Variation of diastereoisomeric pyrrolizidine
alkaloids in
Pleurothallis
(Orchidaceae)
Eduardo L. Borba
!
,
*
, Jose
H
Roberto Trigo
"
,
#
, Joa
8
o Semir
!
!Departamento de BotaLnica, Instituto de Biologia,Universidade Estadual de Campinas, UNICAMP, Cx. P. 6109, 13083-970 Campinas, SP, Brazil
"CPQBA, UNICAMP, Cx. P. 6171, Campinas, SP, 13083-970, Brazil
#Lab. Ecologia Qun&mica, Departamento de Zoologia, Instituto de Biologia, UNICAMP, Cx. P. 6109, Campinas, SP, 13083-970, Brazil
Received 4 November 1999; received in revised form 23 February 2000; accepted 28 February 2000
Abstract
The alkaloids of leaves and#owers of 18 populations of fourPleurothallis(Orchidaceae)
species were characterized. Both leaves and#owers of individuals from all populations have two
diasteroisomers of 1-hydroxymethylpyrrolizidine (RI"1290 and 1311). The 1290 isomer was
the most abundant in all populations ofP. johannensis(74}93%) andP. fabiobarrosii(63}93%),
two morphologically closed species. On the other hand, in almost all populations ofP. teresthe
1311 isomer is the more abundant (61}95%), except in one population occurring disjunct from
the core area distribution of the species. These results support the recent taxonomic revision for
these three species. Almost allP. ochreatapopulations also have the 1290 as the most abundant
isomer, except in one morphologically di!erentiated population situated at the southern
extreme of the distribution of this species. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Pleurothallis; Orchidaceae; Campo rupestre vegetation; Pyrrolizidine alkaloids; 1-Hy-droxymethylpyrrolizidine; Chemosystematic
1. Introduction
The&campo rupestre'vegetation, occurring in the Northeastern and Southeastern regions of Brazil, is characterized by open vegetation growing in a sandy}stony soil
*Corresponding author. Tel.:#55-19-788-7802; fax:#55-19-289-3124. E-mail address:[email protected] (E.L. Borba).
and herbs, sub-shrubs and shrubs growing on&islands'of quartzite, arenite or gneiss rock (Giulietti and Pirani, 1988; Borba and Semir, 1998).
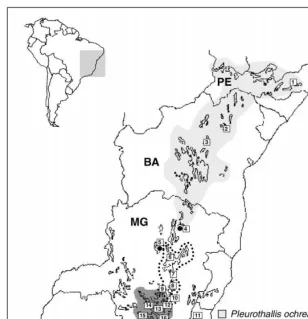
A group of rupicolous species of the genusPleurothallis(Orchidaceae), termed by Pabst and Dungs (1975) as thePleurothallis rupestrisalliance, occurs throughout the &campo rupestre' vegetation. The taxonomy of this group is di$cult and has been controversial among taxonomists. Recently, Borba et al. (2000) made a taxonomic revision of this species group and described a new species,P. fabiobarrosiiBorba and Semir, known only from three localities in north-central Minas Gerais state. Pleur-othallis johannensisBarb. Rodr., a species found only in southeastern Minas Gerais, was re-established, andP. rupestris Lindl. was synonymized under P. teresLindl., a species almost restricted to the&campos rupestres' of the central region of Minas Gerais. Pleurothallis ochreata, a species previously known only from the states of Bahia and Pernambuco, was collected for the"rst time in Minas Gerais in that work; this is the only population of this species in which the individuals have cylindrical leaves (see Fig. 1 for the geographical distribution of these species).
In the course of a study on the volatile#oral compounds of these species (Borba et al., unpublished data), we found two diastereoisomers of a 1-hydroxymethylpyr-rolizidine (laburnine-like) alkaloid, whose relative abundances were variable in di! er-ent populations. In order to verify the usefulness of the alkaloid in the taxonomy of this species group, we analyzed the alkaloid pro"les in several populations of these four species and compared the results to the taxonomic revision proposed by Borba et al.(2000) (See Fig. 2).
2. Materials and methods
2.1. Organisms
We used leaves and#owers (when available) of plants from 18 populations of four Pleurothallisspecies occurring in Minas Gerais (MG), Bahia (BA), Pernambuco (PE) and Rio de Janeiro (RJ) states, in the Northeastern and Southeastern regions of Brazil (Table 1; Fig. 1). Flowers of plants from all populations were collected directly in the "eld. Leaves were collected from individuals of the same populations cultivated in a greenhouse for at least four months. Vouchers were deposited in the herbarium of the Universidade Estadual de Campinas (UEC; Table 1).
