www.elsevier.com / locate / bres
Research report
Immuno-electron microscopic localization of the
a
1and
b
1-subunits
of soluble guanylyl cyclase in the guinea pig organ of Corti
a ,
*
a b a c¨
¨
Ulf-Rudiger Heinrich
, Jan Maurer , Doris Koesling , Wolf Mann , Ulrich Forstermann
a
Department of Otolaryngology – Head and Neck Surgery, Johannes Gutenberg University Medical School, 55131 Mainz, Germany
b
Department of Pharmacology and Toxicology, University of Bochum, 44780 Bochum, Germany
c
Department of Pharmacology, Johannes Gutenberg University, 55101 Mainz, Germany
Accepted 8 August 2000
Abstract
Guanylyl cyclases (GC) catalyze the formation of the intracellular signal molecule cyclic GMP from GTP. For some years it has been known that the heme-containing soluble guanylyl cyclase (sGC) is stimulated by NO and NO-containing compounds. The sGC enzyme consists of two subunits (a1andb1). In the present study, thea1andb1-subunits were identified in the guinea pig cochlea at the electron microscopic level using a post-embedding immuno-labeling procedure. Ultrathin sections of LR White embedded specimens were incubated with various concentrations of two rabbit polyclonal antibodies to thea1- andb1-subunit, respectively. The immunoreactivity was visualized by a gold-labeled secondary antibody in an energy-filtering transmission electron microscope (EFTEM). Marked immunoreactivity for both antibodies was found in the inner and outer hair cells, with numerous gold particles at the border of the cuticular plates, associated with the cell nuclei or attached to electron-dense parts of the cytoplasm. In the pillar cells and apical Deiters cells, soluble guanylyl cyclase immunoreactivity was located at the rim of the cuticular plates and between the microtubuli bundles. Together with the recently identified nitric oxide synthase isoforms [Eur. Arch. Otorhinolaryngol. 254 (1997) 396; Eur. Arch. Otorhinolaryngol. 255 (1998) 483], the soluble guanylyl cyclase may be involved in signalling processes in the organ of Corti. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory system
Topic: Auditory, vestibular and lateral line: periphery
Keywords: Guinea pig; Soluble guanylyl cyclase; Hair cell; Signal transduction; Immunocytochemistry; Nitric oxide synthase; Post-embedding immuno-labeling
1. Introduction immunoreactivities were found in the cytoplasm and
associated with the nuclei of inner and outer hair cells. Nitric oxide (NO) is an intra- and intercellular signaling NOS immunoreactivity was also located to the cuticular molecule that exerts important biological functions in plates of inner and outer pillar cells and to the cytoskeletal many cells and tissues [9,21]. In mammalian cells, NO is elements of apical and basal Deiters cells [15,16]. synthesized from L-arginine by the enzyme NO synthase Recently, NO has been implicated in some physiological
(NOS). Three isoforms of NOS have been identified: the effects in the inner ear. The signalling molecule NO seems neuronal-type NOS I, the inducible-type NOS II, and the to contribute to the basal tone of cochlear blood vessels endothelial-type NOS III [9,21]. In the inner ear, two [1]. Furthermore, sodium nitroprusside, an activator of isoforms of NOS, NOS I and NOS III, have been localized soluble guanylyl cyclase (sGC), suppressed sound-evoked immunochemically at the light- and electron microscopic cochlear potentials [2]. This cochlear neuromodulation was level [8,11,12,15,16]. Fine structure immunocytochemical partially inhibited by the NO scavenger and inhibitor of analyses identified both isoforms in largely the same cell sGC, methylene blue (MB) [3]. sGC is the major target types in the organ of Corti. Abundant NOS I and NOS III enzyme of NO in many tissues. In the rat, the product of sGC, cGMP, has been identified immunohistochemically in
*Corresponding author. Fax:149-76-120-350-16. some structure of the inner ear, namely in pericytes of the
spiral ligament and in nerve fibers innervating the outer In control experiments, the primary antisera (GCpep8 or hair cells [6]. In the guinea pig, NO-stimulated cGMP GCpep3) were omitted. An additional control for specificity formation has been detected in Hensen’s and Deiter’s cells of cell labeling is inherent in the method itself, as the as well as in vascular pericytes of the inner ear [7]. The immunolabeling observed in the embedding resin or in enzyme sGC consists of the two subunits (a and b), both acellular structures such as the tectorial or basilar mem-of which are required for catalytic activity [18]. Recently, branes represents nonspecific background labeling. Finally, the transcription pattern the mRNAs of all known guanylyl the labeling of the same structures by antibodies to thea1 -cyclase isoforms were examined in different tissues of the andb1-subunits of sGC, respectively, is a further indica-inner ear of the rat by the reverse-transcription polymerase tion of specificity.
