www.elsevier.com / locate / bres
Interactive report
Muramyl dipeptide potentiates cytokine-induced activation of inducible
1nitric oxide synthase in rat astrocytes
a b b a
ˇ
ˇ ˇ
ˇ ˇ
ˇ ˇ
ˇ
Vladimir Trajkovic , Tatjana Samardzic , Stanislava Stosic-Grujicic , Zorica Ramic ,
a ,
*
ˇ
Marija Mostarica Stojkovic
a
Institute of Microbiology and Immunology, Dr. Subotica 1, School of Medicine, University of Belgrade, 11000 Belgrade, Yugoslavia
b
Institute for Biological Research, Belgrade, Yugoslavia
Accepted 13 September 2000
Abstract
We investigated the influence of muramyl dipeptide (MDP), a cell wall component of Gram-positive bacteria, on cytokine-induced nitric oxide (NO) production in rat primary astrocytes. MDP alone did not induce NO release in astrocyte cultures. However, MDP increased astrocyte NO production and subsequent nitrite accumulation triggered by IFN-g. IFN-g-activated expression of mRNA for inducible NO synthase (iNOS) and iNOS transcription factor interferon regulatory factor-1 (IRF-1) was markedly enhanced in astrocytes treated with MDP. The potentiating effect of MDP on IFN-g-induced NO production in astrocytes was completely blocked with protein tyrosine kinase (PTK) inhibitor genistein or mitogen activated protein kinase (MAPK) inhibitor PD98059. In contrast, protein kinase C (PKC) inhibitor calphostin C did not affect ability of MDP to augment IFN-g-triggered astrocyte NO synthesis. These results suggest that MDP synergizes with IFN-gin the induction of iNOS gene in astrocytes through mechanisms involving PTK and MAPK, but not PKC activation. Finally, MDP also augmented NO production and iNOS mRNA expression in astrocytes treated with IL-1b. 2000 Elsevier Science B.V. All rights reserved.
Theme: Cellular and molecular biology
Topic: Neuroglia and myelin
Keywords: Muramyl dipeptide; Astrocyte; Nitric oxide; iNOS; Rat
1. Introduction compounds have been shown to trigger the transcription of
inducible NO synthase (iNOS), the enzyme responsible for High levels of pro-inflammatory cytokines and gaseous generation of high amounts of NO from intracellular L -free radical nitric oxide (NO) are proposed to orchestrate arginine. Bacterial endotoxin or lipopolysaccharide (LPS) pathophysiologic mechanism(s) associated with bacterial is the major NO-inducing component of Gram-negative infection of the central nervous system (CNS). The main bacteria [24], but the components of Gram-positive bac-pathogens causing acute bacterial meningitis belong to teria that are responsible for iNOS activation are poorly both Gram-positive and Gram-negative bacteria such as understood. There are reports showing that different
Gram-Escherichia coli and group B streptococci in neonates, positive bacteria or their cell walls can induce iNOS
Streptococcus pneumoniae and Neisseria meningitidis in activity and the production of NO in smooth muscle cells, children and adults, and intracellular Listeria monocyto- macrophages or macrophage-cell lines [4,13,23,31]. In the
genes mainly causing meningitis among newborns, elderly attempts to delineate the molecular substrate responsible people, and immunocompromised patients [33]. for such activity of Gram-positive bacteria, several com-A range of bacterial products and related synthetic ponents were found capable of inducing NO production either in vivo or in vitro: lipoteichoic acid, acting alone [7]
1 or in synergism with peptidoglycan [5,23], superantigens
Published on the World Wide Web on 2 October 2000.
such as streptococcal pyrogenic exotoxins A and C [3], and *Corresponding author. Tel. / fax:1381-11-657-258.