2.2. Extraction of pyrrolizidine alkaloids
Fig. 1. Proposed distribution mapof Pleurothallis teres,P. ochreata, P. johannensisandP fabiobarrosii based on herbarium material and"eld collection. The occurrence area of the&campos rupestre'vegetation is shown: 1*Camocim de Sa8o FeHlix, 2*Jacobina, 3*Morro do ChapeHu, 4*Gra8o Mogol, 5*Serra do Cabral, 6*Diamantina, 7*Serra do CipoH, 8*CaeteH, 9*Serra do Rola Moc7a, 10*Ouro Preto, 11
*Santa Maria Madalena, 12*Sa8o Joa8o Del Rei, 13*Nazareno, 14*Itutinga, 15*Carrancas, and 16
*Santa Rita do Ibitipoca.
Table 1
Populations ofPleurothallis johannensis,P. teres P. ochreata and P. fabiobarrosiiused in this study. Vouchers deposited in the herbarium of the Universidade Estadual de Campinas (UEC)
Species/population Location Voucher
The total alkaloid extracts were diluted in MeOH and analyzed on a GC system HP-5890 series II, equipped with a fused silica capillary column (SA-5, Sigma, 5% diphenyl}95% dimethylsiloxane, 30 m]0.25 mm]0.25lm) coupled to an HP-5970 mass detector. Alkaloid characterization was based on its mass fragmentation (Neuner-Jehle et al., 1965). A retention index (RI) was calculated by co-injection of n-alkanes following van den Dool and Kratz (1963). To quantify the alkaloids, they were analyzed in a GC system as above, equipped with a fused silica capillary column (DB1, J&W Scienti"c, 100% polydimethylsiloxane, 30 m]0.25 mm]0.25]lm) coupled with a FID; methyl stearate was used as external reference substance. The GC conditions were: injector temperature, 2503C; detector temperature, 2803C; temper-ature program, 403C (5 min), 40}3003C, 63C/min; splitless, 0.5 min; carrier gas, He, 1 ml/min.
2.4. High-resolution mass spectrometry
2.5. Statistical analysis
We tested the means of relative abundance of one diastereosiomer betweenP. teres and P. johannensis and tissues (#owers and leaves), using di!erent populations as samples. An arcsin transformation was performed and the data tested for normality and homogeneity of variances. Since the data agreed with both parameters, two-way ANOVA was performed (Zar, 1984).
3. Results and discussion
We found pyrrolizidine alkaloids in#owers and leaves of all populations studied. The total alkaloid content ranged from 0.31 to 6.12 mg/g of dry tissue (Table 2). The total alkaloid content of all populations corresponds to two diastereoisomers of a 1-hydroxymethylpyrrolizidine (1and 2, Fig. 2) [RI"1290 and 1311, HRMSm/z 141.1135 (C
8H15NO, M`, 19, calc. 141.1154), 124.1109 (M-OH, 15, calc. 124.1127),
110.0944 (M-CH
2OH, 10, calc. 110.0970), 83.0718 (C5H9N, 100, calc. 83.0736),
82.0641 (C
5H8N, 62, calc. 82.0657)]. No further NMR and optical rotation analysis
were performed to determine the four possible isomers (laburnine, isoretronecanol, trachelantamidine, and lindelo"dine). These alkaloids or derived substances have been found in several orchid species (LuKning and TraKnkner, 1965, 1968; Leander and LuKning, 1967; Nishikawa and Hirata, 1967, 1968; Nishikawa et al., 1967, 1969; BrandaKnge and LuKning, 1969; LindstroKm and LuKning, 1969, 1971, 1972; BrandaKnge et al., 1970, 1971, 1972; LindstroKm et al., 1971; BrandaKnge and Granelli, 1973).
Leaves and#owers of all populations ofP. johannensis and the morphologically relatedP. fabiobarrosiihave a higher abundance of the isomer RI"1290 (74}93% and 63}93%, respectively). On the other hand, in both leaves and #owers of most populations of P. teres, except the population from Santa Maria Madalena-RJ, the RI"1311 is the more abundant isomer (61}95%; Table 2). The latter population is disjunct from the core area distribution ofP. teres(Fig. 1), and occurs in a di!erent environment from all other populations, a granite rock in the Atlantic Rain Forest of Easthern Brazilian. The two-way ANOVA showed that the relative abund-ance of the diastereoisomers in tissues as signi"cantly di!erent betweenP. teresandP. johhannensis (P(0.01); there were no signi"cant di!erences between leaves and
#owers of the same species (p"0.192) and no interactions between species and tissues (p"0.213).