chain reaction (RT-PCR) [22]. Using specific primers, it As reported previously [15,16], the ultrathin sections could be demonstrated that the sGC-a1and -b1subunits as were analyzed by an energy-filtering transmission electron well as the sGC-a2and -b2were expressed in the organ of microscope (EFTEM) that excludes inelastically scattered Corti [22]. electrons and enhances the contrast. The density of gold The current study was undertaken to map the spatial particles was determined on various ultrathin sections in a
2 distribution of the sGC protein itself in the organ of Corti frame of 1mm . and to determine its subcellular localization utilizing
antibodies to both the a1-and the b1-subunits of the
enzyme. 3. Results
3.1. Localization of the anti-sGC antibodies
2. Materials and methods
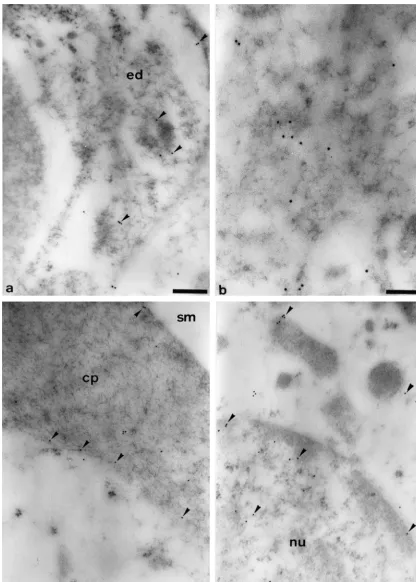
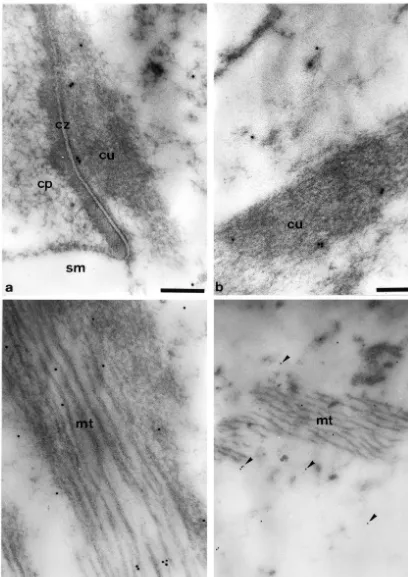
Antibodies to the a1- and b1-subunits of sGC always 2.1. Animals labeled the same cell types and subcellular structures. Electron microscopic analyses localized sGC immuno-All experiments were conducted in accordance with the reactivity to the cytoplasm of the endothelial cells of the German Prevention of Cruelty to Animals Act and were blood-vessel under the basilar membrane (Fig. 1a) and to approved by the supervising authorities. Healthy guinea endothelial cells in the stria vascularis (not shown). Within pigs weighing 200–400 g with good Preyer’s reflexes and the inner and outer hair cells, significant amounts of no evidence of middle ear disease were used for the study. gold-labeled anti-sGC antibodies were found attached to After decapitation, both cochleae were rapidly removed electron-dense regions of the structureless cytoplasm (Fig. (within 3 min of sacrifice), fixed and embedded in London 1b). In addition, gold particles were also seen at the rim of Resin (LR) White as described previously [15,16]. the cuticular plates and in smaller amounts within these cell structures (Fig. 1c). Immunoreactivity was also found 2.2. Antibodies associated with the nuclei of the hair cells. This was located more to the periphery of the karyoplasm (Fig. 1d). An antibody raised against a synthetic peptide corre- Within the lamina reticularis, gold-labeled anti-sGC sponding to a sequence of the a1-subunit of bovine lung antibodies were identified at the contact zone between the sGC (GCpep8) [14] and an antibody to a peptide sequence cuticular plates of the hair cells and the cuticular structures of the b1-subunit of bovine lung sGC (GCpep3) were of the Deiters cells (Fig. 2a). Immunoreactivity was not utilized in the current study. directly located to the cell membranes, but was found about 20–50 nm away from the intercellular space. sGC 2.3. Electron microscopic immunocytochemistry was also identified in the electron-dense, actin-rich parts of the cuticular structures of the Deiters cells (Fig. 2b) and Post-embedding immuno-labeling was performed using within the microtubuli bundles which are connected to ultrathin tissue sections of the second turn of the cochlea these structures (Fig. 2c). Within the structureless cyto-of the guinea pig. The sections were pre-incubated for 5 plasm of the Deiters cells, the amounts of the gold-labeled min in NH Cl, washed three times in phosphate-buffered4 anti-sGC antibodies were less compared to the immuno-saline (PBS) and incubated for 10 min with bovine serum reactivity identified in the hair cells. Nevertheless, a certain albumin (2%) before an overnight incubation at 48C with amount of sGC was always found in or around the either GCpep8 (diluted to 1:500, 1:750, 1:1000 or 1:2000) microtubuli bundles which were present in some basal or GCpep3 (diluted to 1:10 000 or 1:15 000). Then the Deiters cells near to the third row of outer hair cells (Fig. sections were washed six times in PBS and incubated for 2d).
