www.elsevier.com / locate / bres
Research report
Fibroblast growth factor-2-producing fibroblasts protect the
nigrostriatal dopaminergic system from 6-hydroxydopamine
a,b ,
*
c a,b cClifford W. Shults
, Jasodhara Ray , Kyoko Tsuboi
, Fred H. Gage
a
Neurology Service, Veterans Affairs San Diego Healthcare System, VA Medical Center, 3350 La Jolla Village Drive, San Diego, CA 92161, USA b
Department of Neurosciences, University of California, San Diego, La Jolla, CA, USA c
Laboratory of Genetics, The Salk Institute for Biological Studies, La Jolla, CA, USA
Accepted 22 August 2000
Abstract
We tested the hypothesis that fibroblasts, which had been genetically engineered to produce fibroblast growth factor-2 (FGF-2), can protect nigrostriatal dopaminergic neurons. Three groups of rats received either a burr hole only (n55) or implantation of fibroblasts, which had been genetically engineered to produceb-galactosidase (b-gal) (n58) or FGF-2 (n58), at two sites in the right striatum. Two weeks later, the animals received an injection of 25mg of 6-hydroxydopamine hydrobromide (6-OHDA) midway between the two implant sites. The group that received FGF-2-fibroblasts had significantly fewer apomorphine-induced rotations than the groups that received a burr hole only or b-gal-fibroblasts at weeks 2 and 3 following lesioning with 6-OHDA. Testing for amphetamine-induced rotation revealed a mild reduction in rotation in theb-gal-fibroblast group compared to the burr hole only group, but a striking attenuation of amphetamine-induced rotation in the FGF-2-fibroblast group. There was also preservation of TH-IR neurons on the lesioned side relative to both control groups. The size of the grafts and the gliosis surrounding the injection sites did not differ between the FGF-2-fibroblast andb-gal-fibroblast groups. To further characterize the production of FGF-2 by the FGF-2-fibroblasts, we implanted FGF-2-fibroblasts andb-gal-fibroblast into the striatum of rats but did not lesion the animals with 6-OHDA. The animals were then sacrificed at 1, 2 and 5 weeks following implantation. Prior to implantation the FGF-2 fibroblasts contained 148 ng / mg of FGF-2-immunoreactive (FGF-2-IR) material per mg of protein of cell lysate. After implantation FGF-2-IR material was noted in the grafts of FGF-2-fibroblasts, most conspicuously at 1 and 2 weeks following implantation. We also noted FGF-2-IR material in the nuclei of reactive astrocytes adjacent to the implants, and OX-42-immunoreactive (OX-42-IR) cells adjacent and occasionally within the implants. Our work indicates that fibroblasts genetically engineered to produce FGF-2 and implanted in the striatum can protect the nigrostriatal dopaminergic system and may be useful in the treatment of Parkinson’s disease. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Degenerative disease: Parkinson’s
Keywords: Parkinson’s disease; Fibroblast growth factor; Dopamine; Substantia nigra
1. Introduction shown to have a trophic effect on the nigrostriatal dopa-minergic system in vivo. Implantation of FGF-2-treated gel A number of studies have indicated that fibroblast foam into one striatum in 1-methyl-4-phenyl-1,2,3,6-tetra-growth factor-2 (FGF-2) can have trophic effects on hydropyridine (MPTP)-treated mice increased the levels of mesencephalic, dopaminergic neurons [2,21,44]. FGF-2 dopamine and the activity of tyrosine hydroxylase (TH) in increases dopamine uptake and / or survival of fetal dopa- the striatum bilaterally [31]. The effect diminished if FGF-minergic neurons in vitro, but the effect appears to require 2 was administered 7 days after treatment with MPTP [32]. the presence of glia [10,12,28]. FGF-2 has also been The ability of intrastriatal administration of FGF-2 to enhance the recovery of dopaminergic axons in MPTP-treated mice appears to be less in aged than in young mice
*Corresponding author. Tel.:11-858-552-8585 ext. 3685; fax: 1
1-[9]. Similar to the effect of intrastriatal injection,
intraven-858-552-7513.
E-mail address: [email protected] (C.W. Shults). tricular infusion of FGF-2 with heparin attenuated the
behavioral effects and the loss of striatal dopaminergic for 5 min. The cells were centrifuged and resuspended at a fibers and nigral dopaminergic neurons in MPTP-treated final concentration of 100,000 cells /ml in PBS with D -mice [7]. A trophic effect of FGF-2 on the nigrostriatal glucose 1.0 g / l and 2% normal rat serum.
dopaminergic system is not surprising in light of the Female Sprague–Dawley rats, which weighed approxi-presence of both FGF-2 and the FGF receptor-1 in the mately 225 g, were housed under a 12 h light / dark cycle neurons of the substantia nigra pars compacta (SNpc) with free access to food and water and were cared for
[4,6,20]. according to NIH guidelines. Prior to surgical procedures,
A focus of our research has been to develop methods by the animals were anesthetized with a mixture of ketamine, which trophic factors, such as FGF-2, can be used effec- xylazine and acepromazine. Twenty-four animals were tively in the treatment of Parkinson’s disease. The cardinal used in the study. The animals received one of three pathological feature of Parkinson’s disease is loss of treatments: burr hole only (two of these animals died prior dopaminergic neurons in the SNpc and their axons to the to lesioning with 6-OHDA (n56), implantation of b -gal-striatum [17]. Pertinent to Parkinson’s disease are the fibroblasts (n58), or implantation of FGF-2-fibroblasts observations that most of the neurons in the human SNpc (n58). The burr hole was made at: anterior20.5 mm and are immunoreactive for FGF-2 and in Parkinson’s disease lateral (right) 22.4 mm from bregma. The cells were there is disproportionate loss of FGF-2-immunoreactive implanted using a 10ml Hamilton syringe with a 28-gauge
neurons in the SNpc [47]. needle. The cells were implanted at two depths: 6.4 mm
Although certain trophic factors, such as FGF-2, hold and 4.8 mm from the top of the skull. At each site 1.5ml promise as treatments in Parkinson’s disease, the optimal of cells (150,000) were implanted. The cells were injected method of using trophic factors as treatments for Parkin- at a rate of 0.5ml / min, and the needle was left in place for son’s disease remains uncertain. Some of the issues that 5 min after each injection.
must be addressed in development of trophic factors as Approximately 2 weeks later, the animals received a treatments for neurological disorders include: delivery to single, intrastriatal injection of 6-OHDA. Twenty-five the central nervous system, limitation of delivery to micrograms of 6-OHDA hydrobromide (RBI-Sigma, St. specific regions of the central nervous system, and delivery Louis, MO), which was dissolved in 1.5 ml of normal of the optimal dose of trophic factor. A promising tech- saline with 0.2% ascorbic acid, was injected at a depth of nique for sustained delivery of a trophic factor to a specific 5.6 mm ventral to the skull at the same anterior / posterior region of the central nervous system is to genetically and medial / lateral coordinates at which the cells had been engineer cells, such as fibroblasts, to produce trophic injected. The injection site was chosen to be midway factors and implant the cells into specific brain region, e.g., between the two implant sites. 6-OHDA was injected over the striatum [13]. We have found that fibroblasts ge- 5 min, and the needle was left in place for an additional 5 netically engineered to produce FGF-2 and implanted into min before withdrawal of the needle.
the striatum can protect the nigrostriatal system from Beginning the following week, the animals were tested
intrastriatal injections of 6-OHDA. weekly for apomorphine-induced rotation (0.1 mg / kg for
30 min) and amphetamine-induced rotation (1.3 mg / kg of amphetamine sulfate for the 20–60 min epoch following 2. Materials and methods injection).
