www.elsevier.nlrlocateraqua-online
ž
/
Medaka Oryzias latipes as a model for
hypoosmoregulation of euryhaline fishes
Tatsuya Sakamoto
a,), Tomohiro Kozaka
a, Akiyoshi Takahashi
b,
Hiroshi Kawauchi
b, Masaaki Ando
aa
Faculty of Integrated Arts and Sciences, Hiroshima UniÕersity, 1-7-1 Kagamiyama, Higashi-Hiroshima 739-8521, Japan
b
School of Fisheries Sciences, Kitasato UniÕersity, Sanriku, Iwate 022-0101, Japan
Received 30 January 2000; received in revised form 31 May 2000; accepted 6 July 2000
Abstract
Ž .
We examined the hypoosmoregulatory ability of a model fish, medaka Oryzias latipes , in Ž .
relation to the gill-chloride cells or mitochondrion-rich MR cells, and to cortisol. When the
Ž . Ž .
medaka were transferred from freshwater FW to 30‰ seawater SW , muscle water content decreased by 8% after 2 h and normalized within 1 week. Size and density of MR cells in the gill
Ž filament increased after 1–2 weeks in SW. Immersion of medaka in FW containing cortisol 10
.
mgrml for 1–2 weeks also doubled the number of MR cells, and abolished the decrease in Ž
muscle water content after SW transfer. In SW-adapted medaka, prolactin PRL; 10mgrg body
. Ž .
weight injection reduced muscle water content. However, when cortisol 50mgrg was injected simultaneously with PRL, cortisol abolished the decrease in muscle water. We concluded that cortisol plays an important role in the SW adaptation of the medaka, whereas PRL may be involved in ion uptake. Medaka seems to be a good model fish useful for the study of osmoregulatory mechanisms in general.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Osmoregulation; Cortisol; Prolactin; Chloride cell; Medaka; Oryzias latipes
1. Introduction
Ž . Ž .
Recently, medaka Oryzias latipes and zebrafish Brachydanio rerio are emerging as model organisms not only for applications in marine biotechnology but also for
)Corresponding author. Tel.:q81-824-24-6566; fax:q81-824-24-0759.
Ž .
E-mail address: [email protected] T. Sakamoto .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved.
Ž .
Ž . Ž .
general biology for the following reasons: 1 breeding is easy; 2 it is possible to inject
Ž .
DNA into hundreds of embryos; 3 whole-mount analysis is easy because of the transparent body. An embryonic stem-cell line for gene-targetingrknockout technology
Ž .
has also been derived from the medaka Hong et al., 1998 . By using these gene-manipu-lation techniques, analyses of genes involved in development and growth have been
Ž Ž ..
reported see Powers 1989 . However, genes related to osmoregulation have not been analyzed with these techniques, although osmoregulation is critical for aquaculture, and euryhaline fishes are suitable models for vertebrate osmoregulation. This is partly because little has been examined on the osmoregulation in these model fishes since the
Ž . Ž .
earlier report that prolactin PRL is involved in the freshwater FW adaptation of
Ž . Ž .
medaka as in other fishes Utida et al., 1971 . On the other hand, seawater SW -adapt-ing functions of cortisol, angiotensin II and growth hormone are extensively studied
Ž .
mostly in some aquaculture fishes see Takei, 1993; McCormick, 1995; Bentley, 1998 . The medaka is a euryhaline FW fish and can reproduce even in SW, whereas
Ž .
zebrafish is a stenohaline FW fish our unpublished observation . In order to establish the medaka as a model fish for the study of osmoregulatory mechanisms, we examined first the adaptive response of the medaka after SW transfer, and the effects of cortisol on
Ž .
hypoosmoregulation. Density and size of chloride cells or mitochondrion-rich MR cells
Ž
in gills, which are the sites of excess salt secretion Foskett and Scheffey, 1982;
.
Zadunaisky, 1984; Evans et al., 1999 , and muscle water content were analyzed after the treatments.
2. Materials and methods
2.1. Animals
Ž .
Adult fish of commercial orange-red variety of the medaka 0.2–0.3 g were kept for
Ž . Ž
about a month in FW aquaria 0‰ at 228C, and fed on Tetrafin flakes TetraWerke,
.
Melle, Germany . Lighting was by incident natural light and overhead fluorescent lights during the daytime. Experiments were conducted between March and May 1998. The daily feeding regime and water temperature were maintained during the experiments.
