255 (2000) 21–36
www.elsevier.nl / locate / jembe
Growth, metabolic rate, and digestive enzyme activity in the
white shrimp Litopenaeus setiferus early postlarvae fed
different diets
a b b b ,*
´
Roberto Brito , Marıa Eugenia Chimal , Gabriela Gaxiola , Carlos Rosas
a
Center of Marine Research, Havana University, Havana, Cuba
b
Group of Experimental Marine Biology, Laboratory of Ecophysiology, Faculty of Sciences, UNAM. Apdo.
Post. 69, Ciudad del Carmen, Campeche, Mexico
Received 10 March 2000; received in revised form 27 June 2000; accepted 27 August 2000
Abstract
Growth rate, soluble-protein content, oxygen consumption, ammonia excretion, and digestive-enzyme activity were studied in Litopenaeus setiferus early postlarvae under four feeding regimens that included combinations of freshly hatched Artemia nauplii, microparticulate commercial diet, and algae. Growth and of postlarvae fed a mixed diet were significantly higher. Artificial diet used alone caused the lowest growth, lowest soluble-protein content, higher ammonia excretion, lowest O:N ratio, and higher proteolytic and amylase activities. The artificial diet stimulated proteolytic activity and ammonia excretion of postlarvae, apparently in response to some deficiency in protein composition of the diet. Based on results in growth, soluble-protein content, enzymatic activity, and metabolic substrate, we determined that partial substitution of
Artemia nauplii by artificial diet, with or without addition of algae when rearing early postlarval
stages, will benefit the growth and nutritional state of L. setiferus postlarvae. 2000 Elsevier
Science B.V. All rights reserved.
Keywords: Shrimp; Litopenaeus setiferus; Growth; Metabolic rate; Enzymes
1. Introduction
Penaeid larval development is among the most complicated processes in decapods. Penaeid shrimp have three planktonic larval stages, nauplius, protozoea, and mysis, each with a number of instars, and then metamorphosis into postlarva. A gradual shift from
*Corresponding author. Tel.:152-938-28730; fax:152-298-908-544.
E-mail address: crv@hp.fciencias.unam.mx (C. Rosas).
planktonic to benthonic behavior occurs during the first 2 weeks of postlarval life while shrimp migrate to shallow inshore, brackish nursery grounds in estuaries, accompanied
´
by changes in their feeding habits (Perez-Farfante, 1969).
Coinciding with the shift in behavior and feeding habits in penaeid early postlarvae, a wide range of changes in morphology and physiology that permit adaptation to a varying environment is produced. Felder et al. (1985) and Lovett and Felder (1989, 1990a,b) have described ontogenetic changes associated with the development of the digestive tract, kinematics in the gut, and changes in digestive-enzyme activity of larval and postlarval stages of the white shrimp Litopenaeus setiferus. Modifications in digestive-enzyme activity in different penaeid shrimp larvae have also been related to the quantity
´
or quality of some component of the diet (Le-Vay et al., 1993; Rodrıguez et al., 1994; Le Moullac et al., 1996; Lemos and Rodriguez, 1998). Rosas et al. (1995) have associated the metabolic rate with ingestion rate and type and concentration of food provided during larval development of L. setiferus. Physiological and biochemical changes associated with the development of the shrimp Metapenaeus ensis have been studied by Chu and Ovsianico-Koulikowsky (1994) using the O:N ratio as an index of substrate used for oxidative metabolism. Ontogenetic changes in respiratory metabolism of L. setiferus and L. schmitti larvae under optimum feeding conditions have been described by Rosas et al. (1997a,b). In addition, Rosas et al. (1995, 1996) have analyzed the effect of protein levels of purified test diets on substrate metabolism and on apparent heat increment and postprandial nitrogen excretion in postlarvae of four penaeid species. The atomic ratio between oxygen consumption and N-ammonia excretion has been widely used as an index of the metabolic substrate that is oxidized by crustaceans (Regnault, 1981; Dall and Smith, 1986; Rosas et al., 1995). Theoretical values of O:N between 3 to 16 have been suggested for catabolism of proteins, whereas catabolism of equal quantities of proteins and lipids yield O:N values between 50 and 60. Greater values of O:N correspond to an increase in lipid and carbohydrate catabolism.
Sandifer et al. (1993) and Hopkins et al. (1993) stated that L. setiferus might be an alternative species for intensive culture on the Atlantic coast of the Americas. This agrees not only with commercial interests, but also with the ecological interest in the development of an aquaculture based on local species. The present lack of knowledge of seed production and lower growth rate found in L. setiferus as compare with other shrimps are some of the limiting factors in the commercial culture of this species. Low growth reported by Samocha et al. (1998) when studied the feasibility of culture L. setiferus as bait in Texas, USA, could be related with the improper diet used and inadequate pond management. Studies focused on better diet combinations for post-larvae, based on biochemical and physiological methods, will help improve the culture of L. setiferus.