After 3 weeks of behavioral testing, the animals were
2.1. Effects in 6-OHDA lesion model anesthetized and perfused with 4% paraformaldehyde in
phosphate buffer (PB). The brains were sectioned at 25 Fibroblasts from Fischer 344 rats were genetically mm intervals. Sections from the forebrains and midbrains engineered to express either FGF-2 or b-galactosidase were stained for Nissl substance at 100 mm and 200 mm (b-gal), as previously described [37]. Theb-gal fibroblasts intervals, respectively. At 200 mm intervals sections from were used after passage 10, and the FGF-2 fibroblasts were the forebrains and midbrains were immunolabeled for TH. used after passage 11. The cells were grown to confluence The sections were rinsed three times in PBS, quenched in
2
DAB reaction. After five rinses in PBS, sections were performed using the34 objective. The evaluator outlined mounted on gelatin-coated slides, air-dried, dehydrated and the region with GFAP-IR fibers, measured that region in
coverslipped. the three sections and summed the areas for the three
Nissl-stained sections of the striatum from each animal sections.
were evaluated by an observer, who was blinded to the Estimation of number of TH-IR neurons in the SNpc treatment, to determine whether the injection track was in was achieved by using the optical fractionator procedure the proper location and whether the brain was free of [49]) with the assistance of a semiautomatic stereology changes suggesting infection or other abnormality. One of system (StereoInvestigator version 3.0, MicroBrightField, the animals that received a burr hole only had a congenital Inc, Brattleboro, VT). The sections from the midbrains abnormality of the cortex, and this animal was not included were 25 mm in thickness, and every eighth section was in the analyses. The final number of animals in each group immunolabeled and evaluated. Analysis began at the was: burr hole — 5, b-gal-fibroblasts — 8, and FGF-2- rostral border of the SNpc (approximately24.8 mm caudal
fibroblasts — 8. to bregma) [35]) and continued to the caudal extent of the
In the five consecutive, TH-immunolabeled striatal SNpc. Seven or eight sections were analyzed in each brain. sections, we used an image analysis system to determine Images were acquired on an Olympus BH2 microscope the area in the injected striatum that lacked TH-IR fibers (Tokyo, Japan) equipped with appropriate filter sets using and the total area of the injected striatum. The system has a single-chip CCD camera and displayed by using the been previously described [43]. An observer outlined the StereoInvestigator software driving a Ludl X–Y–Z motor-regions of the striatum that were devoid of TH-IR axons ized stage (Ludl Electronic Products, Ltd., Hawthorn, NY). and the total area of the striatum. The percentage of the Rough boundaries to delimit the optical fractionator area striatum that was devoid of TH-IR fibers was calculated sampling fraction were drawn by using a 310 objective for each animal. In addition, in three sections, which were and subsequently were sampled by using a360 objective centered on the implant, we measured the width of the (oil; SPlanApo, 1.4 N.A.). The area evaluated included region that lacked TH-IR fibers at its greatest length and both the SNpc and the substantia nigra pars lateralis. A averaged this length for each animal. All quantitative guard focus height of 2mm was set in the software, which studies of anatomical changes, such as this one, were would then focus through each sample region for sections carried out by an observer, who was unaware of the determined to be on average 9 mm in thickness with
treatment group assignment. markings made on cells meeting the sampling criteria
In each of the Nissl-stained sections in which a graft interactively throughout the focus session. Two dimension-was present, we used the image analysis system to measure al counting rules state that cells that lie entirely within the the cross-sectional area of the graft. The cross-sectional sampling frame are counted, whereas those that lie outside areas were summed for each animal. Injection of 6-OHDA are not. For cells that intersect the sampling frame, those and destruction of the dopaminergic axons typically caused that intersect the green lines are counted, whereas those inflammation, which could obscure the grafted cells. The that intersect the red lines are excluded. By using these graft size was only measured in areas in which there was counting rules, one counts real cells in a volumetric sub-no inflammation. In two animals, which had received sample of the entire tissue without making assumptions grafts ofb-gal-fibroblasts, in some of the sections the graft regarding the size, shape, or orientation of cells. This raw had detached from the section during the staining process. count of cells is used to estimate total neuronal number Although the cavity left outlined the graft site, we ex- using mathematical calculation from tissue volume. cluded these animals in the analysis. In two animals, which
received grafts of FGF-2 fibroblasts, the implanted cells 2.2. FGF-2 were obscured by inflammatory cells or the needle track,
and these two animals were not included in this analysis. 2.2.1. Expression in intact rats
Although, the grafts were obscured in the sections avail- To determine the time-course of expression of FGF-2 able and could not be adequately evaluated, the number of and reaction to the implants, in 18 intact female Sprague– dopaminergic neurons in the SN and preservation of Dawley rats (approximately 225 g) we implanted FGF-2-dopaminergic axons in the striatum was similar to that of fibroblasts (n59) and b-gal-fibroblasts (n59) by the the other animals that received FGF-2-fibroblasts. technique described above. At 1, 2 and 5 weeks, three In three sequential striatal sections, which were 200 mm animals from each group were sacrificed. Two of the apart and in which the implant was clearly visible, we animals in each group were fixed with paraformaldehyde labeled the tissue for glial fibrillary acidic protein (GFAP) as described above, and the other one was fixed with using a monoclonal antibody against GFAP (Amersham, periodate–lysine–paraformaldehyde fixative [29]. The Newark, NJ) at a dilution of 1:1000 by the technique brains were immunolabeled for FGF-2-IR material using a described above. We used the image analysis system to monoclonal antibody (Upstate Biotechnology, Lake Placid, measure the area of increased GFAP-IR material along the NY) at a concentration of 12.5mg / ml.