Effects of SW acclimation were examined by transferring the fish from FW to
Ž .
artificial 30‰ SW Sealife, Marinetech, Tokyo . Fish were decapitated 30 min, 1, 2, 4
Ž .
and 8 h, 1 day and 1 week after transfer, and samples of the paraxial muscle ca 100 mg were dissected out. Gill filaments were sampled 1 and 2 weeks after transfer. Muscles and gills of control fish were sampled before transfer or 1 and 2 weeks after transfer to another FW aquaria.
In a parallel experiment, the effects of long-term treatment of cortisol were examined
Ž
by immersing the fishes for 1 and 2 weeks in FW with cortisol
Na-hydrocortisone-21-.
hemisuccinate at a concentration of 10 mgrml. We chose this concentration because immersion in 1, 10 and 100 mg cortisolrml showed similar effects in a preliminary study. To maintain a fresh supply of cortisol, the water was changed daily. After the
Ž .
with cortisol for 1 week, four to five fishes were transferred to SW, and the muscles were sampled after 2 h.
Ž .
A single intraperitoneal injection of PRL or cortisol 3–100mgrg body weight into FW, intact fish did not have a significant effect on muscle water content 2 h after SW transfer. Therefore, to examine the effects of a single injection of these hormones, fish
Ž
adapted to SW for 1 week were injected intraperitoneally with saline 0.9% NaCl, 0.5%
. Ž .
bovine serum albumin, pH 9.0 , 10 mg ovine PRLrg NIH P-S-9 , or 10 mg ovine PRLrgq50 mg cortisol in a volume of 30 mlrg. All hormones were dissolved in saline. The muscles were sampled and weighed 20 h after the injection.
2.2. Muscle water content
Muscle water content was used as an indicator of osmoregulatory capability because it is difficult to collect blood from small medaka. Since in several teleost species, changes in muscle water content after salinity changes are inversely related to changes
Ž
in plasma osmolality, sodium and chloride Madsen, 1990a,b; Jensen et al., 1998; Kelly
.
et al., 1999 , the muscle water content of small fishes like the medaka seems to be a useful indicator of osmoregulatory capacity. Samples of muscle were immediately weighed after decapitation. The dry weights were obtained after drying at 1058C until
Ž .
constant weight was attained 2–5 h , and the water content was expressed as a percentage of wet weight.
2.3. Gill histology
To assess quantitatively the MR cells in gill filaments, a laser scanning confocal
Ž .
imaging system with dimethylaminostyrylethylpyridiniumiodine DASPEI -labeling was used. This technique is fast and powerful compared to laborious standard microscopical
Ž .
techniques Li et al., 1995; Van Der Heijden et al., 1997 .
The first and second gill arches were dissected and incubated for 1 h in Ringer
Ž .
solution, containing in mM 120 NaCl, 4.7 KCl, 3 CaCl , 1 MgSO , 1 KH SO , 24.92 4 2 4
Ž .
NaHCO , and 10 glucose, pH 7.7, to which DASPEI 23 mM; Sigma had been added to
Ž .
label the MR cells Bereiter-Hahn, 1976 . After washing briefly, the gill was placed on a glass slide in a drop of Ringer, surrounded by silicone grease. A coverslip was placed gently on top to prevent fluid evaporation. The fluorescence by DASPEI was recorded
Ž
with an MRC-600 Bio-Rad, Tokyo, Japan; dichroic reflector 510 nm; emission filter
. Ž
515 nm equipped with a Kr–Ar laser 5470 K, Ion Laser Technology, Salt Lake, UT;
. Ž .
excitation 488 nm and a microscope Axiovert 135 MTV, Zeiss, Tokyo, Japan as
Ž .
described previously Yokota et al., 1997 . For quantitative analyses, consecutive optical
Ž .
MR cells along a 100-mm length of a filament, and expressed as cell numberrmm gill
Ž
filament. The size of the MR cell was measured by image analysis NIH image, NIH,
.
Bethesda, MD .
Compared with this orange-red variety of medaka, gills of the black variety of
Ž .
medaka wild population are not suitable for DASPEI staining due to the
autofluo-Ž .
rescence of the pigment cells unpublished observation .
2.4. Statistics
Comparisons of size of MR cells between groups were assessed using the Kol-mogorov–Smirnov test. Significant difference was determined by the Student’s t-test or the least significant difference test after variance analysis.