2. Materials and methods
2.1. Experimental animals
Larvae of Litopenaeus setiferus were bred in the Laboratory of Ecophysiology, ´
Faculty of Sciences, UNAM, Ciudad del Carmen, Campeche, Mexico, from spawners captured along the coasts of Isla del Carmen, Gulf of Mexico. Larvae were reared until mysis I (M ) in 400-l fiberglass tanks, using the feeding schedule proposed by GallardoI
21 et al. (1995) based on diatoms (Chaetoceros gracilis, nauplius V (NV) 30 000 cell ml ,
21 21
protozoea I-II (PZ I-II) 30 000 cell ml , PZ –MIII I 40 000 cell ml ), flagellates
21 21
(Tetraselmis chuii, NV –PZII 2000 cell ml , PZ –M 3000 cell mlIII I ), and freshly
21 hatched Artemia nauplii (Aquatic Eco-Systems Inc.), (PZIII 0.5 nauplii ml , M 1I
21 nauplii ml ).
2.2. Experimental conditions
At stage M , larvae were transferred to 35-l plastic tanks and stocked at an initialII
21
density of 60 larvae l . Temperature and salinity during the experiments were 28618C and 3561‰. A photoperiod of 13:11-h light / dark, and constant aeration was maintained throughout the experiments. Daily 50–70% water exchanges were made using a 400-mm mesh to eliminate molts, debris, and remaining Artemia nauplii. Larvae were reared in these conditions from MII until postlarva 10 (PL , 10 days after metamorphosis)10 following different feeding regimes (Table 1). Food density was adjusted four times daily during all the experiments.
A microparticulate commercial diet (MCD) was used (Microfeast MY-100, Burns Philp Food Inc.). This diet has a proximate composition of 50% protein, 12% lipids, 12% ash, and 5% moisture, with 50–100 mm particle size and contains the following
Table 1
a
Feeding scheme used in the different treatments
Treatment Food Stages
MII MIII PL1 PL2 – 6 PL7 – 10
1 Artemia nauplii 1 1.5 1.5 2 2.5
2 MCD 0.04 0.04 0.04 0.04 0.04
3 MCD 0.02 0.02 0.02 0.02 0.02
Artemia nauplii 0.5 0.75 0.75 1 1.25
4 MCD 0.02 0.02 0.02 0.02 0.02
Artemia nauplii 0.5 0.75 0.75 1 1.25
Chaetoceros gracilis 30 000 30 000 30 000 35 000 40 000
Tetraselmis chuii 3000 3000 3000 3500 4000
a 21
Alga concentration in cell ml , freshly hatched Artemia nauplii (Aquatic Eco-Systems Inc.) concentration
21
in nauplii ml , microparticulate commercial diet (MCD, Microfeast MY-100, Burns Philp Food Inc.) in
21
ingredients; squid meal, fish and vegetable protein, processed fish oil, yeast, lecithin, cholesterol, stabilized vitamin C, vitamins, minerals, and ethoxyquin as an antioxidant. For growth rate and enzymatic activity analysis, a single batch of larvae was used for the different diet treatments. Because the time needed to obtain enough number of oxygen consumption and ammonia excretion data, different batches of larvae maintained in the same environmental conditions, were used for oxygen consumption and ammonia excretion measurements in each diet treatment.
From MIII to PL10 stages, samples of ten shrimp were collected daily in each treatment for determination of dry weight. Shrimp were washed with distilled water and oven-dried at 608C for 24 h. Dry weights were measured using a CAHN model C-33 microbalance with 0.001 mg precision.
2.3. Enzyme analysis
Between 30 and 50 shrimp, depending on the development stage, were collected at M , PLIII 1 – 3, PL , and PL5 10 stages from each treatment for enzyme assays. All samples were collected between 10:00 and 12:00 h, 2–4 h after feeding. Shrimp were washed with distilled water and immediately frozen at 2708C in 1-ml micro-test tubes until enzyme assays were done. Frozen samples were homogenized in 500-ml ice-cold de-ionized water. Homogenates were centrifuged at 16 0003g for 6 min at 48C. Part of the supernatant was diluted in 10 volumes of ice-cold de-ionized water. Homogenates (crude or diluted) were immediately used for enzyme analysis. (van Wormhoudt, 1999, personal communication).
The soluble-protein content was measured in diluted homogenates by the Bradford method (Bradford, 1976) using the Sigma micro protein determination kit (Procedure No. 610). Samples were read in a Biorad model 550 microplate reader at 495 nm. Duplicate assays for each sample were made.
General protease activity was estimated in crude homogenates using azocoll (Sigma A4341) as substrate in phosphate buffer, pH 7.5 (Todd, 1949). Absorbance was measured in a spectrophotometer Spectronic model 21D at 520 nm. For this method, one unit is defined as the amount of enzyme which catalyzes the release of azo dye causing a
21
DA /Dt50.001 min (Walter, 1988). Each sample was assayed in duplicate.
Amylase activity was assayed in diluted homogenates according to Bernfeld (1955) with 1% oyster glycogen (Sigma G8751) as substrate in 10 mM phosphate buffer, pH 7. One unit of amylase activity was defined as 1 mg of maltose liberated in 1 min at 378C. Each sample was assayed in duplicate.
2.4. Oxygen consumption and ammonia excretion
Isotemp refrigerated circulator, model 900) maintaining a constant temperature in the chambers.
Larvae were acclimated for 30 min in the chambers fallowed by measurement of oxygen consumption for a 10-min period using an oxygen microelectrode (model 781, Strathkelvin Instrument, Glasgow, UK).