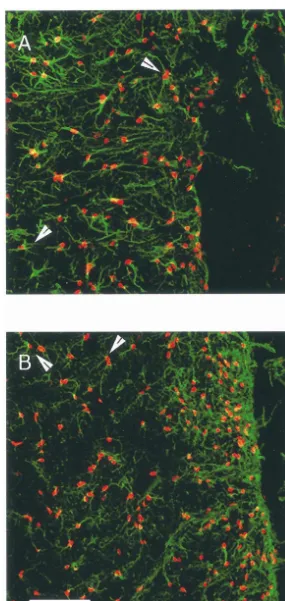
the implanted cells but also in cells adjacent to the implant, was taken for cell counting. The cell suspension was so we sought to further define the cell type containing the centrifuged at 6003g for 5 min at room temperature. The FGF-2-IR material by performing labeling of the tissue for dissociation solution was aspirated and the cells were both FGF-2 and GFAP using fluorescent immunohisto- resuspended in 1 ml of PBS and transferred to two 1.5 ml chemistry. Striatal sections were immunolabeled with a Eppendorf tubes. The cells were then centrifuged at 6003g mouse monoclonal raised against FGF-2 (Upstate Bio- for 5 min. In one of the tubes the PBS was aspirated and technology, Lake Placid, NY) at a concentration of 12.5 the cells were resuspended in 100ml of lysis buffer. The mg / ml and a rabbit polyclonal antiserum raised against lysis buffer was composed of: 137 mM NaCl, 20 mM Tris GFAP (Sigma, St. Louis, MO, 1:80 dilution). The sections (pH 8.0), 1% NP-40, 10% glycerol, 1 mM phenylmethyl-were subsequently incubated with fluorescein-labeled an- sulfonyl fluoride, 10mg / ml aprotinin, 1 mg / ml leupeptin tiserum raised in goat against rabbit IgG (Jackson Im- and 0.5 mM sodium vanadate. The tubes were kept on ice munoresearch, West Grove, PA, 1:100 dilution) and for 30 min and were vortexed for 10 s at 5 min intervals. Rhodamine Red-X-labeled antiserum raised in goat against The contents were sonicated on ice three times for 5 s. The mouse IgG (Jackson Immunoresearch, 1:100 dilution). We tubes were then centrifuged at 12,0003g for 15 min at evaluated seven animals (FGF-2-fibroblasts sacrificed at 48C. The supernatant was transfered to an Eppendorf tube weeks 1 (1) and 5 (2) and b-gal-fibroblasts sacrificed at and was assayed immediately for FGF-2-immunoreactive weeks 1 (2), 2 (1) and 5 (1). The double-labeled sections material using the FGF-2 ELISA system (R&D, Min-were examined using a Zeiss Laser Scanning Microscope neapolis, MN) and assayed for protein content by the BCA
(LSM510) with Argon / Krypton laser. technique (Sigma Chemicals, St. Louis, MO).
In these sections we also immunolabeled the tissue for Statistical analyses were performed with GB-STAT, OX-42, which recognizes microglia and macrophage as version 5.0.8 (Silver Spring, MD). Rotational data was well as monocytes and neutrophils [39]. The immuno- analyzed by application of two factor (treatment and time) labeling was described above for TH. The monoclonal repeated measures analysis of variance (ANOVA) and post antibody was obtained from Serotec (Raleigh, NC), and hoc Newman–Keuls tests. Other analyses were performed sections were incubated overnight with the antibody at a by application of one factor (treatment) randomized
1:250 dilution. ANOVA with post hoc Newman–Keuls tests.
2.2.2. Measurement of FGF-2 produced by the FGF-2
fibroblasts 3. Results
In cells grown in parallel with the FGF-2-fibroblasts and
b-gal-fibroblasts implanted into the striatum of 18 intact Animals receiving FGF-2-fibroblasts prior to treatment rats, we determined the amount of FGF-2-IR material with 6-OHDA had significantly less amphetamine-induced released from the cells and the amount present in the lysate rotation than the animals that received either a burr hole
2
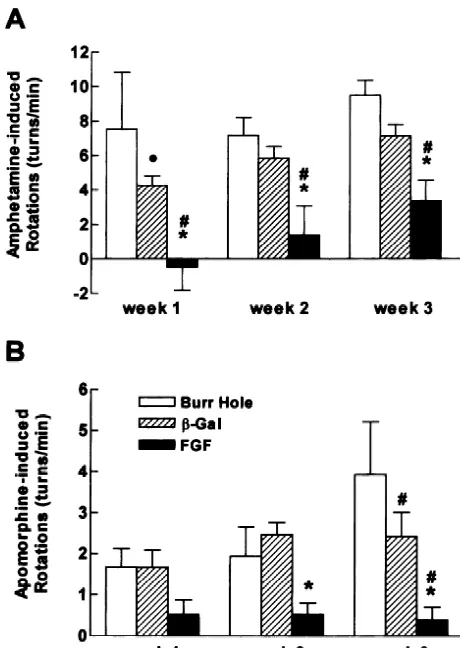
of the cells. Two 75 cm flasks each of FGF-2 and only or implants of b-gal-fibroblasts prior to treatment b-gal-fibroblasts were grown as described above to 90% with 6-OHDA (F2,6259.37, P,0.01) (Fig. 1A). Similarly confluence. On the evening prior to harvesting of the cells, the animals that received FGF-2-fibroblasts had signifi-the media was removed from one of signifi-the flasks containing cantly less apomorphine-induced rotation than either con-FGF-2 fibroblasts and one containingb-gal fibroblasts and trol group (F2,6256.57, P,0.01) (Fig. 1B). The group replaced with 10 ml of media lacking fetal bovine serum. treated with b-gal-fibroblasts had less apomorphine-in-The cells were returned to the incubator for 30 min and duced rotation than the group that received a burr hole then the process was repeated. The cells were then left only group at week three only.
overnight in 10 ml of media lacking fetal bovine serum. Consistent with the reduction in amphetamine and The next morning the media was removed, aliquoted into apomorphine-induced rotation in the animals receiving Eppendorf tubes and stored at 2808C. FGF-2-fibroblasts, the extent of striatal innervation with To determine the amount of FGF-2-IR material in TH-IR fibers was significantly greater in the animals that lysates of the cells, in the remaining two flasks (one FGF-2 received FGF-2-fibroblasts than in the groups that received fibroblasts and the other b-gal fibroblasts) the cells re- either a burr hole only or b-gal-fibroblasts (F2,20516.82, mained in the usual media with fetal bovine serum until P,0.001) (Figs. 2 and 3). In each animal we also the morning of harvesting of cells. The media was then measured the width of the region lacking TH-IR fibers in replaced with media lacking fetal bovine serum and the three sections in which the injections were centered. The cells were returned to the incubator for 30 min. This mean width of the area without TH-IR fibers was sig-process was repeated. The media was then removed and nificantly smaller (F2,2056.66, P,0.01) in the group that washed once with PBS, and 10 ml of enzyme-free cell received FGF-2-fibroblasts (5916234mm, mean6S.E.M.) dissociation solution (Specialty Media, Lavallette, NJ) was than in the burr hole only group (1533668 mm) and the added to each flask and returned to the incubator for 10 b-gal-fibroblast group (1316669 mm).