3. Results
3.1. Effect of SW adaptation
Ž .
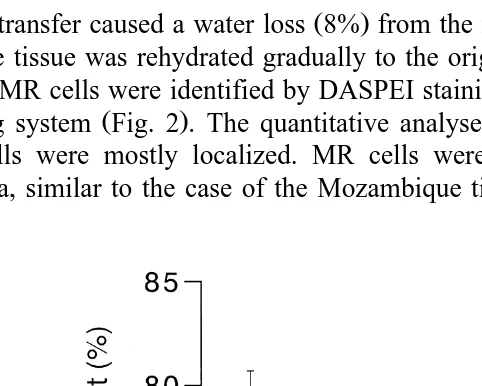
SW transfer caused a water loss 8% from the muscles of the medaka after 2 h, and
Ž .
then the tissue was rehydrated gradually to the original level Fig. 1 .
The MR cells were identified by DASPEI staining using the laser-scanning
confocal-Ž .
imaging system Fig. 2 . The quantitative analyses were made on the filament, where MR cells were mostly localized. MR cells were scarcely observed on the lamellar
Ž .
epithelia, similar to the case of the Mozambique tilapia Uchida et al., 2000 . Both the
Ž . Ž .
Fig. 2. a Micrograph of medaka gill filament indicating the analyzed area rectangle . L, lamellae; LE,
Ž .
leading edge; TE, trailing edge. Confocal laser scanning micrograph of optical sections 0.5 mm thick of the
Ž . Ž . Ž .
gill filaments of medaka in b FW, in c SW and d after 1-week immersion in FW with cortisol following DASPEI staining. MR cells are found on the trailing edge and the interlamellar spaces of the primary epithelium. Note that the MR cells in SW-adapted fish are larger than that in FW fish. Filament tips point to the right of the pictures. Scale bar, 100mm.
number and the size of the MR cells significantly increased 1–2 weeks after SW transfer, however electron microscopy would be required to determine the exact nature
Ž .
of MR cell–MR cell interactions like the multicellular complexes Figs. 2, 3, Table 1 .
Ž .
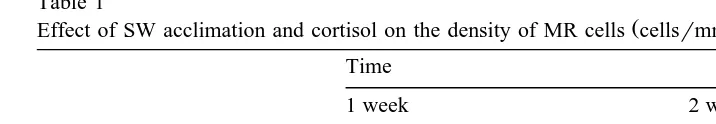
Table 1
Ž .
Effect of SW acclimation and cortisol on the density of MR cells cellsrmm filament in the medaka gills Time
Cortisol 112"13 111"20
All data are expressed as means"S.E. of 4–10 fishes. Asterisks indicate significant differences compared to the corresponding FW control.
3.2. Long-term effects of cortisol
The density of MR cells in the gill filament of cortisol-immersed fish was
signifi-Ž
cantly higher than that in the FW controls and similar to that in SW fish Fig. 2, Table
.
1 . The size of MR cells tended to increase as observed in SW fish but the difference
Ž .
was not significant Figs. 2, 3 .
Ž .
The muscle water content of cortisol-treated fish 77.4"0.6, ns5 was higher than
Ž . Ž .
that of controls 72.7"0.9, ns4 2 h after SW transfer P-0.01 .
3.3. Short-term effects of PRL and cortisol
Ž .
The muscle water content of SW fish treated with PRL 76.3"0.4, ns6 was lower
Ž .
than that of saline-injected SW controls 78.8"0.3, ns6; P-0.05 . The muscle water
Ž .
content of PRLqcortisol-injected fish 79.5"0.7, ns6 was higher than that of
Ž .
PRL-injected fish P-0.001 .
4. Discussion
In the present study, hypoosmoregulation of a FW fish, medaka, was examined in relation to gill chloride cell and their response to cortisol. Our results were compared with the reports using other euryhaline fishes for aquaculture, and suggest the utility of the medaka as a model system to study osmoregulation.
The muscle water content decreased temporarily after transfer of medaka from FW to SW, and was restored to the control level thereafter. This change confirmed the euryhalinity of the medaka, and it is essentially similar to the body-weight change
ŽHaruta et al., 1991 and to those reported in other euryhaline teleosts during SW.
