf Equal variances assumed 1,261 ,294 -19,7 70 8 Equal variances not assumed -19,7 70 6,30 6 Vd Equal variances assumed ,006 ,940 -,020 8 Equal variances not assumed -,020 7,99 9 Cl Equal variances assumed 3,934 ,083 3,83 6 8 Equal variances not assumed 3,83 6 5,49 9
5. Hasil uji t test tidak berpasangan pengaruh pemberian minyak jelantah terhadap profil farmakokinetik parasetamol
Independent Samples Test
t-test for Equality of Means
Sig. (2-tailed) Mean Difference Std. Error Difference K Equal variances assumed ,000 ,0013826078 00 ,0001410509 73
Equal variances not assumed ,000 ,0013826078 00 ,0001410509 73 t1/2 e Equal variances assumed ,000 -87,14952182 1608290 8,402861400 885635
Equal variances not
assumed ,000 -87,14952182 1608290 8,402861400 885635 Ka Equal variances assumed ,630 ,012856000 ,025661348
Equal variances not assumed ,630 ,012856000 ,025661348 t1/2 a Equal variances assumed ,661 -,4909136684 66710 1,078057566 400849
Equal variances not
assumed ,661 -,4909136684 66710 1,078057566 400849
tmax Equal variances
assumed ,267 -4,945122615 514890 4,142275769 558204
Equal variances not
assumed ,267 -4,945122615 514890 4,142275769 558204
Cp max Equal variances
assumed ,000 -8,730948836 191395 ,7868507734 45966
Equal variances not
assumed ,000 -8,730948836 191395 ,7868507734 45966
AUC 0-inf Equal variances assumed ,000 -8214,388181 949930000 415,4954814 95351500
Equal variances not
assumed ,000 -8214,388181 949930000 415,4954814 95351500 Vd Equal variances assumed ,985 -,4542686401 49280 22,75294281 0826816
Equal variances not
assumed ,985 -,4542686401 49280 22,75294281 0826816 Cl Equal variances assumed ,005 ,3746323224 83623 ,0976637704 38932
Equal variances not
assumed ,010
,3746323224 83623
,0976637704 38932
Lampiran 13. Dokumentasi
Pencampuran pakan dengan minyak jelantah Pembuatan minyak jelantah
Hewan coba dikandangkan individual Pembuatan sediaan parasetamol
Sentrifuge sampel
darah+EDTA+TCA5% pada 2500 rpm
Pengambilan supernatan
Penambahan etil asetat pada plasma Pencarian panjang gelombang maksimal dan pemeriksaan spektrofotometri
Lampiran 14. Biodata mahasiswa
I. Identitas
1. Nama Lengkap : Faizurrahman Andi Kusuma
2. NIM : 22010112140182
3. Tempat, Tanggal lahir : Grobogan, 14 April 1994 4. Jenis Kelamin : Laki-laki
5. Alamat Rumah : Jl. Ahmad Yani 269 Purwodadi, Kab.Grobogan
6. E-mail : faizurrahmanandi@ymail.com
7. Telepon rumah/HP : 08574012498
II. Pendidikan
No. Tingkat Nama & Tempat Tahun Lulus
1. Sekolah Dasar SD Negeri 1 Kuripan 2006
2. Sekolah Menengah Pertama SMP Negeri 1 Purwodadi 2009 3. Sekolah Menengah Akhir SMA Negeri 1 Purwodadi 2012
4. Perguruan Tinggi Fakultas Kedokteran UNDIP 2012 - sekarang
III. Pengalaman Kepanitiaan
No Jabatan Tahun Nama Kepanitiaan
1. Sie Perlengkapan Mei 2014 Scientific Fair
2. Koordinator Sie Lomba September - Desember 2014 Porseni
3. Ketua Panitia September - Oktober 2015 Kemah Bakti
IV. Pengalaman Bekerja
No. Jabatan Tahun Nama Instansi