1.5–2.5 h with a gold-labeled goat anti-rabbit secondary
antibody (10-nm particles, diluted to 1:40, Sigma Bio- 3.2. Cellular differences in sGC content sciences, St. Louis, MO, USA). After additional rinses in
2 21
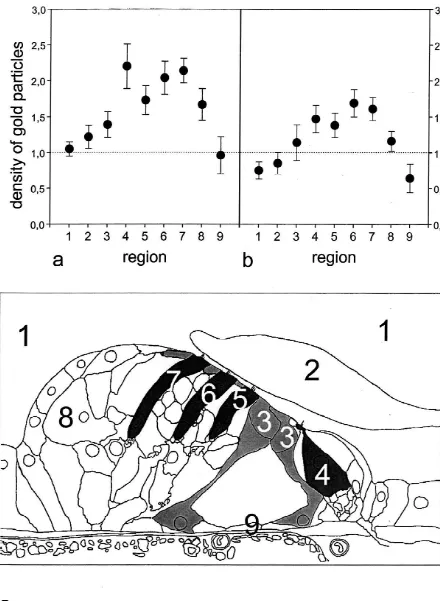
of gold particles within a constant area of 1 mm was bound by fixed stereociliary buffers and mobile Ca -determined for both antibodies in nine different regions binding molecules [19]. These ion-regulating processes (for numbering see Fig. 3c). The cellular labeling pattern might also exist in other regions of the hair cell. The local
21
was similar for antibodies to thea1-subunit (Fig. 3a) and Ca increase may activate other processes in the outer the b1- subunit of sGC (Fig. 3 b). Significant immuno- hair cells, such as the NO / cGMP signaling pathway. reactivity was always found in the inner hair cells (region 4 Using immunolabeling procedures, NOS I and NOS III in Fig. 3c) and in the three rows of the outer hair cells (first have been identified at an ultrastructural level in the organ row of outer hair cells5region 5, second row of outer hair of Corti [15,16]. Biochemical studies performed some cells5region 6, third row of outer hair cells5region 7 in years ago had also shown the formation of cGMP in this Fig. 3c). Some immunoreactivity was also identified in the tissue [13,23]. These findings suggest the presence of an cuticular plates of the inner and outer pillar cells (region 3 NO / cGMP signalling pathway in the organ of Corti in Fig. 3c) and in Deiters cells with visible microtubuli [6,8,13,23]. In the current study, we determined the bundles (region 8 in Fig. 3c, see also Fig. 2d). The modest cellular and subcellular distribution of the a1- and b1 -immunoreactivity seen in the scala media (region 1 in Fig. subunits of sGC – the target enzyme of NO – at an 3c), in the tectorial membrane (region 2 in Fig. 3c) and in ultrastructural level. With several concentrations of pri-the basilar membrane (region 9 in Fig. 3c) represents mary antisera against the two sGC subunits, we obtained non-specific background labeling. consistent results. Both sGC-subunits were always found With the Student–Newman–Keuls multiple range test, co-expessed in the same cell types and colocalized in the significant differences were identified for both antibodies same subcellular structures. Thea1- andb1-subunits were when comparing the immunoreactivity in the various seen in the endothelial cells under the basilar membrane regions and cell types. When analyzing the immuno- (cf. Fig. 1a). This cell type has long been known to also reactivity of the a1-subunit of sGC (Fig. 3a), significant express NOS III [10]. Both sGC subunits were also differences were found between region 1 (scala media / localized in the inner and outer hair cells, in the pillar cells background) and region 3 (P,0.001) and regions 4, 5, 6, 7 and in cytoskeletal-containing regions of those Deiters and 8 (P,0.0004). There was no significant difference cells that form the lamina reticularis. Quantification of the between region 1 (scala media / background) and region 9 gold particles coupled to the anti-sGC antibodies revealed (P50.54) and only a small difference between regions 1 marked immunoreactivity in the inner and – more promi-and 2 (P50.08). nently – in the outer hair cells. In the pillar cells and in the A similar labeling profile was found for the immuno- cytoskeletal-containing regions of the Deiters cells the reactivity of the b1-subunit of sGC (Fig. 