These results agree with the delimitation of these species as proposed by Borba et al.(2000). Comparable results with other orchids were found with diastereoisomers of 1-hydroxymethylpyrrolizidine (laburnine and lindelo"dine) in two species of Vandop-sis, with 1:3 (V. lissochiloides) and 10 : 1 (V. gigantea) ratios (BrandaKnge and Granelli, 1973).
Table 2
Pyrrolizidine alkaloids found in leaves and#owers ofPleurothallis johannensis,P. teres,P. ochreataandP. fabiobarrosiipopulations!
Population Tissue Total alkaloid Relative abundance (%) Proportion of
Content (mg/g diastereisomers
dry weight) RI 1290 RI 1311 (1290:1311)
P. johannensis
Carrancas Leaf 3.61 75.6 24.4 3.1:1
Flower Nc 93.3 6.7 14.0:1
Itutinga Leaf 4.90 73.5 26.5 2.8:1
Flower Nc 82.7 17.3 4.8:1
Santa Rita do Ibitipoca Leaf Nc 87.0 13.0 6.7:1
Flower 4.89 88.3 11.7 7.6:1
Serra do CipoH/APA Leaf 1.87 37.9 62.1 1:1.6
Flower Nc 26.7 73.3 1:2.7
Serra do CipoH/Congonhas Leaf Nc 30.2 69.8 1:2.3
Flower Nc 22.9 77.1 1:3.4
Ouro Preto Leaf Nc 11.1 88.9 1:8.0
CaeteH Leaf 1.44 38.8 61.2 1:1.6
Diamantina Leaf 4.54 22.8 77.2 1:3.4
Flower Nc 27.1 72.9 1:2.7
Santa Maria Madalena Leaf 1.63 95.8 4.2 22.8:1
P. ochreata
Gra8o Mogol Leaf 1.11 16.8 83.2 1:4.9
Flower Nc 18.3 81.7 1:4.5
Jacobina Leaf 0.93 90.2 9.8 9.2:1
Camocim de Sa8o FeHlix Leaf Nc 95.7 4.3 22.3:1
Morro ChapeHu Leaf 0.74 85.9 14.1 6.1:1
P. fabiobarrosii
Gra8o Mogol Leaf 0.64 93.4 6.6 14.2:1
Serra do Cabral Leaf 0.31 63.1 36.9 1.7:1
!RI"retention index, nc: not calculated, tr: trace.
individuals have linear cylindrical leaves while the individuals of the other popula-tions have large conduplicate leaves. Our results, together with the morphological data, suggest that this population could represent a distinct sub-species.
di!erentiation, such as the formation of chemotypes or even geographical speciation. At least in the population ofP. teresfrom Santa Maria Madalena this conclusion is supported by population genetics studies using isozyme markers (Borba et al., unpub-lished data), in spite of which we could not detect any morphological di!erence in this population.
Acknowledgements
We thank CleHcio F. Klitzke for the HRMS analysis, Adilson Sartoratto for tech-nical support, and Keith S. Brown Jr., MaHrcio Z. Cardoso and two anonymous reviewers for improvements on the manuscript. We are grateful to Vera N. Solferini, Veridiana N. Vacarelli, A)ngela B. Martins, Rosana Romero for collecting some of the Pleurothallis, and Juliana M. Felix, MaHrcio Lucca and Graciela S. Oliver for helping in"eld trips. ELB received a fellowship from the Fundac7a8o de Amparo a` Pesquisa do Estado de Sa8o Paulo (FAPESPd97/08800-1). This work was funded by a FAPESP grant to JS (FAPESPd97/08795-8) and JRT (FAPESPd98/01065-7).
References
Borba, E.L., Semir, J., 1998. Wind-assisted#y pollination in three Bulbophyllum(Orchidaceae) species occurring in the Brazilian campos rupestres. Lindleyana 13, 203}218.
Borba, E.L., Felix, J.M., Semir, J., Solferini, V.N., 2000.Pleurothallis fabiobarrosii, a new Brazilian species: morphological and genetic data, and notes on the taxonomy of Brazilian rupicolousPleurothallis. Lindleyana15, 2}9.
BrandaKnge, S., Granelli, I., 1973. Studies on Orchidaceae alkaloids XXXVI. Alkaloids from someVandaand Vandopsisspecies. Acta Chem. Scand. 27, 1096}1097.
BrandaKnge, S., LuKning, B., 1969. Studies on Orchidaceae alkaloids XII. Pyrrolizidine alkaloids from Phalaenopsis amabilisBl. andPh. manniiRchb. f. Acta Chem. Scand. 23, 1151}1154.