ˇ
E-mail address: bstojko@eunet.yu (M.M. Stojkovic). toxic shock syndrome toxin 1 [44], cell wall serotype
polyosides [25], as well as some water soluble protein dislodge microglia and oligodendrocytes and astrocytes fractions [40]. were further purified by repetition of trypsinization (0.25% It has been shown that several cell types within the trypsin and 0.02% ethylene diamine tetra-acetic acid) and central nervous system, including endothelial cells, neu- replating. The cells used in experiments described here rons, microglial cells and astrocytes can be induced to were obtained after third to fourth passage when they were produce NO after stimulation with Gram-positive bacteria more than 97% positive for glial fibrillary acidic protein, [1,21], or their cell wall components [10,19]. Besides an astrocyte-specific intermediate filament component, and being one of the major mechanisms employed by non- less than 1% positive for microglial surface molecule specific immune system to combat microorganisms, in- CD11b. For nitrite measurement, after conventional
4
cluding gram-positive bacteria [24], high amounts of trypsinization procedure, astrocytes (8310 / well) were iNOS-derived NO are toxic for neurons and oligoden- seeded in flat bottomed 96-plates (Flow) in 200ml medium drocytes [22]. Thus, NO produced by astrocytes, the in the presence of cytokines, MDP, and inhibitors of predominant cell type in the CNS, in response to bacteria intracellular signaling pathways (GEN, CAL or PD98059), and / or proinflammatory cytokines, may play an important as indicated in Results. For RNA isolation, astrocytes
5
role in the pathophysiology of bacterial meningitis. How- (5310 / well) were incubated in 24-well plates (Flow), in ever, the exact nature of NO-inducing component of Gram- the presence of cytokines and MDP.
positive bacteria and the requirements for their activation
of iNOS in astrocytes have not been delineated. 2.3. Nitrite measurement The aim of the present study was to investigate the role
of muramyl dipeptide (N-acetylmuramyl-L-alanyl-D-iso- Nitrite accumulation, an indicator of NO production, glutamine, MDP), the minimal bioactive structure of was measured in 72 h culture supernatants using the Griess bacterial cell wall peptidoglycan [6], in the L-arginine- reagent [17]. Briefly, 50ml aliquots of culture supernatants dependent biotransformation pathways leading to forma- were mixed with an equal volume of Griess reagent (a tion of NO in rat primary astrocytes. mixture at 1:1 of 0.1% naphthylenediamine dihydro-chloride and 1% sulfanilamide in 5% H PO ) and incu-3 4 bated at room temperature for 10 min. The absorbance at
2. Materials and methods 570 nm was measured in an automated Titertek Multiscan
reader (Flow). The nitrite concentration was calculated
2.1. Reagents from a NaNO standard curve.2
Fetal calf serum (FCS), RPMI-1640, and phosphate- 2.4. Determination of iNOS and IRF-1 mRNA by buffered saline (PBS) were from Flow Laboratories (Ir- reverse transcription-polymerase chain reaction
vine, UK). Recombinant rat IFN-g was obtained from (RT-PCR) Holland Biotechnology (Leiden, Netherlands). Rat
recom-binant IL-1b was from Genzyme (Cambridge, MA). N- After 6 h of incubation total RNA was isolated with acetylmuramyl-L-alanyl-D-isoglutamine (MDP) was from RNA Isolator, according to manufacturer’s instruction. GIRPI (Paris, France). Naphthylenediamine dihydro- RNA was reverse transcribed, using Moloney leukemia chloride, sulfanilamide, genistein (GEN), calphostin virus reverse transcriptase and random primers. PCR (CAL), polymyxin B sulfate, and L-leucine-methyl-ester amplification of cDNA with primers specific for iNOS / (L-LME) were from Sigma (St. Louis, MO). PD98059 was IRF-1 and GAPDH as a house-keeping gene, was carried from Research Biochemicals International (Natic, MA). out in the same tube in a Thermojet (Eurogentec, Seraing, Moloney leukemia virus reverse transcriptase was obtained Belgium) thermal cycler as follows: 30 s of denaturation at from Eurogentec (Seraing, Belgium). RNA Isolator was 958C, 30 s of annealing at 538C, and 30 s of extension at purchased from Genosys (Woodlands, TX). Random prim- 728C. Number of cycles (30 for both iNOS and iRF-1, and ers were from Pharmacia (Uppsala, Sweden). 25 for GAPDH) ensuring non-saturating PCR conditions was established in preliminary experiments. For iNOS,
2.2. Cell cultures the sense primer was 59-AGAGAGATCCGGTTCACA-39,
and the antisense primer was 59 -CACAGAACTGAG-Astrocytes were isolated from mixed glial cell cultures GGTACA-39corresponding to positions 88–105 and 446– prepared from brains of newborn DA rats, as previously 463, respectively, of the published rat iNOS mRNA described [28]. Cells were maintained in HEPES-buffered sequence (GenBank accession number S71597); the PCR RPMI 1640 medium supplemented with 5% FCS, 2 mM product was 376 bp long. For IRF-1, the primers were: glutamine, antibiotics, sodium pyruvate and 6 g / l glucose sense, 59-GACCAGAGCAGGAACAAG-39; antisense, 59
-2
was 417 bp long. The primers for GAPDH were: sense, 59-GAAGGGTGGGGCCAAAAG-39; antisense, 59 -GGA-TGCAGGGATGATGTTCT-39, corresponding to positions 371–388 and 646–665 of the published rat GAPDH mRNA sequence (AB017801); the PCR product was 295 bp long. PCR products were visualized by electrophoresis through agarose or polyacrylamide gel stained with ethidium bromide. Gels were photographed and results were analyzed using NIH Image 1.61 PPC software. Relative expression of iNOS and IRF-1 mRNA was calculated as a ratio between densities of the iNOS / IRF-1 and GAPDH bands.