3b). Significant amount of the gold particles was less, but significantly differences were seen between region 1 (scala media / above background, whereas the modest immunreactivity background) and region 3 (P,0.002) as well as regions 4, identified in the scala media, in the acellular tectorial 5, 6, 7 and 8 (P,0.0004). No significant differences were membrane and basilar membrane represents nonspecific found between region 1 (scala media / background) and background labeling. Virtually the same cellular labeling regions 2 or 9 (which all represent nonspecific background profile has been obtained previously with an anti-NOS I labeling, P50.3 and P50.4, respectively). antibody [15] and an anti-NOS III antibody [16]. Using quantitative RT-PCR, Seebacher et al. demonstrated re-cently [22] that there was a nonstoichiometric expression
4. Discussion of theaandbsubunits in the rat inner ear. In the organ of
Corti, the a1- andb1-subunits were the commonly found In outer hair cells, calcium ions participate in multiple isoforms. The identified a2-subunit may potentially com-signalling pathways located in different regions of the cell. plement the poora1 expression compared tob1, whereas
21
When analyzing the possible Ca sources in outer hair the barely detectable level of the b2-subunit transcripts cells, it has been shown that focal application of extracellu- suggests that it is functionally not important or only lar ATP to the hair bundles of the outer hair cells lead to a expressed within a few cell types [22]. In a previous study,
21
two-component increase in [Ca ] . After an initial entryi Fessenden and Schacht [7] utilized an antibody to cGMP 21
of Ca through the apical membrane, a second and larger, to monitor sGC activity in the inner ear. They found 21
inositol triphosphate (InsP )-gated3 [Ca ]i increase significant NO-stimulated cGMP-immunoreactivity in occurred at the base of the hair bundle [20]. Recently, we Hensen’s and Deiters cells and in vascular pericytes, but proposed a model suggesting that the high content of little or no cGMP in hair cells. This seems to be at
21
loosely bound Ca in the nearby tectorial membrane may variance with our current finding of sGC-immunoreactivity be used for regulation of the ion channels located in the in this cell type. However, cGMP can be subject to rapid hair bundles [17]. In addition, four regulatory mechanisms degradation by phosphodiesterases. These can be activated
21
seem to determine the concentration of free Ca in the during tissue preparation and sectioning even before the 21
hair bundle region. Ca may be cleared from the bundle tissue is exposed to a phosphodiesterase inhibitor [4,7]. 21
gold-la-Fig. 3. Density of the gold-labeled antibodies (mean values with 5% confidence intervals) against thea1-subunit (panel a) andb1-subunit (panel b) in nine selected regions of the second turn of the cochlea (panel c). Region 15scala media, region 25tectorial membrane, region 35cuticular plates of the pillar cells, region 45inner hair cell, region 55outer hair cell of the first row, region 65outer hair cell of the second row, region 75outer hair cell of the third row, region 85Deiters cell, region 95basilar membrane. The dotted lines represent the threshold for specific labeling commonly defined as 1 gold
2
belled anti-sGC antibodies, the electron microscopic analy- References
ses revealed that the immunoreactions were not
homoge-[1] P.B. Brechtesbauer, A.L. Nuttall, J.M. Miller, Basal nitric oxide
neously distributed within the outer hair cells. The gold
production in regulation of cochlear blood flow, Hear. Res. 77
particles were located mostly at the apical, basal and lateral
(1994) 38–42.