For the determination of ammonia excretion, the same larvae used for oxygen consumption were kept in the respirometric cells for 2 h. Ammonia excretion was the difference of the ammonia concentration in the water in the cells between the beginning and end of this period. Samples for the determination of ammonia were taken in Ependorff tubes with sublimated iodine and they were preserved frozen until they were processed, always less than 12 h after sampling.
Dissolved total ammonia in samples was determined using the flow injection-gas diffusion technique (Hunter and Uglow, 1993), which permits the use of small samples with low ammonia concentrations. Concentrations of samples were measured with reference to a calibration curve derived using accurate dilutions of (NH ) SO4 2 4 as standard solutions. The equipment was coupled to a PC and used software made by Dispositivos NaFri S.A. de C.V.
One control chamber without shrimp for each five experimental chambers with larvae was used in the measurements of oxygen consumption and ammonia excretion. In all the treatments, ten to 15 measurements were made from MIII to PL . Using individual10 oxygen consumption and ammonia excretion data, the O:N ratio was calculated. After the measurements of oxygen consumption and ammonia excretion, individual larva dry weights were determined.
The relation between dry weight and age in days of the postlarvae in different treatments was analyzed with a simple linear correlation and regression analysis with logarithmic transformation of weight data. Regression coefficients were contrasted by analysis of covariance. Post-hoc comparisons between slopes and intercepts were made using the Tukey–Kramer method (Sokal and Rohlf, 1981). The same procedure was used for the analysis of the relation between logarithmically transformed soluble-protein content per larvae and age in days of postlarvae. Oxygen consumption, ammonia excretion, and O:N ratio data were contrasted with two-factor analysis of variance (Zar, 1984). Differences were reported as statistically significant when P,0.05.
3. Results
3.1. Growth rate and soluble protein content
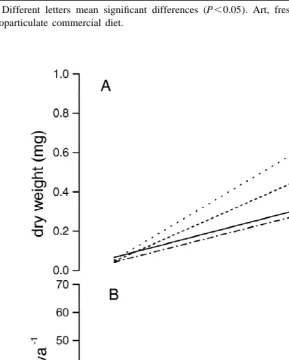
Postlarval growth, measured as an increase in dry weight, was affected by different diets (Table 2, Fig. 1a). Analysis of covariance indicated that postlarvae fed a mixture of Artemia nauplii, MCD, and algae (treatment 4), showed significantly highest growth rate
21 21
(0.12 mg dw day ), followed by treatment 3 (0.11 mg dw day ). Postlarvae fed on Artemia nauplii or artificial diet alone showed the significantly lowest growth rate (0.08
21
Table 2
Correlation and regression coefficients, and covariance analysis for the relation between dry weight (mg) and age in days for L. setiferus postlarvae fed different diets
Treatment Diet r P Slope F( 3, 601 ) Intercept F( 2,310 )
b a9
1 Art 0.93 ,0.001 0.08 – 0.07 –
b b9
2 MCD 0.91 ,0.001 0.09 92.02 0.04 18.15
a b
3 MCD1Art 0.95 ,0.001 0.11 – 0.04 –
a a
4 MCD1Art1algae 0.95 ,0.001 0.12 – 0.05 –
a,b
Different letters mean significant differences (P,0.05). Art, freshly hatched Artemia nauplii; MCD, microparticulate commercial diet.
Table 3
Correlation and regression coefficients, and covariance analysis for the relation between soluble-protein
21
content (mg protein larvae ) and age in days for L. setiferus postlarvae fed different diets
Treatment Diet r P Slope F( 3, 40 ) Intercept F( 3,44 )
Different letters mean significant differences (P,0.05). Art, freshly hatched Artemia nauplii; MCD, microparticulate commercial diet.
and was also affected by diet (Table 3, Fig. 1b). Postlarvae fed on MCD alone showed the significantly lowest soluble-protein content.
3.2. Enzyme activity
General protease activity increased in PL in treatments 1 and 4, with low values from1 PL2 to PL . In treatment 3, general protease activity decreased from M10 III to PL ,2 remaining low thereafter. In the animals fed on artificial diet alone (treatment 2), general protease activity is high until PL and decreased in the rest of the stages. MCD caused2 higher proteolytic activity during all the stages when compared with the other treatments (Fig. 2a).
Amylase activities decreased from MIII to the intermediate stages and increased in PL10 in all treatments (Fig. 2b). Postlarvae fed on Artemia nauplii (treatment 1) and on MCD (treatment 2) had higher amylase activity in MIII and PL than the postlarvae in1 other treatments, with amylase activity remaining high until PL when the postlarvae3 were fed on the artificial diet.
3.3. Oxygen consumption and ammonia excretion
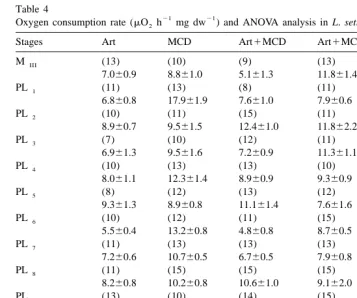
There were significant effects of different diets and stages on the weight specific-rate of oxygen consumption of postlarvae, as well as significant interaction among this two factors. Oxygen consumption did not show a clear pattern through postlarval stages in all
21 treatments (Table 4). Oxygen consumption varied between 4.8 and 17.9 mO h2 mg
21
dw . There was no decrease in the weight specific-rate of oxygen consumption as postlarvae increased in weight.