2
fibroblasts (929,773 mm 6108,296; mean6S.E.M.) did not differ significantly from that in the group that received FGF-2-fibroblasts (1,129,0036257,662) (F1,1150.51, P. 0.5). Gliosis, as reflected by the area with GFAP-IR fibers adjacent to the injection sites (Figs. 5 and 6), did not differ significantly among the three groups (F2,2051.29, P.0.2).
3.1. Production of FGF-2 in vitro
In media collected after the FGF-2-fibroblasts had been left overnight in chemically defined media without fetal bovine serum, we detected 155 pg / ml of FGF-2-IR material. No FGF-2-IR material was detected in the media from the b-gal-fibroblasts. In the lysate from the FGF-2-fibroblasts we detected a concentration of FGF-2-IR material of 148 ng / mg protein. This amount of FGF-2-IR material is very similar to the amount reported in the original description of these cells (151 ng / mg protein) [37]. In the b-gal-fibroblasts we also detected a small amount of FGF-2-IR material (7.6 ng / mg protein). We believe that this small amount of FGF-2-IR material represents the residual from that contained in the fetal bovine serum, as FGF-2-IR material has been shown to be present in fetal serum [22]. We chose to measure the FGF-2-IR material content after only a brief removal of the media containing fetal bovine serum to try to mimic the conditions of transplantation of the cells as closely as possible.
Fig. 1. (A) Amphetamine-induced rotation was significantly lower in the FGF-2-fibroblast group than in either the burr hole group or theb
-gal-3.2. Expression of FGF-2-IR material in vivo at 1, 2
fibroblast group at each week of testing. (B) Similarly,
apomorphine-and5 weeks after implantation
induced rotation was significantly lower in the group that received FGF-2-fibroblasts than either control group.d different from the burr hole group, P,0.05; [different from the burr hole group, P,0.01; *
We noted the presence of FGF-2-IR material in the
different from theb-gal group, P,0.01.
implanted FGF-2-fibroblasts at week 1 (data not shown) and week 2 after implantation into the striatum in intact ipsilateral to the implants and injection of 6-OHDA was rats (Fig. 7A). The FGF-2-IR material was noted on cells greater in the animals that received FGF-2 fibroblasts than scattered through the implant of FGF-2-fibroblasts and either the animals that received a burr hole only or b-gal- appeared to be present in the cytoplasm and on the cell fibroblasts (F2,2059.44, P,0.01) (Fig. 4A). Similarly, the surface. Such immunolabeling was not noted in theb -gal-number of TH-IR neurons in the SNpc on the side fibroblasts (Fig. 7B). FGF-2-IR material was less con-ipsilateral to the implants and injection of 6-OHDA as a spicuous in the implanted fibroblasts at week 5 (data not percentage of the number of TH-IR neurons on the intact shown).
side was significantly greater in animals that received After implantation of both FGF-2-fibroblasts andb -gal-FGF-2-fibroblasts than in the animals that received either a fibroblasts, we also noted an increase in the number of burr hole or implant of b-gal-fibroblasts (F2,20519.70, FGF-2-IR nuclei adjacent to the implant site at weeks 1
P,0.001) (Fig. 4B). and 5 (Fig. 8A and B). These cells had the appearance of
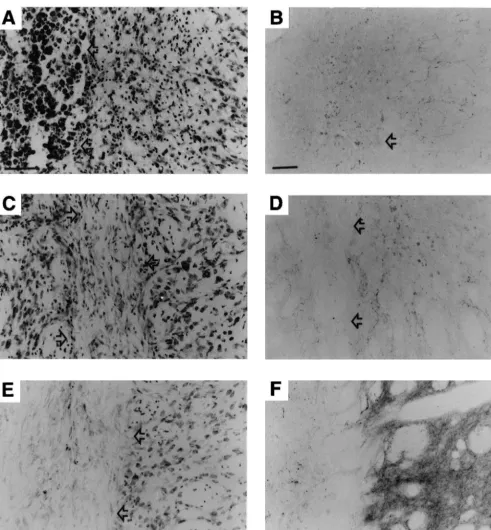
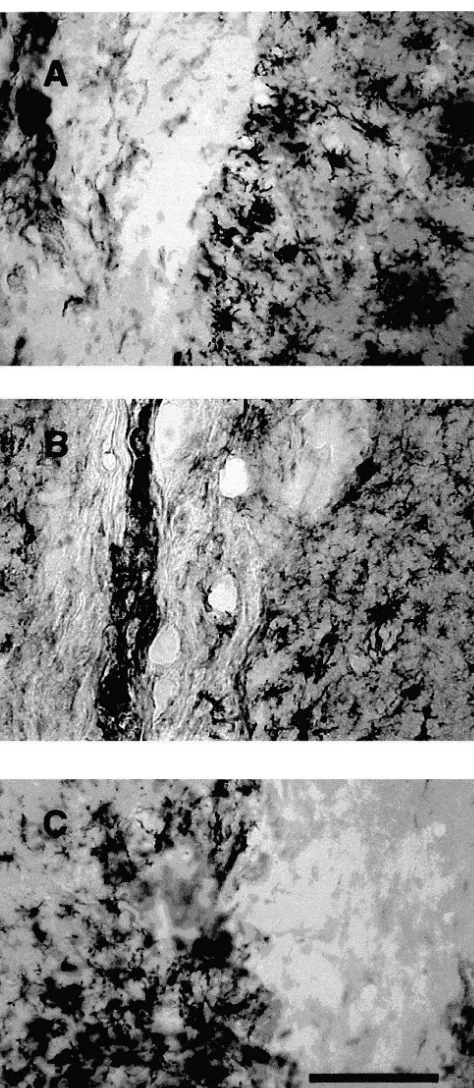
Fig. 2. Representative sections of the striatum from animals treated with burr hole only (A, B),b-gal-fibroblasts (C, D), and FGF-2-fibroblasts (E, F). The sections had been stained for Nissl substance (A, C, E) or immunolabeled for TH (B, D, F). Arrows indicate the border of areas containing predominantly inflammatory cells in animals that received burr hole only (A, B), the border of the graftedb-gal-fibroblast grafts (C, D) or FGF-2-fibroblast grafts (E). Scale bar in A indicates 30mm and is representative also for C–F. Scale bar in B indicates 50mm.