rims of the cuticular plates. This localization was similar to [2] C. Chen, A. Nenov, R. Skellett, M. Fallon, L. Bright, C.H. Norris, NOS I [15] and NOS III [16]. Also similar to NOS I and R.P. Bobbin, Nitroprusside suppresses cochlear potentials and outer NOS III, significant sGC immunoreactivity was found hair cell responses, Hear. Res. 87 (1995) 1–8.
[3] C.G.D. Dais, J. Prazma, S.S. Ball, C. Zdanski, V. Carrasco, H.C.
associated with the cell nuclei, in the apical electron-dense
Pillsbury, Effect of sodium nitroprusside on compound action
cytoskeletal elements and in the microtubuli bundles of the
potential thresholds in the gerbil cochlea, Hear. Res. 99 (1996) 1–6.
apical Deiters cells in the lamina reticularis. Thus NOS and [4] J. De Vente, D.A. Hopkins, M. Markerink-van Ittersum, P.C. Emson, sGC immunoreactivities are not only located in the same H.H.H.W. Schmidt, H.W.M. Steinbusch, Distribution of nitric oxide synthase and nitric oxide-receptive, cyclic GMP-producing
struc-cells types, but also in the same subcellular regions within
tures in the rat brain, Neurosci. 87 (1998) 207–241.
these cells. This strongly suggests the existance of an
[5] D. Dulon, G. Zajic, J. Schacht, Increasing intracellular free calcium
autocrine NO / cGMP pathway in the guinea pig organ of induces circumferential contractions in isolated cochlear outer hair Corti. cells, J. Neurosci. 10 (1990) 1388–1397.
The functional significance of this pathway remains to [6] J.D. Fessenden, R.A. Altschuler, A.F. Seasholtz, J. Schacht, Nitric oxide / cyclic guanosine monophosphate pathway in the peripheral
be elucidated. It is assumed that cGMP is involved in the
21 and central auditory system of the rat, J. Comp. Neurol. 404 (1999)
regulation of intracellular Ca concentration in cells of
52–63.
the inner ear [12]. In a common model of hair cell [7] J.D. Fessenden, J. Schacht, Localization of soluble guanylate cyclase 21
function, Ca cycles in and out of the cell. The level of activity in the guinea pig cochlea suggests involvement in regulation
21
intracellular free Ca can be regulated by different of blood flow and supporting cell physiology, J. Histochem. Cytochem. 45 (1997) 1401–1408.
processes. At the apical cell side of the outer hair cells, the
21 [8] J.D. Fessenden, J. Schacht, The nitric / cyclic GMP pathway: a
intracellular free Ca content might be controlled by the
potential major regulator of cochlear physiology, Hear. Res. 118
hair bundles that are in contact with the tectorial mem- (1998) 168–176.
brane. This acellular structure has a high content of loosely [9] U. Forstermann, H. Kleinert, Nitric oxide synthase: expression and¨
21
bound Ca which may enter the outer hair cells [17]. An expressional control of the three isoforms, Naunyn-Schmiedebergs
21 Arch. Pharmacol. 352 (1995) 351–364.
increase in intracellular free Ca leads to a contraction of
¨
[10] U. Forstermann, J.S. Pollock, H.H.H. Schmidt, M. Heller, F. Murad,
the cells thereby altering the stiffness of the plasma
Calmodulin-dependent endothelium-derived relaxing factor / nitric
membrane and the turgor of the cells [5]. In addition, the oxide synthase activity is present in the particulate and cytosolic 21
increased intracellular Ca could activate the calmodulin- fractions of bovine aortic endothelial cells, Proc. Natl. Acad. Sci.
regulated constitutive NOS isoform(s) identified in these (USA) 88 (1991) 1788–1792. ¨
[11] P. Franz, C. Hauser-Kronberger, P. Bock, C. Quint, W.D.