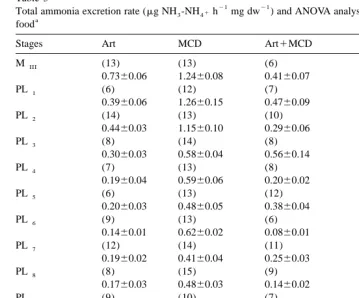
Ammonia excretion rate (Table 5) was also significantly affected by diet and postlarvae stages, and there was a significant interaction between this factors. Ammonia excretion decreases from MIIIto PL9 – 10 stages, in all diets, with higher values observed in organisms fed on MCD (treatment 2). PL and PL9 10 from treatments fed on Artemia nauplii plus MCD (treatment 3) and Artemia nauplii plus MCD plus algae (treatment 4) had the lowest total ammonia excretion rate with values between 0.04 and 0.07 mg
21 21 NH -NH3 41 h mg dw .
Fig. 2. General protease (A) and amylase (B) activity in L. setiferus fed different diets. (h) Artemia nauplii, (m) MCD, (1) Artemia nauplii plus MCD (♦) Artemia nauplii plus MCD plus algae. Each point represents the mean of two observations.
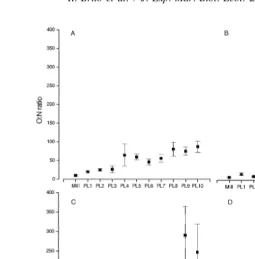
(Fig. 3a), the O:N ratio remained low from MIIIto PL with values between 10 and 27,3 and thereafter the ratio increased significantly reaching values between 56 (in PL ) and7 87 (in PL ). Treatment 2 (Fig. 3b) had low O:N ratios from M10 III to PL (O:N ratios3 between 8 and 16), then the O:N ratio increased, remaining between 25 and 30 from PL4 to PL . Higher values were found in PL and PL8 9 10(39 and 51). In treatment 3 (Fig. 3c), the O:N ratio fluctuated between MIIIand PL , with values between 16 and 84, then rose8 in PL and PL9 10 with values of 290 and 247. In treatment 4 (Fig. 3d), the O:N ratio was low from MIII to PL (values between 22 and 53). From PL , the O:N ratio increased,3 4 with higher values in PL (162), PL (193), and PL6 9 10 (252).
Table 4
21 21 a
Oxygen consumption rate (mO h2 mg dw ) and ANOVA analysis in L. setiferus fed different food
Stages Art MCD Art1MCD Art1MCD1algae
MIII (13) (10) (9) (13)
7.060.9 8.861.0 5.161.3 11.861.4
PL1 (11) (13) (8) (11)
6.860.8 17.961.9 7.661.0 7.960.6
PL2 (10) (11) (15) (11)
8.960.7 9.561.5 12.461.0 11.862.2
PL3 (7) (10) (12) (11)
6.961.3 9.561.6 7.260.9 11.361.1
PL4 (10) (13) (13) (10)
8.061.1 12.361.4 8.960.9 9.360.9
PL5 (8) (12) (13) (12)
9.361.3 8.960.8 11.161.4 7.661.6
PL6 (10) (12) (11) (15)
5.560.4 13.260.8 4.860.8 8.760.5
PL7 (11) (13) (13) (13)
7.260.6 10.760.5 6.760.5 7.960.8
PL8 (11) (15) (15) (15)
8.260.8 10.260.8 10.661.0 9.162.0
PL9 (13) (10) (14) (15)
6.460.5 11.560.7 10.160.4 9.760.7
PL10 (11) (8) (15) (15)
9.660.5 9.960.5 7.560.3 7.660.7
Source df F P
Diet 3 23.58 ,0.001
Stages 10 2.73 0.002
Diet3Stages 30 4.89 ,0.001
a
Mean values6standard error, number of observations in parenthesis. Art, freshly hatched Artemia nauplii; MCD, microparticulate commercial diet.
had higher O:N ratios during all the stages, when compared with the other two treatments.
4. Discussion
Table 5
21 21
Total ammonia excretion rate (mg NH -NH3 41h mg dw ) and ANOVA analysis in L. setiferus fed different
a
food
Stages Art MCD Art1MCD Art1MCD1algae
MIII (13) (13) (6) (14)
0.7360.06 1.2460.08 0.4160.07 0.7360.08
PL1 (6) (12) (7) (6)
0.3960.06 1.2660.15 0.4760.09 0.1860.05
PL2 (14) (13) (10) (9)
0.4460.03 1.1560.10 0.2960.06 0.3060.03
PL3 (8) (14) (8) (8)
0.3060.03 0.5860.04 0.5660.14 0.4860.08
PL4 (7) (13) (8) (9)
0.1960.04 0.5960.06 0.2060.02 0.3260.10
PL5 (6) (13) (12) (5)
0.2060.03 0.4860.05 0.3860.04 0.0660.02
PL6 (9) (13) (6) (10)
0.1460.01 0.6260.02 0.0860.01 0.0660.01
PL7 (12) (14) (11) (11)
0.1960.02 0.4160.04 0.2560.03 0.1360.01
PL8 (8) (15) (9) (11)
0.1760.03 0.4860.03 0.1460.02 0.1360.06
PL9 (9) (10) (7) (9)
0.0860.01 0.4160.06 0.0560.01 0.0760.01
PL10 (10) (9) (9) (6)
0.1460.02 0.2260.02 0.0560.01 0.0460.01
Source df F P
Diet 3 132.21 ,0.001
Stages 10 39.25 ,0.001
Diet3Stages 30 5.93 ,0.001
a
Mean values6standard error, number of observations in parenthesis. Art, freshly hatched Artemia nauplii; MCD, microparticulate commercial diet.