2.5. Statistical analysis
To analyze the significance of the differences between various treatments performed in triplicates, we used analy-sis of variance (ANOVA), followed by Student–Newman– Keul’s test. A P-value less than 0.05 was considered
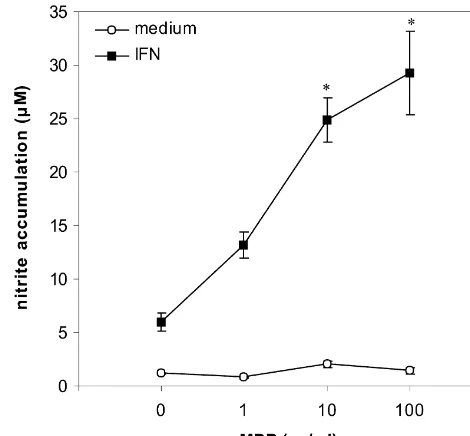
significant. Fig. 1. MDP stimulates IFN-g-induced NO production in astrocytes.
4
Astrocytes (8310 / well) were incubated with or without IFN-g(1000 U / ml), in the presence or absence of various concentrations of MDP. After 72 h of cultivation, nitrite concentration in cell culture supernatants
3. Results was determined. Results from one of five independent experiments with
similar results are presented as mean6S.E.M. of triplicate observations 3.1. MDP enhances NO production in IFN-g-stimulated (*P,0.05 refers to treatment with IFN-galone).
rat primary astrocytes
Non-stimulated astrocytes did not produce detectable abolished this LPS effect (10.260.2 mM; P,0.05 in levels of nitrites, and MDP by itself, in a wide range of comparison with IFN-g1LPS-treatment). In addition, concentrations (1–100mg / ml), did not have any stimulat- similar potentiation of NO release in IFN-g-treated as-ory effects on NO production in astrocytes. Proinflammat- trocyte cultures was obtained with MDP purchased from ory cytokine IFN-g(1000 U / ml) induced by itself signifi- another supplier (Sigma; St. Louis, MO). Finally, we cant up-regulation of NO production, measured as a rise of excluded the influence of residual microglial contamination nitrite concentrations in astrocyte cultures (Fig. 1), in by using lysosomotropic agent LLME, which is selectively accordance with previous results showing iNOS expression toxic for macrophages and microglia [16]. While peritoneal and NO production in cytokine-stimulated rodent primary macrophages were all killed upon 60 min exposure to 10 astrocytes [11,15]. Protein synthesis inhibitor cyclohex- mM LLME (not shown), 60 min preincubation with LLME imide (1 mg / ml), as well as aminoguanidine (1 mM), a did not affect IFN-g-induced nitrite accumulation in as-NOS inhibitor which preferentially inhibits its inducible trocyte cultures (4.160.5mM vs. 4.160.8mM for control isoform [27], both completely prevented IFN-g-induced and LLME-treated cells, respectively; P.0.05), indicating nitrite accumulation (not shown), indicating that NO in that microglia did not contribute to NO generation in our astrocytes was generated via iNOS-dependent L-arginine- experimental system. Similarly, LLME did not alter MDP-NO pathway. We next examined nitrite levels in astrocyte mediated augmentation of NO release in IFN-g-treated culture supernatants after stimulation with IFN-gin combi- astrocytes (14.760.6mM vs. 16.461.2mM for control and nation with MDP (1, 10 and 100 mg / ml), and the results LLME-treated cells, respectively; P.0.05).