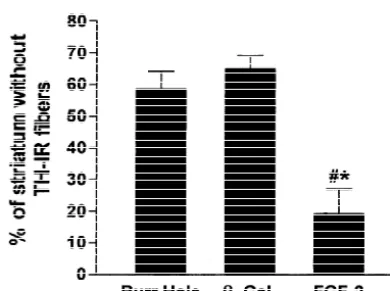
Fig. 3. Five sections of the striatum, in which 6-OHDA had been injected were immunolabeled for TH. The total area of the injected striatum and the area devoid of TH-IR fibers were determined for each animal, and the percentage of the striatum devoid of TH-IR fibers was calculated for each animal. This percentage was significantly lower in the group that received FGF-2-fibroblasts.[different from burr hole, P,0.01; * different from b-gal group, P,0.01.
Fig. 5. Representative sections of the striatum from animals treated with a burr hole only (A), b-gal-fibroblasts (B), and FGF-2-fibroblasts (C) were immunolabeled for GFAP. Scale bar indicates 100mm.
Fig. 4. (A) The number of TH-IR neurons in the SNpc were quantitated in both the side ipsilateral to injection and the intact side. The number on
4. Discussion TH-IR neurons on the injected side in the group that received
FGF-2-fibroblasts was significantly greater than the number in the groups that
received burr hole only ([P,0.01) orb-gal-fibroblasts (*P,0.05). (B) We tested the hypothesis that delivery of FGF-2 to the The number of TH-IR neurons in the SNpc ipsilateral to injection, striatum by intrastriatal implantation of FGF-2-fibroblasts expressed as a percentage of the number in the SNpc contralateral to
could reduce the damage caused by intrastriatal injection
injection, was significantly greater in the groups of animals treated with
of 6-OHDA. The study demonstrated that animals that
FGF-2-fibroblasts than the groups treated withb-gal-fibroblasts or burr
neuroprotective treatment during the course of that process would slow the progression of the disease and be useful in slowing the deterioration of the patients. We employed a model in which we could determine whether the intrastriat-al implantation of FGF-2-fibroblasts could intervene in the degeneration caused by the neurotoxin 6-OHDA. In this model the initial injury occurs in the terminals of the nigrostriatal axons with subsequent retrograde degenera-tion of the cell bodies in the SNpc [40]. Whether the injury to the nigrostriatal dopaminergic system in Parkinson’s disease begins initially in the axon terminals in the striatum or in the cell bodies in the SNpc remains unknown. However, autopsy studies in Parkinsonian brains
Fig. 6. Three striatal sections that were adjacent to those in which the have demonstrated greater loss of dopamine in the striatum density of TH-IR material had been measured were immunolabeled for
than in the SNpc, and one interpretation of this data is that
GFAP, and area of intense gliosis was measured. The gliosis did not differ
the initial injury is greater in the nerve terminals [23]. The
significantly among the three groups.
model that we employed reflects this pattern of injury to the nigrostriatal dopaminergic system.
asymmetry, loss of striatal dopaminergic innervation and We chose to study fibroblasts that had been genetically loss of nigral dopaminergic neurons than either control engineered to produce native FGF-2, rather than a form group. These results are consistent with previous reports of that was modified to also express the signal sequence of a protective effect of direct administration of FGF-2 on the nerve growth factor (NGF) to obtain secretion of the nigrostriatal dopaminergic system [31,32] and the report FGF-2 from the transfected cells. Cells expressing native that FGF-2-fibroblasts can have a trophic effect on fetal FGF-2 had been demonstrated in previous studies to be dopaminergic neurons grafted to the striatum in rodent more potent in promotion of survival and fiber outgrowth models of Parkinson’s disease [45]. Similarly, Uteza and when grafted with fetal dopaminergic neurons [45]. None colleagues reported that implantation of fibroblasts secret- of the FGF-2 isoforms contain a signal sequence. But ing FGF-2 delayed the degeneration of photoreceptors in a previous studies have shown that FGF-2 can be released
rat model of retinal degeneration [48]. into the extracellular medium by mechanisms, which are
We employed a model in which the FGF-2-fibroblasts not yet fully understood [26,30]. Our study did detect were implanted in the striatum prior to administration of FGF-2-IR material in the medium after the FGF-2-fi-the neurotoxin. Parkinson’s disease is a chronic neurode- broblasts had been left in chemically defined medium generative disorder in which there is progressive loss of overnight.
dopaminergic axons in the striatum and neurons in the The mechanism by which the FGF-2-fibroblasts substantia nigra [17]. It is plausible that administration of a protected the nigrostriatal system remains to be fully
elucidated. A trivial explanation would be that FGF-2 simply bound the 6-OHDA and prevented its access to the dopamine transporter and uptake into the dopaminergic axons. This seems unlikely, since a number of studies have indicated that FGF-2 does not impede, rather it increases
3
uptake of H dopamine in cultures of dopaminergic neurons [5,10,28,34]. Other trivial explanations, such as difference in the size of the grafts, also did not appear to explain the effect.
One consideration is the role that glia might have played in the effect of the FGF-2-fibroblasts. Previous in vitro studies have demonstrated that FGF-2 can enhance the survival of mesencephalic dopaminergic neurons, and the trophic effect appeared to be mediated through glia present in the cultures. An in vivo study reported that addition of FGF-2, but not NGF, to nigral grafts in hemiparkinsonian grafts resulted in a more rapid reduction in amphetamine-induced rotational asymmetry, an increase in the number of TH-IR cells in the grafts, larger graft volume and longer neurite outgrowth [52]. The effects correlated with the number of GFAP-IR astrocytes suggesting that the actions of FGF-2 may have been mediated through glial cells. Our data indicated that at 5 weeks after the implantation of the genetically engineered fibroblasts and 3 weeks after lesion-ing with 6-OHDA, the level of reactive gliosis did not differ among the three treatment groups. It is conceivable that after implantation of the fibroblasts and before lesion-ing with 6-OHDA, there may have been more gliosis in the group that received FGF-2-fibroblasts than in the group that received b-gal-fibroblasts. Given the reactive gliosis caused by injection of 6-OHDA alone, we cannot comment on this possibility. However, pertinent to this possibility were the reports from Unsicker’s group [33] that quantita-tive determination of GFAP and immunohistochemical analyses of GFAP-IR cells in the striatum did not differ significantly between mice treated with 14 days of intra-striatal FGF-2 or cytochrome c (the time after grafting of cells at which the animals were treated with 6-OHDA in our study) but that at earlier time-points (18 h and 2 days) there was a significant increase in the number of GFAP-IR cells in the animals treated with FGF-2 [51]. Our study of FGF-2 expression by FGF-2-fibroblasts implanted in the intact striatum was not powered to quantitatively evaluate reactive gliosis caused by implantation of FGF-2- and b-gal-fibroblasts, but we noted substantial gliosis in early time-points in both groups.