Artemia nauplii, artificial diet, and algae, showing that this diet combination was better for postlarval nutritional requirements than the other diets used in our experiments. When postlarvae were fed on Artemia nauplii or artificial diet alone, the lower growth rate found denoted that these two diets did not fulfill L. setiferus nutritional require-ments. These results are supported by significantly lower soluble-protein content in postlarvae fed on MCD. In the case of the postlarvae fed with Artemia nauplii alone,
Table 6
Results of two factorial ANOVA for O:N ratio in L. setiferus fed different diets from mysis III to postlarva 10
Source df F P
Diet 3 33.40 ,0.001
Stages 10 22.17 ,0.001
Fig. 3. O:N ratio (mean6standard error) for L. setiferus fed different diets. (A) Artemia nauplii, (B) MCD, (C)
Artemia nauplii plus MCD, (D) Artemia nauplii plus MCD plus algae.
was evident that this type of food was nutritionally unbalanced, increasing the soluble protein level without affecting the growth rate. In this sense Mayzaud and Conover (1988) demonstrated that when the diet is unbalanced the protein metabolism can be increased as a consequence of excess protein catabolism. A low O:N values observed in L. setiferus postlarvae fed with Artemia nauplii alone in the present study show that a high soluble protein content can be reflect how an unbalanced diet affected both growth and protein metabolism of shrimp.
Recent results obtained in our laboratory have demonstrated that diet that included Artemia nauplii, artificial food, and algae enhanced growth and soluble-protein content in the early postlarvae of L. vannamei, whereas artificial diet or Artemia nauplii alone produced low growth and low soluble-protein content (Brito, unpublished results).
nutritional requirements. This did not occur when we tested artificial diet or Artemia nauplii alone.
Higher proteolytic activity found in postlarvae fed on an artificial diet may be correlated with the protein composition of MCD. Van Wormhoudt et al. (1986) stated that squid meal increased protease activity in Marsopenaeus japonicus juveniles when it is incorporated in the diet along with other protein sources. Squid meal also stimulated chymotrypsin activity in L. vannamei larvae (Le Moullac and Van Wormhoudt, 1994; Le Moullac et al., 1996). Squid meal, as a component of MCD, could have increased general protease activity when postlarvae fed on MCD alone. Several authors have proposed that higher protease activity found in postlarvae fed on artificial diet may be caused by an adjustment mechanism to low availability of dietary protein or by the
´
relative poor digestibility of the diet (Le-Vay et al., 1993; Rodrıguez et al., 1994; Kumlu and Jones, 1995, Lemos and Rodriguez, 1998).
Gaxiola (1994) found protein requirements of L. setiferus is 40% for PL10 – 40 and 50% for PL10 – 30, showing a decrease in protein requirements according to age. The protein requirements of early postlarvae of L. setiferus (PL1 – 10) are unknown, but results from our study indicate the 50% protein content in MCD may not be enough to fulfill protein requirements of these stages. The requirements of these early stages could also have been affected by the quality of protein and lipid present in the MCD. Shrimp fed on mixed live and artificial diet could have obtained a higher quality and quantity of nutrients from food, which improved their physiological state and growth (protein
´
content of Artemia nauplii 55–56.5%, Leger and Sorgeloos, 1992; Rodrıguez et al., 1994; Lemos and Rodriguez, 1998). In the present study, L. setiferus fed on MCD alone, in the absence of live food, were affected in their growth rate and protease activity, suggesting that nutritional requirements may not have been met by this diet. In other shrimp larvae, feeding on artificial diets combined with live food enhanced growth and decreased protease activity, as shown by Le-Vay et al. (1993) in Marsopenaeus japonicus, and Kumlu and Jones (1995) in Fenneropenaeus indicus.
Amylase activity in our experiments decreased from MIII to PL3 – 5 and then increased in PL , independently of the diet. Lovett and Felder (1990b) obtained similar results in10 the same species feeding on live food. These authors found a significant ontogenetic change in digestive enzyme activity though they held the postlarval diet constant in their study. Increased amylase activity in PL10 stage has been found in Penaeus monodon (Fang and Lee, 1992) and in L. vannamei, (Brito, unpublished results). Lovett and Felder (1990b) stated that the substantial increase in amylase activity observed during postlarval development in L. setiferus might be a response to low levels of carbohydrate
´
in the diet. Rodrıguez et al. (1994) also explained high levels of amylase activity in early myses of M. japonicus as a function of low substrate availability. Mixed diets used in our experiments caused a decline in amylase activity, showing that perhaps the combination of starch from different origins enhances digestibility of diet starch. Le Moullac and Van Wormhoudt (1994) remarked on the importance of the origin of the starch as a factor of amylase variation in L. vannamei larvae.