revealed statistically significant dose-dependent rise in NO
production. The potentiation of NO production was similar 3.2. MDP stimulates IFN-g-induced expression of iNOS at 10 and 100 mg / ml of MDP and ranged between twofold and IRF-1 mRNA in astrocytes
to fivefold in 5 independent experiments (Fig. 1). The
expres-5
Fig. 2. MDP stimulates IFN-g-induced expression of iNOS and IRF-1 mRNA in astrocytes. Astrocytes (5310 / well) were incubated with or without IFN-g(1000 U / ml), in the presence of absence of MDP (10mg / ml). After 6 h, RNA was isolated and the expression of IRF-1 and iNOS mRNA was assessed by RT-PCR. Results from the representative of three separate experiments are presented as relative expression of iNOS / IRF-1 mRNA in comparison to GAPDH.
sion of iNOS mRNA in IFN-g-treated astrocytes (Fig. 2). or PD98059 (40 mM) (Fig. 3). In contrast, although Since transcription factor IRF-1 has a cardinal role in suppression of IFN-g-activated NO production was com-IFN-g-mediated induction of iNOS in macrophages [24], parable in astrocytes treated with GEN, PD98059, or CAL we assessed the influence of MDP on IRF-1 mRNA
expression in IFN-g-treated astrocytes. Under the PCR conditions used, we could not detect IRF-1 message in unstimulated astrocytes or cultures treated with MDP alone (Fig. 2). Interestingly, IFN-g-triggered induction of IRF-1 in astrocytes was augmented in the presence of MDP (Fig. 2). These results indicate that MDP might enhance iNOS transcription by stimulating IRF-1 activation, but the interference of MDP with the iNOS mRNA stability can not be excluded.
3.3. Signaling pathways involved in IFN-g1 MDP-induced NO production in astrocytes
To gain some insight into intracellular signals respon-sible for IFN-g1MDP-mediated NO production, we used inhibitors of protein tyrosine kinase (PTK), protein kinase C (PKC), and mitogen activated protein kinase (MAPK) pathway–GEN, CAL and PD98059, respectively. Very low
Fig. 3. Effect of PTK, MAPK, and PKC inhibition on IFN-g/
MDP-basal NO production was not affected by any of the agents 4
induced NO production in astrocytes. Astrocytes (8310 / well) were tested (data not shown). Each of the three inhibitors almost incubated with IFN-g(1000 U / ml) or IFN-gand MDP (10mM), in the completely blocked NO synthesis in IFN-g-treated as- presence or absence of PTK, MAPK, or PKC inhibitors GEN (20mm), PD98059 (40mM), or CAL (100 nM). Control cultures contained same trocyte cultures (Fig. 3), without reducing cell viability
amount of vehicle (DMSO) as GEN, PD98059, or CAL-treated cultures. (not shown), indicating that activation of PTK, PKC, and
(100 nM), inhibition of PKC activity with CAL did not production and further elevated the expression of iNOS significantly affect NO synthesis induced by combination mRNA in IL-1-treated astrocytes (Fig. 4).
of IFN-g and MDP (Fig. 3). These results suggest that cooperation between IFN-gand MDP in the iNOS
induc-tion in astrocytes involves PTK and MAPK, but not PKC 4. Discussion
activation.