Baumgar-cells [15,16]. Subsequently, sGC would be stimulated and
21 tner, Localization of nitric oxide synthase I and III in the cochlea,
the cGMP formed might be involved in the Ca handling
Acta. Otolaryngol. 116 (1996) 726–731.
of the outer hair cells. Interference with the intracellular [12] K. Gosepath, I. Gath, J. Maurer, J.S. Pollock, R. Amedee, U. 21
Ca homeostasis could change both the mechanical Forstermann, W. Mann, Characterization of NO synthase isoforms¨
properties of the outer hair cells, as well as modulate expressed in different structures of the guinea pig cochlea, Brain Res. 747 (1996) 26–33.
neurotransmitter release at the synapse of both hair cells
[13] P.S. Guth, M. Stockwell, Guanylate cyclase and cyclic guanosine
types.
monophosphate in the guinea-pig cochlea, J. Neurochem. 28 (1977)
In conclusion, the current study demonstrates the pres- 263–265.
ence of significant sGC immunoreactivity in the outer hair [14] F. Guthmann, B. Mayer, D. Koesling, W.R. Kukovetz, E. Bohme,¨
cells. Immunoreactivity in other cell types such as the Characterization of soluble platelet guanylyl cyclase with peptide antibodies, Naunyn-Schmiedebergs Arch. Pharmacol. 346 (1992)
inner hair cells, the pillar cells and the
cytoskeletal-con-537–541.
taining Deiters cells was less, but still above background.
[15] U.-R. Heinrich, J. Maurer, K. Gosepath, W. Mann, Electron
micro-Together with the recently identified NOS isoforms in the scopic localization of nitric oxide I synthase in the organ of Corti of same cell types, the sGC may form an autocrine signalling the guinea pig, Eur. Arch. Otorhinolaryngol. 254 (1997) 396–400.
system in the organ of Corti. [16] U.-R. Heinrich, J. Maurer, K. Gosepath, W. Mann, Electron micro-scopic localization of nitric oxide synthase III in the guinea pig organ of Corti, Eur. Arch. Otorhinolaryngol. 255 (1998) 483–490.
21
[17] U.-R. Heinrich, J. Maurer, W. Mann, Possible Ca -dependent
Acknowledgements mechanism of apical outer hair cell modulation within the cochlea of
the guinea pig, Cell Tiss. Res. 292 (1998) 57–65. ¨
This study was supported by Grant Ma 1343 / 2-3 from [18] D. Koesling, E. Bohme, G. Schultz, Guanyly cyclase, a growing family of signal-transducing enzymes, FASEB 5 (1991) 2785–2791.
the Deutsche Forschungsgemeinschaft (DFG) and by the 21
[19] E.A. Lumpkin, A.J. Hudspeth, Regulation of free Ca
concen-Collaborative Research Centers SFB 428 (Project A2) and
tration in hair-cell stereocilia, J. Neurosci. 18 (1998) 6300–6318.
SFB 553 (Project A1). The authors wish to thank Mrs. K. [20] F. Mammano, G.I. Frolenkov, L. Lagostena, I.A. Belyantseva, M.
21
Backes and Mrs. E. Schill-Wendt for their invaluable Kurc, V. Dodane, A. Colavita, B. Kachar, ATP-induced Ca release
triphosphate-21
gated Ca store to the base of the sensory hair bundle, J. Neurosci. J.E. Schultz, Expression of membrane-bound and cytosolic guanylyl 19 (1999) 6918–6929. cyclases in the rat inner ear, Hear. Res. 127 (1999) 95–102. [21] H.H.H.W. Schmidt, S.M. Lohmann, U. Malter, The nitric oxide and [23] R. Thalmann, S. Paloheimo, I. Thalmann, Distribution of cyclic
cGMP signal transduction system: regulation and mechanism of nucleotides in the organ of Corti, Acta Otolaryngol. 87 (1979) action, Biochem. Biophys. Acta 1178 (1993) 153–175. 375–380.