Section B provides a description of a simple and practical synthetic protocol for the thioacetalization of carbonyl compounds using acetyl chloride under solvent-free conditions. The potential of the present method is that various dithioacetals can be obtained on a large scale from the corresponding carbonyl compounds using bromodimethylsulfonium. Chapter II, Part I provides a brief overview on the resolution of various dithioacetals into the corresponding carbonyl compounds using various reagents and their drawbacks.
Section A tells about a suitable and efficient method for the cleavage of dithioacetals to the corresponding carbonyl compounds using organic ammonium tribromides (OATB), namely by using cetyltrimethylammonium tribromide (CetTMATB) and tetrabutylammonium tribromide (TBATB) as new reagents. PART B Simple and practical synthetic protocol for thioacetalization of carbonyl compounds using acetyl chloride under solvent-free conditions. PART C New synthetic methodology for thioacetalization of the carbonyl compounds using bromodimethylsulfonium bromide (Me2S+BrBr-) as a pre-catalyst.
SECTION A A convenient and efficient method for the resolution of dithioacetals into the corresponding carbonyl compounds using organic ammonium tribromides (OATB) as novel reagents.
GENERAL REMARKS
Introduction
This method has been widely used for the preparation of dithioacetals from the corresponding carbonyl compounds. Ong and Chan have reported the dithioacetalization of carbonyl compounds using Me3SiCl as catalyst.25 Later, Evans et al. Further, Ong demonstrated27 a new method for the thioacetalization of carbonyl compounds using AlCl3 as an efficient catalyst.
Garlaschelli and Vidari developed30 the thioacetalization of carbonyl compounds using the anhydrous catalyst LaCl3as shown in Scheme 17. Later, Patney also reported46 the thioacetalization of carbonyl compounds using CoBr2-impregnated silica gel as a catalyst. The catalytic converter is very expensive, which is one of the disadvantages. have shown55 that molybdenyl acetylacetonate mediates the thioacetalization of carbonyl compounds.
This method has shown for the first time that metal acetylacetonate can be used for the thioacetalization of carbonyl compounds.
SECTION A)
Results and Discussion
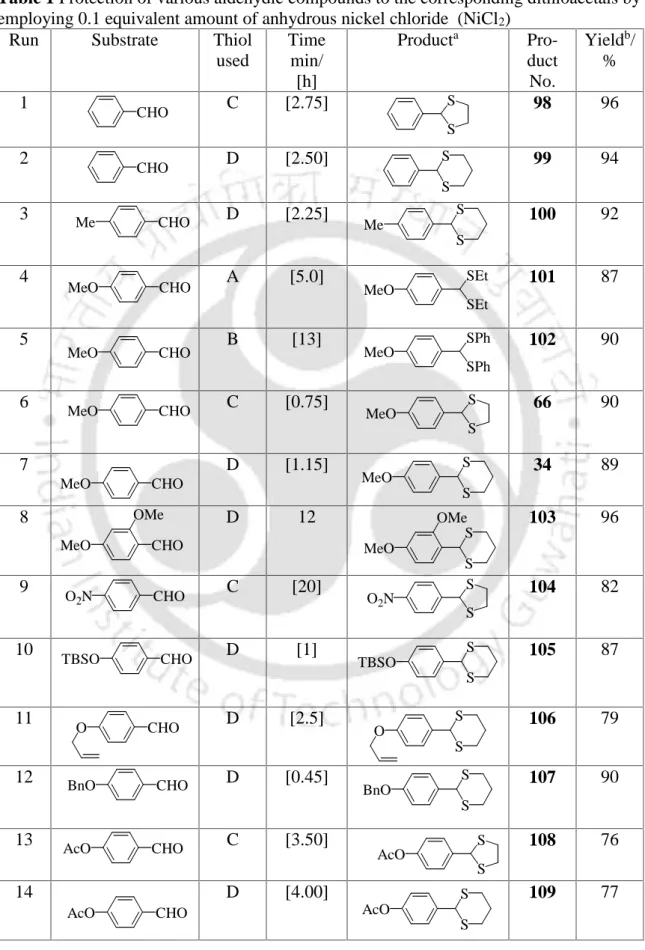
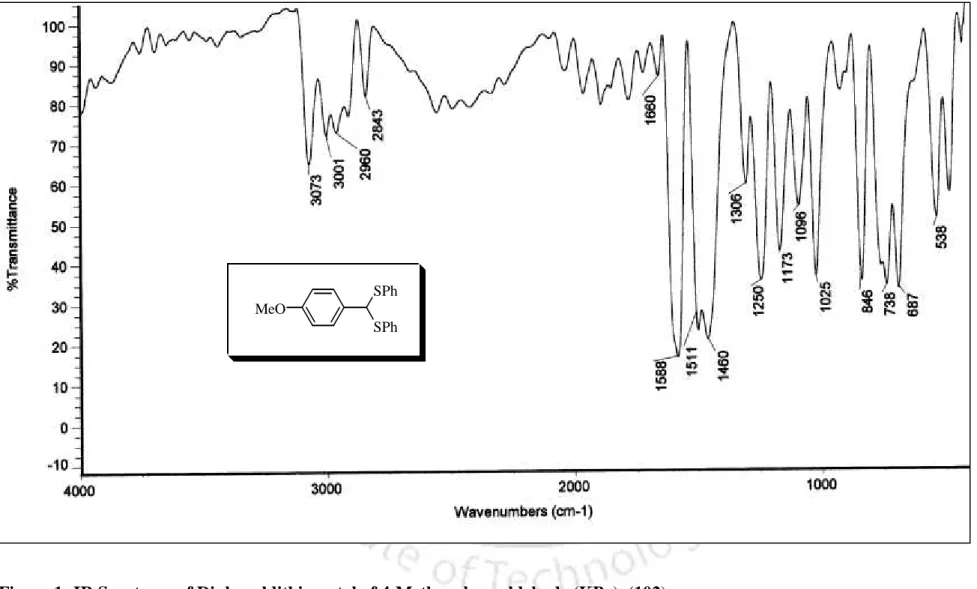
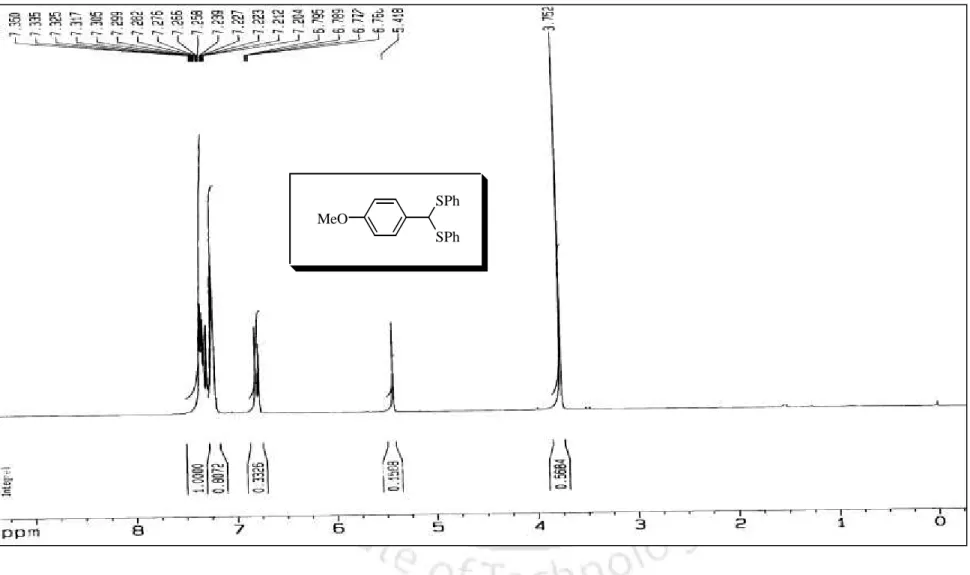