BrandaKnge, S., Granelli, I., LuKning, B., 1970. Studies on Orchidaceae alkaloids XVIII. Isolation of phalaenopsin fromKingiella taenialis(Lindl.) Rolfe. Acta Chem. Scand. 24, 354}355.
BrandaKnge, S., LuKning, B., Moberg, C., SjoKstrand, E., 1971. Studies on Orchidaceae alkaloids XXIV. A pyrrolizidine alkaloid fromPhalaenopsis cornu-cerviRchb. f. Acta Chem. Scand. 25, 349}350. BrandaKnge, S., LuKning, B., Moberg, C., SjoKstrand, E., 1972. Studies on Orchidaceae alkaloids XXX.
Investigations in fourteenPhalaenopsisspecies. A new pyrrolizidine alkaloid fromPhalaenopsis equestris Rchb. f. Acta Chem. Scand. 26, 2558}2560.
Giulietti, A.M., Pirani, J.R., 1988. Patterns of geographic distribution of some plant species from the Espinhac7o range, Minas Gerais and Bahia, Brazil. In: Heyer, W.R., Vanzolini, P.E. (Eds.), Proceedings
of a Workshop of Neotropical Distribution Patterns. Academia Brasileira de Cie(ncias, Rio de Janeiro, pp. 39}69.
Leander, K., LuKning, B., 1967. Studies on Orchidaceae alkaloids VII. Structure of a glucosidic alkaloid from Malaxis congestacomb. Nov. (Rchb. f.). Tetrahedron Lett. 36, 3477}3478.
LindstroKm, B., LuKning, B., 1969. Studies on Orchidaceae alkaloids XIII. A new alkaloid, laburnine acetate, fromVanda cristataLindl. Acta Chem. Scand. 23, 3352}3354.
LindstroKm, B., LuKning, B., 1971. Studies on Orchidaceae alkaloids XXIII. Alkaloids fromLiparis loeselii(L.) L. C. Rich. andHammarbya paludosa(L.) O. K. Acta Chem. Scand. 25, 895}897.
LindstroKm, B., LuKning, B., Siirala-HanseHn, K., 1971. Studies on Orchidaceae alkaloids XXVI. A new glycosidic alkaloid fromMalaxis grandifoliaSchltr. Acta Chem. Scand. 25, 1900}1903.
LuKning, B., TraKnkner, H., 1965. Studies on Orchidaceae alkaloids II. Structure of alkaloids fromChysis bractescensLindl. Tetrahedron Lett. 14, 921}922.
LuKning, B., TraKnkner, H., 1968. Studies on Orchidaceae alkaloids X. A pyrrolizidine alkaloid fromChysis bractescensLindl. Acta Chem. Scand. 22, 2324}2328.
Neuner-Jehle, N., Nesvadba, H., Spiteller, G., 1965. Anwendung der Massenspektrometrie zur Strukturauf-klaKrung von Alkaloiden, 6.Mitt: Pyrrolizinalkaloide aus dem Goldregen. Mh. Chem. 96, 321}338. Nishida, R., Kim, C., Fukami, H., Irie, R., 1991. Ideamine N-oxides: pyrrolizidine alkaloids sequestered by
the danaine butter#y,Idea leuconoe. Agric. Biol. Chem.55, 1787}1792.
Nishikawa, K., Hirata, Y., 1967. Chemotaxonomical alkaloid studies I. Structure of nervosine. Tetrahedron Lett. 27, 2591}2596.
Nishikawa, K., Hirata, Y., 1968. Chemotaxonomical alkaloid studies III. Further studies of Liparis alkaloids. Tetrahedron Lett. 60, 6289}6291.
Nishikawa, K., Miyamura, M., Hirata, Y., 1967. Chemotaxonomical alkaloid studies II. Structures of kuramerine and kumokirine. Tetrahedron Lett. 27, 2597}2600.
Nishikawa, K., Miyamura, M., Hirata, Y., 1969. Chemotaxonomical alkaloid studies. Structures ofLiparis alkaloids. Tetrahedron 25, 2723}2741.
Pabst, G.F.J., Dungs, F., 1975.Orchidaceae Brasiliensis, Vol. 1. Kurt Schmersow, Hildesheim.
Trigo, J.R., Brown Jr., K.S., Henriques, S.A., Barata, L.E.S., 1996. Quantitative patterns of pyrrolizidine alkaloids in Ithomiinae butter#ies. Biochem. Syst. Ecol. 24, 181}188.