1994; Rosas et al., 1997a). Few attempts have been made to analyze changes in oxygen consumption related to diet or nutritional state in penaeid early stages (Kurmaly et al., 1989; Chu and Ovsianico-Koulikowsky, 1994; Rosas et al., 1995).
As Rosas et al. (1995) stated, larval oxygen consumption is the sum of the apparent heat increment (Beamish and Trippel, 1990) and larval activity, so it can be seen as a reflection of the metabolic cost associated with the ingestion and assimilation of each type of food. According to Kurmaly et al. (1989), the magnitude of oxygen consumption will depend on the type and amount of food ingested, the nutritional quality of the food, and the physiological condition of larvae. In postlarvae however, the metabolic rate may also be influenced by differences in swimming activity in the various stages, as was noted by Chu and Ovsianico-Koulikowsky (1994), who associated lower oxygen consumption in PL than in PL Metapenaeus ensi with transition from a planktonic to a9 3 benthic mode. In larvae of L. setiferus, feeding is continual with a retention time of food less than 15 min. During postlarval development, both retention time of food and the interval between successive feedings increase gradually until the adult pattern is attained (Lovett and Felder, 1990a).
In the present study, differences in oxygen consumption, may be related to postlarvae behavior and differences in energy demand in the different stages. This could also explain the absence of a decrease in the weight-specific respiration rate with increasing animal size, as is generally found in crustaceans.
A change in ammonia excretion according to the diet fed and stages was observed. A higher ammonia excretion was observed in all stages when animals were fed MCD compared to postlarvae fed with the other diets. These differences could be explained as a result of deamination of amino acids derived from the diet, which are related both to quality and quantity of dietary protein. According to Claybrook (1983), deamination occurs when dietary protein is deficient in one or more essential amino acids or there is poor amino acid balance, when excess amounts of protein have been ingested, or when there is insufficient energy from lipids and carbohydrates to support body processes. Higher ammonia excretion observed in postlarvae fed on MCD could be related to some deficiency in the protein balance of the diet, which when mixed with live food produced a decrease in ammonia excretion by L. setiferus postlarvae.
O:N ratio as index of metabolic substrate changed in shrimp from all treatments as postlarvae increased in age, showing a transition in the use of proteins as an energy source in the first stages to a protein–lipid mixture in later stages. Minello and Zimmerman (1991) reported that L. setiferus is the most herbivorous shrimp in the Gulf of Mexico, which could explain its ability to use lipid–protein as a source of dietary energy. As Taboada et al. (1998) stated, this could represent an advantage to L. setiferus because these animals would be able to use low-cost energy sources for their metabolic processes and exploit proteins as a structural component for growth. In a previous study, Rosas et al. (1995) showed that the O:N ratio in L. setiferus PL30 changed according to the protein level of the diet, with low values in shrimp fed on low or excess protein levels, and high values in shrimp fed with an optimum protein level. These results showed the wide ability of this species to use different metabolic substrates as energy sources according to their availability.
postlarvae from all treatments used a mixture of lipid and protein as energy substrate, the proportion of different metabolic substrates used as energy source varied with diet. In postlarvae fed on MCD, the O:N ratios (38 to 51) were related to equal catabolism of fat and protein (Mayzaud and Conover, 1988), whereas in the other treatments, higher O:N ratios (near 100 or higher) indicated greater influence of lipid catabolism.
The increase in ammonia excretion in postlarvae fed on MCD that produced low O:N ratios could be, as is mentioned above a result of an increase of amino acid deamination produced by an unbalanced diet. Mayzaud and Conover (1988) showed that if the protein in the diet is unbalanced, the complete supply of amino acids cannot be used for synthesis, so amino acids will be deaminated and the nitrogen excreted without using the amino acids for tissue growth, what is in agreement with lower growth rate found in postlarvae fed on MCD.
Inclusion of algae in the diet, although enhancing growth, is not reflected in soluble-protein content, enzyme activity, ammonia excretion, or metabolic substrate. Apparently, algae play a role as source of vitamins or other micronutrients for postlarvae.
In postlarvae fed on artificial diet alone, we obtained the lowest growth, low soluble-protein content, high protease and amylase activities, higher ammonia excretion, and lower O:N ratios. These results indicated that this diet did not satisfy the nutritional requirements of L. setiferus postlarvae. However, when we combined the artificial diet with Artemia nauplii and with Artemia nauplii plus algae, postlarvae grew better than when they were fed on MCD or Artemia nauplii alone, showing that MCD contained some nutrients that are not present in Artemia nauplii. We can then recommend partial substitution of Artemia nauplii for MCD and the use of algae in the rearing of early L. setiferus postlarvae, which, besides enhancing postlarvae growth, avoids Artemia overpopulation in rearing tanks, and reduces the cost of the operation with better assimilation of the diet.
Acknowledgements
We express our gratitude to the MUTIS foundation for the scholarship awarded to
´ ´
Roberto Brito. This project was partially financed by Direccion de Atencion al Personal ´
Academico of the UNAM (project No. 214596) and CONACyT (project No. 3293P-9607). We wish to thank Adriana Paredes and Claudia Durrutty for larvae and algae supplied. Thanks to Dr Ellis Glazier for editing this English-language text. [SS]
References
´
Bernfeld, P., 1955. Sur un methode de microdosage des amylases. In: Colowick, N.O.K.S.P. (Ed.), Methods of Enzymology. Academic Press, New York, pp. 149–157.