FGF-2 more likely protected the nigrostriatal dopa-minergic system by more subtle effects on glia and / or a direct effect on the dopaminergic axons and neurons. Of note, Hou et al. [24] have shown that protection of
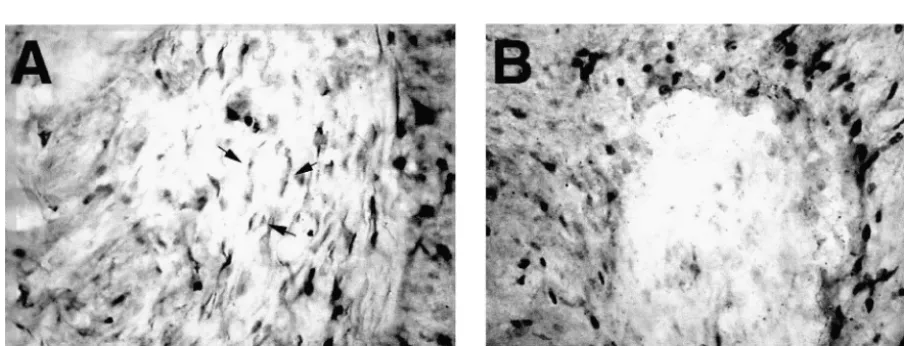
Fig. 9. Sprague–Dawley rats that had received implants of FGF-2- mesencephalic dopaminergic neurons against 6-OHDA
fibroblasts and sacrificed 5 weeks later (A) or b-gal-fibroblasts and toxicity by FGF-2 in vitro is mediated through upregula-sacrificed 1 week later (B) were immunolabeled for OX-42 to identify tion of the glutathione system in glia. Since neurons in the microglia and macrophage. In addition, Fischer 344 rats received implants
SNpc contain FGFR-1 message and protein [6,20], it is
of GDNF-fibroblasts and were sacrificed 4 weeks later (C). In all of the
likely that the nigral, dopaminergic neurons respond to
animals studied we noted OX-42-IR cells surrounding the implant and in
We noted that implantation of FGF-2 and b-gal-fi- only the region affected by the degenerative process, e.g., broblasts into the striatum, not surprisingly, was accom- the nigrostriatal system, and not to other regions. The need panied by reactive astrocytes and OX-42-IR cells adjacent to avoid delivery of the trophic factor outside of that to the implant. We initially thought that this reaction might region is the result of the fact that a trophic factor’s have been due to implantation of fibroblasts derived from distribution and actions typically are not limited to a single the Fischer 344 strain of rats into Sprague–Dawley rats. neural system, rather trophic factors, such as FGF-2, are However, we also noted these changes when we trans- usually present in and affect a number of neural systems. planted Fischer 344-derived fibroblasts, which had been These concerns are more than theoretical as demonstrated genetically engineered to produce glial cell line-derived by the recent experience with NGF. Swedish investigators neurotrophic factor, into Fischer 344 rats. Trophic factors reported that patients with Alzheimer’s disease developed produced by reactive astrocytes [8,38, and see above] or, painful dysesthesias after intraventricular administration of GDNF produced by microglia [3] as part of the reaction to NGF [25]. Winkler and colleagues found in rats that implantation of fibroblasts into the striatum may have intraventricular administration of NGF caused hyperplasia contributed to the effect of the FGF-2-fibroblasts. Because of Schwann cells [50].
we also noted the presence of reactive astrocytes and Genetically engineered fibroblasts provide an attractive microglia after implantation ofb-gal-fibroblasts, the pres- vehicle, as they can be developed from an individual and ence of these cells appears not to be sufficient to protect thereby avoid the problem of immunological rejection. The the dopaminergic system in the model that we used. fibroblasts, which have been genetically engineered to Previous work from our group had utilized direct produce a trophic factor such as FGF-2, can be grafted into administration of trophic factors to enhance the activity of the striatum of parkinsonian patients. The degenerative or to protect the nigrostriatal dopaminergic system [41– process that occurs in Parkinson’s disease appears to first 43]. As the understanding of trophic factors has increased, begin in the dopaminergic axons in the striatum and later we and others have begun to increasingly appreciate that a to result in death of the neurons in the SNpc [23]. We and number of issues need to be addressed before trophic others have shown that although the majority of FGF-2 factors can be used safely and effectively [44]. Two of the injected into the striatum remains in this region, some is major issues are delivery to the central nervous system and transported retrogradely to the SNpc [11,19].
delivery limited to specific regions of the central nervous In summary, our study demonstrated that fibroblasts,
system. which had been genetically engineered to produce FGF-2
In certain diseases, such as peripheral neuropathy or and implanted into the striatum, attenuated the damage perhaps amyotrophic lateral sclerosis, systemically ad- caused by intrastriatal injection of 6-OHDA. This study is ministered trophic factor may be able to gain access to the a proof of principle that fibroblasts that have been ge-peripheral nerves and exert a trophic effect on the injured netically engineered to produce FGF-2, or perhaps other nervous system. However, in degenerative disorders of the trophic factors, can protect the nigrostriatal dopaminergic brain such as Parkinson’s disease, the blood–brain barrier system in a model of PD and suggests that intrastriatal will prevent access of the trophic factor to the area of implantation of cells, which had been genetically en-degeneration. A number of strategies have been developed gineered to produce FGF-2 or other trophic factors, may be to overcome this barrier. The most straightforward ap- useful in the treatment of Parkinson’s disease.
proach has been intrastriatal administration of a trophic factor [31–33,42,43,46]. This technique may be useful for short duration administration but is not currently feasible
Acknowledgements for prolonged administration. A second straightforward
approach is intraventricular administration of the trophic
The work was supported by a Merit Review Grant from factor [1,16]. However, the fact that all the trophic factors
the Department of Veterans Affairs and a Center of that have been identified to date affect multiple neural
Excellence Award from the National Parkinson Federation systems will likely limit the use of this technique. Also,
to C.S. and F.G and NIH / NIA PO1 AG 10435 to F.G. We experience indicates that repeated infusion either into the
thank Tess Kimber and Quentin Heinemann for their brain parenchyma or the ventricular system is too often
technical assistance and Barb Reader for her help in associated with complications [27,36]. A technique
de-preparation of the manuscript. veloped to deliver trophic factors across the blood–brain
barrier is to couple the trophic factor to a molecule for which there is a transport system, e.g., NGF coupled to an
antibody to the transferrin receptor [14]. Again the wide- References spread actions of most trophic factors may limit the
usefulness of this technique. [1] P. Aebischer, M. Schluep, N. Deglon, J.-M. Joseph, L. Hirt, B.´ Not only must the trophic factor be delivered to the Heyd, M. Goddar, J.P. Hammang, A.E. Zurn, A.C. Kato, F. Regli, E.