In this study we showed that muramyl dipeptide, the minimal bioactive structure of bacterial cell wall peptido-3.4. MDP enhances NO production and iNOS mRNA glycan potentiates cytokine-induced iNOS mRNA
expres-expression in IL-1-treated astrocytes sion and NO production by rat astrocytes. There are
numerous data showing that MDP can induce NO pro-Finally, in order to find out whether the potentiating duction in macrophages either alone [29,32], or in the effect of MDP on NO production was selectively ex- combination with various proinflammatory cytokines pressed only when astrocytes were exposed to IFN-g, we [34,35]. However, so far the only known effects of MDP analyzed the NO production and iNOS mRNA expression on astrocytes are those showing that MDP induces IFN-g -in astrocytes treated with IL-1 -in the presence of MDP (10 independent MHC class II expression and production of
mg / ml). Exposure of rat astrocytes to IL-1b (10 ng / ml) prostaglandine D2 in rat astrocyte cultures [26,43]. There-alone caused an accumulation of nitrite in culture super- fore, the potentiation of cytokine (IL-1, IFN-g)-induced natants and induced expression of iNOS mRNA (Fig. 4) in NO production in astrocytes by MDP, to the best of our cultivated cells. Similarly to the effects observed in IFN-g- knowledge, is the first report of that kind.
stimulated astrocytes, MDP enhanced IL-1-induced NO Potentiation of astrocyte NO release by MDP was accompanied by enhanced expression of iNOS mRNA, confirming that MDP affected the induction phase of iNOS activation. Previous results indicate that different signaling pathways involving PTK, PKC, and MAPK activation, are involved in the induction of astrocyte iNOS [2,9,14]. Indeed, interference with either PTK, PKC, or MAPK activity, almost completely prevented IFN-g-triggered astrocyte NO release in our study. This is consistent with findings that tyrosine phosphorylation of STAT1, leading to activation of iNOS transcription factor IRF-1, is neces-sary for IFN-g induction of iNOS in rat glial cells [20]. Recently, activation of MAP kinase pathway also has been linked to the IRF-1 induction [8], while it has been well documented that iNOS activation in IFN-g-stimulated macrophages depends on translocation of PKC from cytosol to cell membrane [37]. However, our data suggest that synergistic cooperation between IFN-g and MDP in astrocytes, while being completely dependent on PTK or MAPK activity, probably does not involve activation of PKC. Since NO synthesis in astrocytes treated with IFN-g
alone was abolished by PKC inhibition, it seems that MDP provides signal(s) that can replace iNOS-inducing activity of PKC. This is concordant with observation that PKC activation might lead to, but is not absolutely required for iNOS induction in astrocytes [38].
The downstream events responsible for the enhancement of iNOS induction by MDP might involve activation of Fig. 4. MDP stimulates NO production and iNOS mRNA expression in
4 5 IRF-1, as indicated by further increase of IFN-g-triggered
IL-1-treated astrocytes. Astrocytes (8310 or 5310 / well for NO
accumulation of this iNOS transcription factor in MDP-production or RNA isolation, respectively) were incubated with or
without IL-1b (10 ng / ml), in the presence or absence of MDP (10 treated astrocytes. LPS, a cell wall component of
gram-mg / ml). After 6 h, RNA was isolated and RT-PCR for iNOS and negative bacteria and a well known activator of iNOS [24], GAPDH was performed. Nitrite accumulation in cell culture supernatants also has the ability to augment IFN-g-induced activation of was determined after 72 h of cultivation. Results from the representative
IRF-1 [8]. Accordingly, our preliminary results with of three separate experiments are presented as mean nitrite
simultaneous MDP/ LPS treatment indicate that MDP and accumulation1S.E.M. of triplicate observations, or iNOS / GAPDH signal
regulated kinase and p38 subgroups of mitogen-activated protein involved in iNOS activation in astrocytes (unpublished
kinases regulate inducible nitric oxide synthase and tumor necrosis observation). This is consistent with previously published
factor-alpha gene expression in endotoxin-stimulated primary glial ability of both agents to bind to monocyte CD14 [42], a cultures, J. Neurosci. 18 (1998) 1633–1641.
molecule also involved in LPS induction of iNOS in glial [3] E.A. Christ, E. Meals, B.K. English, Streptococcal pyrogenic cells [12]. However, LPS activation of another iNOS exotoxins A (SpeA) and C (SpeC) stimulate the production of inducible nitric oxide synthase (iNOS) protein in RAW 264.7 transcription factor, NF-kB, has been regarded as a major
macrophages, Shock 8 (1997) 450–453. mechanism behind synergistic action of IFN-g/ LPS
[4] F.Q. Cunha, D.W. Moss, L.M. Leal, S. Moncada, F.Y. Liew, combination in the induction of macrophage iNOS [24]. Induction of macrophage parasiticidal activity by Staphylococcus Since MDP can induce NF-kB activity in various cell aureus and exotoxins through the nitric oxide synthesis pathway, types [39], it is possible that activation of this transcription Immunology 78 (1993) 563–567.