Bradford, M.M., 1976. A refined and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 72, 248.
Chu, K.H., 1991. Larval rearing of the shrimps Metapenaeus ensis (de Haan) and Penaeus chinensis (Osbeck) on artificial feed. Aquacult. Fish. Manag. 22, 473–479.
Chu, K., Ovsianico-Koulikowsky, N.N., 1994. Ontogenetic changes in metabolic activity and biochemical composition in the shrimp, Metapenaeus ensis. J. Exp. Mar. Biol. Ecol. 183, 11–26.
Claybrook, D.L., 1983. Nitrogen metabolism. In: Mantel, L.H. (Ed.), The Biology of Crustacea. Internal Anatomy and Physiological Regulation, Vol. 5. Academic Press, New York, pp. 163–213.
Dall, W., Smith, D.M., 1986. Oxygen consumption and ammonia N-excretion in fed and starved tiger prawn
Penaeus esculentus Haswell. Aquaculture 55, 23–33.
Fang, L.S., Lee, B.N., 1992. Ontogenic change of digestive enzymes in Penaeus monodon. Comp. Biochem. Physiol. 103B (4), 1033–1037.
Felder, D.L., Martin, J.W., Goy, J.W., 1985. Patterns in early postlarval development of decapods. In: Wenner, A.M. (Ed.), Crustacean Issues. Larval Growth, Vol. 2. AA Balkema, Rotterdam, pp. 163–225.
Gallardo, P.P., Alfonso, E., Gaxiola, G., Soto, L.A., Rosas, C., 1995. Feeding schedule for Penaeus setiferus larvae based on diatoms (Chaetoceros ceratosporum), flagellates (Tetraselmis chuii ) and Artemia nauplii. Aquaculture 131, 3–4.
Gaudy, R., Sloane, L., 1981. Effect of salinity on oxygen consumption in postlarvae of the penaeid shrimps
Penaeus monodon and P. stylirostris without and with acclimation. Mar. Biol. 65, 297–301.
Gaxiola, G., 1994. Requerimientos nutricionales de postlarvas de Penaeus setiferus y Penaeus duorarum (Crustacea, Penaeidae). Doctoral thesis, Facultad de Ciencias, UNAM, 109 pp.
Hopkins, J.S., Hamilton, II R.D., Sandifer, P.A., Browdy, C.L., Stokes, A.D., 1993. Effect of water exchange rate on production, water quality, effluent characteristics and nitrogen budgets of intensive shrimp ponds. J. World Aquacult. Soc. 24, 304–320.
Hunter, D.A., Uglow, R.F., 1993. A technique for the measurement of total ammonia in small volumes of sea water and hemolymph. Ophelia 37, 31–40.
Jones, D.I., 1984. Penaeid larval culture using microencapsulated diets. In: Proceedings Of The First International Conference On The Culture Of Penaeid Prawns: Shrimps, Iloilo City, Philippines 1985, p. 171.
Jones, D.A., Yule, A.B., Holland, D.L., 1997. Larval nutrition. In: D’Abramo, L.R., Conklin, D.E., Akiyama, D.M. (Eds.), Crustacean Nutrition. Advances in World Aquaculture, Vol. 6. World Aquaculture Society, p. 587.
Kuban, F.D., Lawrence, A.L., Wilkenfeld, J.S., 1985. Survival, metamorphosis and growth of larvae from four penaeid species fed six food combinations. Aquaculture 47, 151–162.
Kumlu, M., Jones, D.A., 1995. The effect of live and artificial diets on growth, survival, and trypsin activity in larvae of Penaeus indicus. J. World Aquacult. Soc. 26 (4), 406–415.
Kurmaly, K., Yule, A.B., Jones, D.A., 1989. An energy budget for the larvae of Penaeus monodon (Fabricius). Aquaculture 81, 13–25.
Leger, P., Sorgeloos, P., 1992. Optimized feeding regimes in shrimp hatcheries. In: Fast, A.W., Lester, L.J. (Eds.), Marine Shrimp Culture. Principles and Practices. Elsevier, Amsterdam, pp. 245–287.
Lemos, D., Rodriguez, A., 1998. Nutritional effects on body composition, energy content and trypsin activity of Penaeus japonicus during early postlarval development. Aquaculture 160, 103–116.
Le Moullac, G., Klein, G.B., Sellos, D., Van Wormhoudt, A., 1996. Adaptation of trypsin, chymotrypsin and
a-amylase to casein level and protein source in Penaeus vannamei (Crustacea, Decapoda). J. Exp. Mar. Biol. Ecol. 208, 107–125.
AQUACOP, Le Moullac, G., Van Wormhoudt, A., 1994. Adaptation of digestive enzymes to dietary protein, carbohydrate and fibre levels and influence of protein and carbohydrate quality in Penaeus vannamei larvae (Crustacea, Decapoda). Aquat. Living Resour. 7 (3), 203–210.
Le-Vay, L., Rodriguez, A., Kamarudin, M.S., Jones, D.A., 1993. Influence of live and artificial diets on tissue composition and trypsin activity in Penaeus japonicus larvae. Aquaculture 118, 287–297.