125 modified xenogeneic cells in amyotrophic lateral sclerosis patient, Shults, A. Baird, Storage, metabolism and processing of [ I]-Nature Med. 2 (1996) 696–699. fibroblast growth factor-2 after intracerebral injection, Brain Res.
665 (1994) 285–292. [2] A. Baird, Fibroblast growth factors: activities and significance of
non-neurotrophin growth factors, Curr. Opin. Neurobiol. 4 (1994) [20] A.M. Gonzalez, M. Berry, P.A. Maher, A. Logan, A. Baird, A
78–86. comprehensive analysis of the distribution of FGF-2 and FGFR1 in
the rat brain, Brain Res. 701 (1995) 201–226. [3] P.E. Batchelor, G.T. Liberatore, J.Y. Wong, M.J. Porritt, F. Frerichs,
G.A. Donnan, D.W. Howells, Activated macrophages and microglia [21] F. Hefti, Neurotrophic factor therapy for nervous system degenera-induce dopaminergic sprouting in the injured striatum and express tive diseases, J. Neurobiol. 25 (1994) 141–143.
brain-derived neurotrophic factor and glial cell line-derived neuro- [22] D.J. Hill, G.J.M. Tevaarwerk, E. Arany, D. Kilkenny, M. Gregroy, trophic factor, J. Neurosci. 19 (1999) 1708–1716. K.S. Langford, J. Miell, Fibroblast growth factor-2 (FGF-2) is [4] A.J. Bean, R. Elde, Y. Cao, C. Oellig, C. Tamminga, M. Goldstein, present in maternal and cord serum, and in the mother is associated
¨
R.F. Pettersson, T. Hokfelt, Expression of acidic and basic fibroblast with a binding protein immunologically related to the FGF receptor-growth factors in the substantia nigra of rat, monkey, and human, 1, J. Clin. Endocrinol. Metab. 80 (1995) 1822–1831.
Proc. Natl. Acad. Sci. USA 88 (1991) 10237–10241. [23] O. Hornykiewicz, Mechanisms of neuronal loss in Parkinson’s ¨
[5] K.D. Beck, B. Knusel, F. Hefti, The nature of the trophic action of disease: a neuroanatomical-biochemical perspective, Clin. Neurol. brain-derived neurotrophic factor, des(1–3)-insulin-like growth Neurosurg. 94 (1992) S9–S11.
factor-1, and basic fibroblast growth factor on mesencephalic [24] J.-G. Hou, G. Cohen, C. Mytilineou, Basic fibroblast growth factor dopaminergic neurons developing in culture, Neuroscience 52 stimulation of glial cells protects dopamine neurons from 6-hy-(1993) 855–866. droxydopamine toxicity: involvement of the glutathione system, J. [6] N. Belluardo, G. Wu, G. Mudo, A.C. Hansson, R. Pettersson, K. Neurochem. 69 (1997) 76–83.
Fuxe, Comparative localization of fibroblast growth factor receptor- [25] M. Jonhagen, L.O. Wahlund, K. Amberla, T. Ebendal, B. Meyer-¨ ˚
1,-2, and -3 mRNAs in the rat brain: in situ hydrization analysis, J. sson, A. Norberg, L. Olson, M. Shigeta, A. Seiger, M. Viitanen, B. Comp. Neurol. 379 (1997) 226–246. Winblad, Nerve growth factor as a treatment of Alzheimer’s disease,
´
[7] G. Chadi, A. Moller, L. Rosen, A.M. Janson, L.A. Agnati, M. Neurobiol. Aging 17 (Suppl.) (1996) 160–161. ¨
Goldstein, S.-O. Ogren, R.F. Pettersson, K. Fuxe, Protective actions [26] J. Jouanneau, J. Plouet, G. Moens, J.P. Thiery, FGF-2 and FGF-1 of human recombinant basic fibroblast growth factor on MPTP- expressed in rat bladder carcinoma cells have similar angiogenic lesioned nigrostriatal dopamine neurons after intraventricular infu- potential but different tumorigenic properties in vivo, Oncogene 14 sion, Exp. Brain Res. 97 (1993) 145–158. (1997) 671–676.
[8] G.Y. Chadi, Y. Cao, R.F. Pettersson, K. Fuxe, Temporal and spatial [27] A. Karavelis, G. Foroglou, P. Selviaridis, G. Fountzilas, Intraven-increase of astroglial basic fibroblast growth factor synthesis after tricular administration of morphine for control of intractable cancer 6-hydroxydopamine-induced degeneration of the nigrostriatal dopa- pain in 90 patients, Neurosurgery 39 (1996) 57–62.
minergic neurons, Neuroscience 61 (1994) 891–910. [28] B. Knusel, P.P. Michel, J.S. Schwaber, F. Hefti, Selective and¨ [9] I. Date, Y. Yoshimoto, T. Imaoka, Y. Miyoshi, T. Furuta, S. Asari, T. nonselective stimulation of central cholinergic and dopaminergic Ohmoto, Enhanced recovery of the nigrostriatal dopaminergic development in vitro by nerve growth factor, basic fibroblast growth system in MPTP-treated mice following intrastriatal injection of factor, epidermal growth factor, insulin and the insulin-like growth basic fibroblast growth factor in relation to aging, Brain Res. 621 factors I and II, J. Neurosci. 10 (1990) 558–570.
(1993) 150–154. [29] I.W. McLean, P.K. Nakane, Periodate-lysine-paraformaldehyde fixa-[10] J. Engele, M.C. Bohn, The neurotrophic effects of fibroblast growth tive. A new fixative for immunoelectron microscopy, J. Histochem.
factors on dopaminergic neuron in vitro are mediated by mesence- Cytochem. 22 (1974) 1077–1083.
phalic glia, J. Neurosci. 11 (1991) 3070–3078. [30] P. Mignatti, T. Morimoto, D.B. Rifkin, Basic fibroblast growth [11] I.A. Ferguson, E.M. Johnson, Fibroblast growth factor receptor- factor, a protein devoid of secretory signal sequence, is released by bearing neurons in the CNS: identification of receptor-mediated cells via a pathway independent of the endoplasmic reticulum–Golgi retrograde transport, J. Comp. Neurol. 313 (1991) 693–706. complex, J. Cell. Physiol. 151 (1992) 81–93.
[12] G. Ferrari, M.-C. Minozzi, G. Toffano, A. Leon, S.D. Skaper, Basic [31] D. Otto, K. Unsicker, Basic FGF reverses chemical and morphologi-fibroblast growth factor promotes the survival and development of cal deficits in the nigrostriatal system of MPTP-treated mice, J. mesencephalic neurons in culture, Dev. Biol. 133 (1989) 140–147. Neurosci. 10 (1990) 1912–1921.