[5] S.J. De Kimpe, M. Kengatharan, C. Thiemermann, J.R. Vane, The factor in astrocytes might be responsible for LPS-like
cell wall components peptidoglycan and lipoteichoic acid from iNOS-inducing action of MDP in these cells. While our
Staphylococcus aureus act in synergy to cause shock and multiple data are consistent with transcriptional regulation of iNOS organ failure, Proc. Natl. Acad. Sci. USA 92 (1995) 10359–10363. by MDP, we can not exclude the possibility that MDP [6] F. Ellouz, A. Adam, R. Ciorbaru, E. Lederer, Minimal structural could have affected stability of iNOS mRNA. Interesting- requirements for adjuvant activity of bacterial peptidoglycan
deri-vates, Biochem. Biophys. Res. Commun. 59 (1974) 1317–1325. ly, it has been proposed that PKC activity is involved in
[7] B.K. English, C.C. Patrick, S.L. Orlicek, R. McCordic, J.L. Shenep, stabilization of IFN-g-induced iNOS message in
macro-Lipoteichoic acid from viridans streptococci induces the production phages [18]. Thus, the assumption that MDP might prevent of tumor necrosis factor and nitric oxide by murine macrophages, J. degradation of iNOS mRNA in astrocytes is actually Infect. Dis. 174 (1996) 1348–1351.
concordant with our observation that MDP stimulates [8] V. Faure, C. Hecquet, Y. Courtois, O. Goureau, Role of interferon regulatory factor-1 and mitogen-activated protein kinase pathways in astrocyte NO production by providing a PKC-replacing
the induction of nitric oxide synthase-2 in retinal pigmented signal.
epithelial cells, J. Biol. Chem. 274 (1999) 4794–4800.
Finally, we have shown that MDP-mediated enhance- [9] D.L. Feinstein, E. Galea, J. Cermak, P. Chugh, L. Lyandvert, D.J. ment of iNOS induction in astrocytes was not specific for Reis, Nitric oxide synthase expression in glial cells: suppression by IFN-g stimulation, as it was readily observed also in tyrosine kinase inhibitors, J. Neurochem. 62 (1994) 811–814.
[10] D. Freyer, M. Weih, J.R. Weber, W. Burger, P. Scholz, R. Manz, A. IL-1-treated cells. Elevated levels of IL-1 were found in
Ziegenhorn, K. Angestwurm, U. Dirnagl, Pneumococcal cell wall cerebrospinal fluid taken from both patients [30] and
components induce nitric oxide synthase and TNF-ain astroglial-animals with bacterial meningitis in the early stage of enriched cultures, Glia 16 (1996) 1–6.
infection [41], and antibodies to IL-1 prevented inflamma- [11] E. Galea, D.L. Feinstein, D.J. Reis, Induction of calcium-indepen-tion in rabbits after intracisternal challenge with pneumo- dent nitric oxide synthase activity in primary rat glial cultures, Proc.
Natl. Acad. Sci. USA 89 (1992) 10945–10949. cocci [36]. Therefore, the synergistic induction of iNOS in
[12] E. Galea, D.J. Reis, E.S. Fox, H. Xu, D.L. Feinstein, CD14 mediate astrocytes by MDP and IL-1, produced intrathecally by
endotoxin induction of nitric oxide synthase in cultured brain glial blood derived neutrophils and macrophages, and resident
cells, J. Neuroimmunol. 64 (1996) 19–28.
cells such as astrocytes and microglia, might be par- [13] K.J. Goodrum, L.L. McCormick, B. Schneider, Group B streptococ-ticularly important in the early phases of infection, during cus-induced nitric oxide production in murine macrophages is CR3 (CD11b / CD18) dependent, Infect. Immun. 62 (1994) 3102–3107. which the IFN-g-producing T cells are absent from the
[14] R.P. Hellendall, J.P. Ting, Differential regulation of cytokine-in-CNS.
duced major histocompatibility complex class II expression and Therefore, our observations suggest that MDP-mediated
nitric oxide release in rat microglia and astrocytes by effectors of induction of astrocyte iNOS may be an important deter- tyrosine kinase, protein kinase C, and cAMP, J. Neuroimmunol. 74 minant of the CNS inflammatory response to Gram-posi- (1997) 19–29.