Lovett, D.L., Felder, D.L., 1989. Ontogeny of gut morphology in the white shrimp Penaeus setiferus (Decapoda, Penaeidae). J. Morphol. 201, 253–272.
Lovett, D.L., Felder, D.L., 1990a. Ontogeny of kinematics in the gut of the white shrimp Penaeus setiferus (Crustacea: Penaeidae). J. Crust. Biol. 10, 53–68.
Lovett, D.L., Felder, D.L., 1990b. Ontogenetic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol. Bull. 178, 144–159.
Mayzaud, P., Conover, R.J., 1988. O:N atomic ratio as a tool to describe zooplankton metabolism. Mar. Ecol. Prog. Ser. 45, 289–302.
Minello, T.J., Zimmerman, R.J., 1991. The role of estuarine habitats in regulating growth and survival of juvenile penaeid shrimp. In: DeLoach, P., Dougherty, W.J., Davison, M.A. (Eds.), Frontiers in Shrimp Research. Elsevier, Amsterdam, pp. 1–16.
´
Perez-Farfante, I., 1969. Western Atlantic shrimps of the genus Penaeus. Fish. Bull. 67, 461–591. Regnault, M., 1981. Respiration and ammonia excretion of sand shrimp Crangon crangon (L). Metabolic
responses to prolonged starvation. J. Comp. Physiol. 141, 549–555. ´
Rodrıguez, A., Le Vay, L., Mourente, G., Jones, D.A., 1994. Biochemical composition and digestive enzyme activity in larvae and postlarvae of Penaeus japonicus during herbivorous and carnivorous feeding. Mar. Biol. 118, 45–51.
Rosas, C., Sanchez, A., Diaz, E., Soto, L.A., Gaxiola, G., Brito, R., 1996. Effect of dietary protein level on apparent heat increment and post-prandial nitrogen excretion of Penaeus setiferus, P. schmitti, P. duorarum, and P. notialis postlarvae. J. World Aquacult. Soc. 27, 92–102.
Rosas, C., Sanchez, A., Diaz-Iglesia, E., Brito, R., Martinez, E., Soto, L.A., 1997a. Critical dissolved oxygen level to Penaeus setiferus and Penaeus schmitti postlarvae (PL10 – 18) exposed to salinity changes. Aquaculture 152, 1–4.
´ ´
Rosas, C., Sanchez, A., Gaxiola, G., Dıaz, E., Brito, R., Soto, L.A., 1997b. Tasa respiratoria de larvas de
Penaeus setiferus y P. schmitti Burkenroad (Decapoda: Penaeidae). Rev. Invest. Mar. 18, 51–57. ´
Rosas, C.S., Dıaz, E., Soto, L.A., Gaxiola, G., Brito, R., Baez, M., Pedroza, R., 1995. Oxygen consumption and ammonia excretion of Penaeus setiferus, P. schmitti, P. duorarum and P.notialis postlarvae fed purified test diets: effect of protein levels on substrate metabolism. Aquat. Living Resour. 8, 161–169.
Samocha, T.M., Burkott, B.J., Lawrence, A.L., Juan, Y.S., Jones, E.R., McKee, D.A., 1998. Management strategies for production of the Atlantic white shrimp Penaeus setiferus as bait shrimp in outdoor ponds. J. World Aquacult. Soc. 29 (2), 211–220.
Sandifer, P.A., Hopkins, J.S., Stokes, A.D., Browdy, C.L., 1993. Preliminary comparisons of the native
Penaeus setiferus and Pacific P. vannamei white shrimp for pond culture in South Carolina, USA. J. World Aquacult. Soc. 24, 295–303.
Sokal, R.R., Rohlf, F.J. (Eds.), 1981. Biometry. The Principles and Practice of Statistics in Biological Research. W.H. Freeman, New York, p. 859.
Sorgeloos, P., Coutteau, P., Dhert, P., Merchie, G., Lavens, P., 1998. Use of brine shrimp, Artemia spp., in larval crustacean nutrition: A review. Rev. Fish. Sci. 6, 1–2.
´ ´
Taboada, G., Gaxiola, G., Garcıa, T., Pedroza, R., Sanchez, A., Soto, L.A., Rosas, C., 1998. Oxygen consumption and ammonia-N excretion related to protein requirements for growth of white shrimp, Penaeus
setiferus (L), juveniles. Aquacult. Res. 29, 823–833.
Todd, E.W., 1949. Quantitative studies on the total plasmin and the trypsin inhibitor of human blood serum. J. Exp. Med. 89, 295–308.
Villarreal, H., Hinojosa, P., Naranjo, J., 1994. Effect of temperature and salinity on the oxygen consumption of laboratory produced Penaeus vannamei postlarvae. Comp. Biochem. Physiol. A 2–3, 331–336. Van Wormhoudt, A., Cruz, E., Guillaume, J., Favrel, P., 1986. Action de l’inhibiteur trypsique de soja sur la
´ ˆ
croissance et l’activite des enzymes digestives chez Penaeus japonicus (Crustacea, Decapoda): role
´ ´
eventuel des hormones gastro-intestinales. Oceanis 12, 305–319.
Walter, H.E., 1988. Proteinases (proteins as substrates) method with haemoglobin, casein and azocoll as substrate. In: Methods of Enzymatic Analysis. Chemie Verlag, Weinheim, Vol. V, pp. 270–277.