Ž .
adaptation e.g., Madsen, 1990a,b . Both the density and size of MR cells in the gill filament increased significantly during SW adaptation. In the gills of euryhaline fishes,
Ž
the numbers andror sizes of filament MR cells or a-chloride cells possible SW-type
. Ž
MR cells are known to increase in SW Pisam et al., 1987; Uchida et al., 1996, 2000;
.
Ž
cells in FW Uchida et al., 1996; Evans et al., 1999; Hirai et al., 1999; Sasai et al.,
.
1999 .
The results of long-term treatment with cortisol shows the SW adaptive effect of cortisol in the medaka partly through an increase in the density of the MR cells. This is
Ž
also in line with the reports from tilapia and salmonids Foskett et al., 1981; Ouchi,
.
1985; Madsen, 1990a,b . The change in the size of MR cells after cortisol immersion, however, was not comparable to that observed in the SW medaka. Similar results are
Ž .
reported by Foskett et al. 1983 after cortisol treatment of FW tilapia, they observed an increase in MR cell density but no concomitant hypertrophy of the cells. Cortisol alone may be insufficient to evoke maximal hypertrophy or to activate MR cells: the changes
Ž .
in plasma concentrations of electrolytes, hormones e.g., decrease in PRL andror other factors may be involved in the full development of the MR cells. It is also possible that prolonged cortisol treatment down-regulates cortisol receptors.
The ion-retaining effect of PRL has been well established in several euryhaline
Ž .
teleosts e.g., Foskett et al., 1983; Takei, 1993; McCormick, 1995 including the
Ž .
hypophysectomized medaka Utida et al., 1971 . Although there is a risk of GH-like effects of ovine PRL, a single injection of ovine PRL dehydrated the SW-adapted medaka, perhaps reflecting the increased plasma electrolytes, and cortisol restored the normal osmoregulation in SW. The mechanism for this antagonism remains unclear, but
Ž .
this result is similar to the observation in brown trout Seidelin and Madsen, 1997 and also confirms that the SW-adaptive effect may be a fundamental action of cortisol in euryhaline teleosts. There seems to be two modes of action of cortisol: long term and short term.
In conclusion, the effects of SW transfer and cortisol on the osmoregulation of medaka are essentially similar to those of other euryhaline teleosts. The medaka can be a good model fish to study osmoregulation in teleosts, by assessing the MR cells and muscle water content, especially in combination with the gene-manipulation techniques.
Acknowledgements
We thank Dr. Tsuyoshi Ogasawara, Kanagawa University, for his valuable discussion and guidance, Dr. Felix G. Ayson, Southeast Asian Fisheries Development Center, for his critical reading of the manuscript, and the undergraduate students, Faculty of Integrated Arts and Sciences, Hiroshima University. This research was supported in part by grants-in-aid for scientific research from the Society for the Promotion of Science, the Ministry of Education, and the Fisheries Agency, Japan.
References
Bentley, B.J., 1998. Comparative Vertebrate Endocrinology. Cambridge Univ. Press, New York, 526 pp.
Ž .
Bereiter-Hahn, J., 1976. Dimethylaminostyrylmethylpyridiniumiodine DASPMI as a fluorescent probe for mitochondria in situ. Biochim. Biophys. Acta 423, 1–14.
Foskett, J.K., Scheffey, C., 1982. The chloride cell: definitive identification as the salt-secretory cell in teleosts. Science 215, 164–166.
Foskett, J.K., Longsdon, C.D., Turner, T., Machen, T.E., Bern, H.A., 1981. Differentiation of the chloride extrusion mechanism during seawater adaptation of a teleost fish, the cichlid Saratherodon mossambicus. J. Exp. Biol. 93, 209–224.
Foskett, J.K., Bern, H.A., Machen, T.E., Conner, M., 1983. Chloride cells and hormonal control of teleost fish osmoregulation. J. Exp. Biol. 106, 255–281.
Haruta, K., Yamashita, T., Kawashima, S., 1991. Changes in arginine vasotocin content in the pituitary of the
Ž .
medaka Oryzias latipes during osmotic stress. Gen. Comp. Endocrinol. 83, 327–336.
Hirai, N., Tagawa, M., Kaneko, T., Seikai, T., Tanaka, M., 1999. Distributional changes in branchial chloride cells during freshwater adaptation in Japanese sea bass Lateolabrax japonics. Zool. Sci. 16, 43–49. Hong, Y., Winkler, C., Schart, M., 1998. Production of medaka fish chimeras from a stable embryonic stem
cell line. Proc. Natl. Acad. Sci. U.S.A. 95, 3679–3684.