[13] L. Fisher, F.H. Gage, Intracerebral transplantation: basic and clinical [32] D. Otto, K. Unsicker, FGF-2 modulates dopamine and dopamine-applications to the neostriatum, FASEB J. 8 (1994) 489–496. related striatal transmitter systems in the intact and MPTP-lesioned [14] P.M. Friden, L.R. Walus, R. Watson, S.R. Doctrow, J.W. Kozarich, C. mouse, Eur. J. Neurosci. 5 (1993) 927–932.
¨
Backman, H. Bergman, B. Hoffer, F. Bloom, A.-C. Granholm, [33] D. Otto, K. Unsicker, FGF-2 in the MPTP model of Parkinson’s Blood–brain barrier penetration and in vivo activity of an NGF disease: effects on astroglial cells, Glia 11 (1994) 47–56. conjugate, Science 259 (1993) 373–377. [34] T.H. Park, C. Mytilineou, Protection from 1-methyl-4-[15] K. Fuxe, B. Tinner, M. Zoli, R.F. Pettersson, A. Baird, G. Biagini, phenylpyridinium (MPP1) toxicity and stimulation of regrowth of G. Chadi, L.F. Agnati, Computer-assisted mapping of basic fibro- MPP1-damaged dopaminergic fibers by treatment of mesencephalic blast growth factor immunoreactive nerve cell populations in the rat cultures with EGF and basic FGF, Brain Res. 599 (1992) 83–97. brain, J. Chem. Neuroanat. 11 (1996) 13–35. [35] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [16] D.M. Gash, Z. Zhang, A. Ovadia, W.A. Cass, A. Yi, L. Simmerman, Academic Press, San Diego, 1986.
D. Russell, D. Martin, P.A. Lapchak, F. Collins, B.J. Hoffer, G.A. [36] R.K.B. Pearce, P. Collins, P. Jenner, C. Emmett, C.D. Marsden, Gerhardt, Functional recovery in parkinsonian monkeys treated with Intraventricular infusion of basic fibroblast growth factor (bFGF) in GDNF, Nature 380 (1996) 52–255. the MPTP-treated common marmoset, Synapse 23 (1996) 192–200. [17] W.R.G. Gibb, Neuropathology of the substantia nigra, Eur. Neurol. [37] J. Ray, J. Hogg, A.S. Beutler, T. Hideichi, A. Baird, F.H. Gage, 31 (Suppl. 1) (1991) 48–59. Expression of biologically active basic fibroblast growth factor by [18] V.C. Gomide, G. Chadi, The trophic factors S-100 beta and basic genetically modified rat primary skin fibroblasts, J. Neurochem. 64
fibroblast growth factor are increased in the forebrain reactive (1995) 503–513.
astrocytes of adult callosotomized rat, Brain Res. 835 (1999) 162– [38] J.L. Ridet, S.K. Malhotra, A. Privat, F.H. Gage, Reactive astrocytes:
174. cellular and molecular cues to biological function, Trends Neurosci.
[39] A.P. Robinson, T.M. White, D.W. Mason, Macrophage heterogeneity Hoffer, L. Olson, Protection and repair of the nigrostriatal dopa-in the rat as determdopa-ined by two monoclonal antibodies MRC OX-41 minergic system of GDNF in vivo, Nature 373 (1995) 335–339. and MRC OX-42, the latter recognizing complement receptor type [47] I. Tooyama, T. Kawamata, D. Walker, T. Yamada, K. Hanai et al., 3, Immunology 57 (1986) 239–247. Loss of basic fibroblast growth factor in substantia nigra neurons in [40] H. Sauer, W.H. Oertel, Progressive degeneration of nigrostriatal Parkinson’s disease, Neurology 43 (1993) 372–376.
dopamine neurons following intrastriatal terminal lesions with 6- [48] Y. Uteza, J.-S. Rouillot, A. Kobetz, D. Marchant, S. Pecqueur, E. hydroxydopamine: a combined retrograde tracing and immuno- Arnaud, H. Prats, J. Honiger, J.L. Dufier, M. Abitbol, M. Neuner-cytochemical study in the rat, Neuroscience 59 (1994) 401–415. Jehle, Intravitreous transplantation of encapsulated fibroblasts sec-[41] C.W. Shults, R. Matthews, P. Langlais, F. Gage, A. Baird, Effects of reting the human fibroblast growth factor 2 delays photoreceptor cell BDNF and bFGF on the mesostriatal dopaminergic system in vivo, degeneration in Royal College of Surgeons rats, Proc. Natl. Acad. Mov. Disord. 7 (1992) 291. Sci. USA 96 (1999) 3126–3131.
[42] C.W. Shults, T. Kimber, C.A. Altar, BDNF attenuates the effects of [49] M.J. West, Stereological methods for estimating the total number of intrastriatal injection of 6-hydroxydopamine, NeuroReport 6 (1995) neurons and synapses: issues of precision and bias, Trends Neurosci.
1109–1112. 22 (1999) 51–61.
[43] C.W. Shults, T. Kimber, D. Martin, Intrastriatal injection of GDNF [50] J. Winkler, G.A. Ramirez, G. Kuhn, D.A. Peterson, P.A. Day-Lollini, attenuates the effects of 6-hydroxydopamine, NeuroReport 7 (1996) G.R. Stewart, M.H. Tuszynski, F.H. Gage, L.J. Thal, Reversible 627–631. induction of Schwann cell hyperplasia and sprouting of sensory and [44] C.W. Shults, Neurotrophic factors in neurodegenerative disorder, in: sympathetic neurites in vivo after continuous intracerebroventricular J. Jankovic, E. Tolosa (Eds.), Parkinson’s Disease and Movement administration of nerve growth factor, Ann. Neurol. 41 (1997) Disorders, 3rd Edition, Williams and Wilkins, Baltimore, 1998, pp. 82–93.
105–118. [51] S.B. Wirth, M. Rufer, K. Unsicker, Early effects of FGF-2 on glial [45] H. Takayama, J. Ray, H.K. Raymon, A. Baird, J. Hogg, L.J. Fisher, cells in MPTP-lesioned striatum, Exp. Neurol. 137 (1996) 191–200. F.H. Gage, Basic fibroblast growth factor increases dopaminergic [52] B.-Y. Zeng, P. Jenner, C.D. Marsden, Altered motor function and graft survival and function in a rat model of Parkinson’s disease, graft survival produced by basic fibroblast growth factor in rats with Nature Med. 1 (1995) 53–58. 6-OHDA lesions and fetal ventral mesencephalic grafts are
associ-¨