[15] S.J. Hewett, J.A. Corbett, M.L. McDaniel, D.W. Choi, Interferon-g
tive infection, contributing to both bacterial clearance and
and interleukin-1b induce nitric oxide formation from primary inflammatory destruction of self tissues.
mouse astrocytes, Neurosci. Lett. 164 (1993) 229–232.
[16] J.A. Hewett, S.J. Hewett, S. Winkler, S.E. Pfeiffer, Inducible nitric oxide synthase expression in cultures enriched for mature oligoden-drocytes is due to microglia, J. Neurosci. Res. 56 (1999) 189–198.
Acknowledgements
[17] J.B. Hibbs, R. Taintor, Z. Vavrin, E. Rachlin, Nitric oxide: a cytotoxic activated macrophage effector molecule, Biochem. Bio-This work was supported by grants from Ministry of phys. Res. Commun. 157 (1989) 87–94.
Science and Technology, Republic of Serbia, Yugoslavia. [18] C.D. Jun, B.M. Choi, J.Y. Um, Hoon-Ryu, H.J. Kwak, B.S. Lee, S.G. Paik, H.M. Kim, H.T. Chung, Synergistic cooperation between phorbol ester and IFN-gfor induction of nitric oxide synthesis in murine peritoneal macrophages, J. Immunol. 153 (1994) 3684–
References 3690.
[19] Y.S. Kim, M.G. Tauber, Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production, [1] A. Bernatowicz, U. Koedel, K. Frei, A. Fontana, H.-W. Pfister,
Infect. Immun. 64 (1996) 3148–3153. Production of nitrite by primary astrocytes in response to
oxide synthase in rat glial cells, Eur. J. Pharmacol. 306 (1996) [33] H.-W. Pfister, W.M. Scheld, Brain injury in bacterial meningitis: 297–306. therapeutic implications, Curr. Opin. Neurol. 10 (1997) 254–259. [21] U. Koedel, A. Bernatowicz, R. Paul, K. Frei, A. Fontana, H.-W. [34] N.C. Phillips, L. Gagne, Modulation of murine macrophage nitric
Pfister, Experimental pneumococcal meningitis:cerebrovascular al- oxide synthesis by liposomal phospholipids: correlation with lipo-terations, brain edema, and meningeal inflammation are linked to the some immune adjuvant activity, J. Drug. Target. 3 (1995) 137–147. production of nitric oxide, Ann. Neurol. 37 (1995) 313–323. [35] K. Sarkar, P.K. Das, Protective effect of neoglycoprotein-conjugated [22] J. Licinio, P. Prolo, S.M. McCann, M.L. Wong, Brain iNOS: current muramyl dipeptide against Leishmania donovani infection: the role
understanding and clinical implications, Mol. Med. Today 5 (1999) of cytokines, J. Immunol. 158 (1997) 5357–5365.
225–232. [36] K. Saukkonen, S. Sande, C. Cioffe, S. Wolpe, B. Sherry, A. Cerami, [23] M.O. Lonchampt, M. Auguet, S. Delaflotte, J. Goulin-Schulz, P.E. E. Tuomanen, The role of cytokines in the generation of inflamma-Chabrier, P. Braquet, Lipoteichoic acid: a new inducer of nitric tion and tissue damage in experimental gram-positive meningitis, J. oxide synthase, J. Cardiovasc. Pharmacol. 12 (1992) 20145–20147. Exp. Med. 171 (1990) 439–448.