Jensen, M.K., Madsen, S.S., Kristiansen, K., 1998. Osmoregulation and salinity effects on the expression and
q q Ž .
activity of Na ,K -ATPase in the gills of European sea bass, Dicentrarchus labrax L. . J. Exp. Zool. 282, 290–300.
Ž .
Kelly, S.P., Chow, I.N.K., Woo, N.Y.S., 1999. Haloplasticity of black seabream Mylio macrocephalus : hypersaline to freshwater acclimation. J. Exp. Zool. 283, 226–241.
Li, J., Eygensteyn, J., Lock, R.A.C., Verbost, P.M., Van Der Heijden, A.J.H., Wendelaar Bonga, S.E., Flik, G., 1995. Branchial chloride cells in larvae and juveniles of freshwater tilapia Oreochromis mossambicus. J. Exp. Biol. 198, 2177–2184.
Madsen, S.S., 1990a. Enhanced hypoosmoregulatory response to growth hormone after cortisol treatment in immature rainbow trout, Salmo gairdneri. Fish Physiol. Biochem. 8, 271–279.
Madsen, S.S., 1990b. The role of cortisol and growth hormone in seawater adaptation and development of
Ž .
hypoosmoregulatory mechanisms in sea trout parr Salmo trutta trutta . Gen. Comp. Endocrinol. 79, 1–11. McCormick, S.D., 1995. Hormonal control of gill Naq,Kq-ATPase and chloride cell function. In: Wood,
Ž .
C.M., Suttleworth, T.J. Eds. , Cellular and Molecular approaches to Fish Ionic Regulation. Academic Press, New York, pp. 285–315.
Ž .
Ouchi, K., 1985. Effect of cortisol on the tolerance of masu salmon Oncorhynchus masou parr adapted to different salinities. Bull. Natl. Res. Inst. Aquacult. 7, 21–27.
Pisam, M., Caroff, A., Rambourg, A., 1987. Two types of chloride cells in the gill epithelium of a freshwater-adapted euryhaline fish: Lebistes reticulatus; their modifications during adaptation to saltwater. Am. J. Anat. 179, 40–50.
Powers, D.A., 1989. Fish as model systems. Science 246, 352–358.
Sasai, S., Kaneko, T., Hasegawa, S., Tsukamoto, K., 1999. Morphological alteration in two types of gill
Ž .
chloride cells in Japanese eel Anguilla japonica during catadromous migration. Can. J. Zool. 76, 1480–1487.
Seidelin, M., Madsen, S.S., 1997. Prolactin antagonizes the seawater adaptive effect of cortisol and growth
Ž .
hormone in anadromous brown trout Salmo trutta . Zool. Sci. 14, 249–256.
Ž .
Takei, Y., 1993. Role of peptide hormones in fish osmoregulation. In: Rankin, J.C., Jensen, F.B. Eds. , Fish Ecophysiology. Chapman & Hall, London, pp. 136–160.
Uchida, K., Kaneko, T., Yamauchi, K., Hirano, T., 1996. Morphometrical analysis of chloride cell activity in the gill filaments and lamellae and changes in Naq,Kq-ATPase activity during seawater adaptation in
chum salmon fry. J. Exp. Zool. 276, 193–200.
S. Utida, S. Hatai, T. Hirano, F.I. Kamemoto, 1971. Effect of prolactin on survival and plasma sodium levels in hypophysectomized medaka Oryzias latipes, 16, 556–573.
Van Der Heijden, A.J.H., Verbost, P.M., Eygensteyn, J., Li, J., Wendelaar Bonga, S.E., Flik, G., 1997.
Ž .
Mitochondria-rich cells in gills of tilapia Oreochromis mossambicus adapted to fresh water or seawater: quantification by confocal laser scanning microscopy. J. Exp. Biol. 200, 55–64.
Yokota, S., Iwata, K., Fujii, Y., Ando, M., 1997. Ion transport across the skin of the mudskipper Periophthalmus modestus. Comp. Biochem. Physiol. 118A, 903–910.
Zadunaisky, J., 1984. The chloride cell: the active transport of chloride and the paracellular pathways. In:
Ž .