[24] J. MacMicking, Q.-W. Xie, C. Nathan, Nitric oxide and macrophage [37] A. Severn, M.J. Wakelam, F.Y. Liew, The role of protein kinase C in function, Annu. Rev. Immunol. 15 (1997) 323–350. the induction of nitric oxide synthesis by murine macrophages, [25] V. Martin, A.L. Kleschyov, J.P. Klein, A. Beretz, Induction of nitric Biochem. Biophys. Res. Commun. 188 (1992) 997–1002.
oxide production by polyosides from the cell walls of Streptococcus [38] M.L. Simmons, S. Murphy, Roles for protein kinases in the mutans OMZ 175, a gram-positive bacterium, in the rat aorta, Infect. induction of nitric oxide synthase in astrocytes, Glia 11 (1994)
Immun. 65 (1997) 2074–2079. 227–234.
[26] P.T. Massa, V. ter Meulen, Analysis of Ia induction on Lewis rat [39] R. Schreck, D. Bevec, P. Dukor, P.A. Baeuerle, L. Chedid, G.M. astrocytes in vitro by virus particles and bacterial adjuvants, J. Bahr, Selection of a muramyl peptide based on its lack of activation Neuroimmunol. 13 (1987) 259–271. of nuclear factor-kB as a potential adjuvant for AIDS vaccines, Clin. [27] T.P. Misko, W.M. Moore, T.P. Kasten, G.A. Nickols, J.A. Corbett, Exp. Immunol. 90 (1992) 188–193.
R.G. Tilton, M.L. McDaniel, J.R. Williamson, M.G. Currie, Selec- [40] L. Tuckova, Z. Zidek, M. Hanikyrova, B. Cukrowska, H. Tlas-tive inhibition of the inducible nitric oxide synthase by amino- kalova-Hogenova, R. Barot-Ciorbaru, Macrophage nitric oxide guanidine, Eur. J. Pharmacol. 233 (1993) 119–125. synthase (NOS) activation by Nocardia opaca fractions and 15- and [28] K.D. McCarthy, J. De Vellis, Preparation of separate astroglia and 56-kD isolated antigens, Clin. Exp. Immunol. 104 (1996) 215–220. oligodendroglial cell cultures from rat cerebral tissue, J. Cell. Biol. [41] A. Waage, A. Halstensen, R. Shalaby, P. Brandtzaeg, P. Kierulf, T. 85 (1980) 890–902. Espevik, Local production of tumor necrosis factor-a, interleukin 1, [29] C. Morin, H. Fessi, J.P. Devissaguet, F. Puisieux, G. Barratt, Factors and interleukin 6 in meningococcal meningitis. Relation to the
influencing macrophage activation by muramyl peptides: inhibition inflammatory response, J. Exp. Med. 170 (1989) 1859–1867. of NO synthase activity by high levels of NO, Biochim. Biophys. [42] B. Weidemann, J. Schletter, R. Dziarski, S. Kusumoto, S. Stelter, Acta 1224 (1994) 427–432. E.T. Rietschel, H.D. Flad, A.J. Ulmer, Specific binding of soluble [30] M.M. Mustafa, O. Ramilo, X. Saez-Llorens, K.D. Olsen, R.R. peptidoglycan and muramyldipeptide to CD14 on human
mono-Magness, G.H. McCracken Jr., Cerebrospinal fluid prostaglandins, cytes, Infect. Immun. 65 (1997) 858–864.
interleukin 1b, and tumor necrosis factor in bacterial meningitis. [43] K. Yamamoto, T. Miwa, R. Ueno, O. Hayaishi, Muramyl dipeptide-Clinical and laboratory correlations in placebo-treated and dexa- elicited production of PGD2 from astrocytes in culture, Biochem. methasone-treated patients, Am. J. Dis. Child. 144 (1990) 883–887. Biophys. Res. Commun. 156 (1988) 882–888.
[31] K.L. Orman, J.L. Shenep, B.K. English, Pneumococci stimulate the [44] A. Zembowicz, J.R. Vane, Induction of nitric oxide synthase activity production of the inducible nitric oxide synthase and nitric oxide by by toxic shock syndrome toxin 1 in a macrophage-monocyte cell murine macrophages, J. Infect. Dis. 178 (1998) 1649–1657. line, Proc. Natl. Acad. Sci. USA 89 (1992) 2051–2055.