The disappearance of the carbonyl frequency in the IR spectrum clearly indicates the formation of the benzaldehyde dithioacetal. The diphenyl dithioacetal product of 4-methoxybenzaldehyde (102) was also confirmed by recording IR, 1H NMR and 13C NMR spectra as shown in Figures 1–3, respectively. The results presented in Table 1 clearly show the scope and generality of the reaction with respect to various aromatic, aliphatic and alkenyl aldehydes.
We have also found in the literature69 that the reaction of 1,2-ethanedithiol and nickel(II) chloride gives the polymeric compound [Ni(es)]n and hydrochloric acid. We have observed that the reaction of cyclohexanone and 1,2-ethanedithiol in the presence of catalytic amount of nickel(II) chloride is very slow under the same conditions and the reaction does not complete even after a prolonged reaction time. In addition, an aldehyde group can be chemically protected in the presence of a keto group.
After addition of the catalyst, the reaction mixture turned black in the case of 1,2-ethanedithiol or dark brown for 1,3-propanedithiol.
SECTION B)
The methodology for the thioacetalization of aldehydes that we developed using nickel(II) chloride as a catalyst had some drawbacks, such as the difficulty of preparing dithioketals from the corresponding ketones under the same reaction conditions. Recently, we have been working on the development of new synthetic methodologies for the deprotection of carbonyl compounds as dithioacetals.71 For our needs, we had to prepare various dithioacetals from the corresponding carbonyl compounds as our key starting materials. It is well known from the literature that acetyl chloride reacts with methanol to form dry hydrochloric acid,72 which can be used as a catalyst for the thioacetalization of carbonyl compounds.
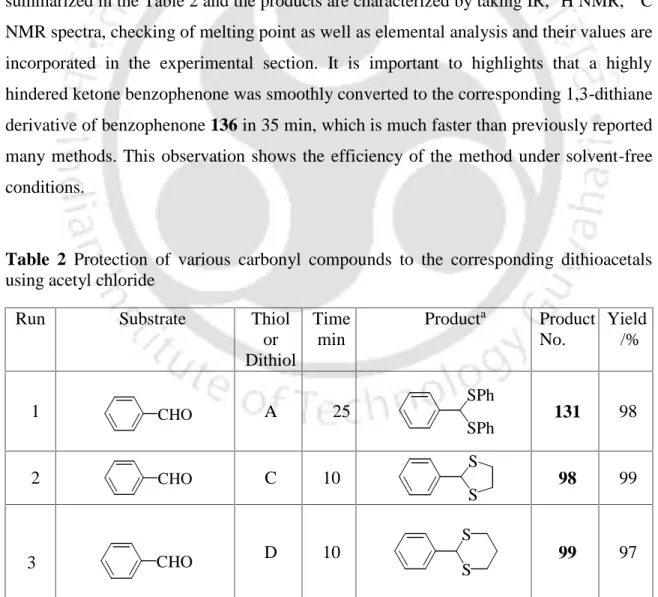
In this chapter we would like to discuss a very mild, very efficient and chemoselective synthetic protocol for the transformation of the carbonyl compounds into the corresponding dithioacetals or dithioketals, involving a catalytic amount of acetyl chloride, as shown in Scheme 37. We then attempted to optimize. conditions for the protection of a wide variety of carbonyl compounds to the corresponding dithioacetals. The new signal appears at 5.43 and the disappearance of the aldehyde signal at 9.80 clearly indicates that the aldehyde group has been damaged.
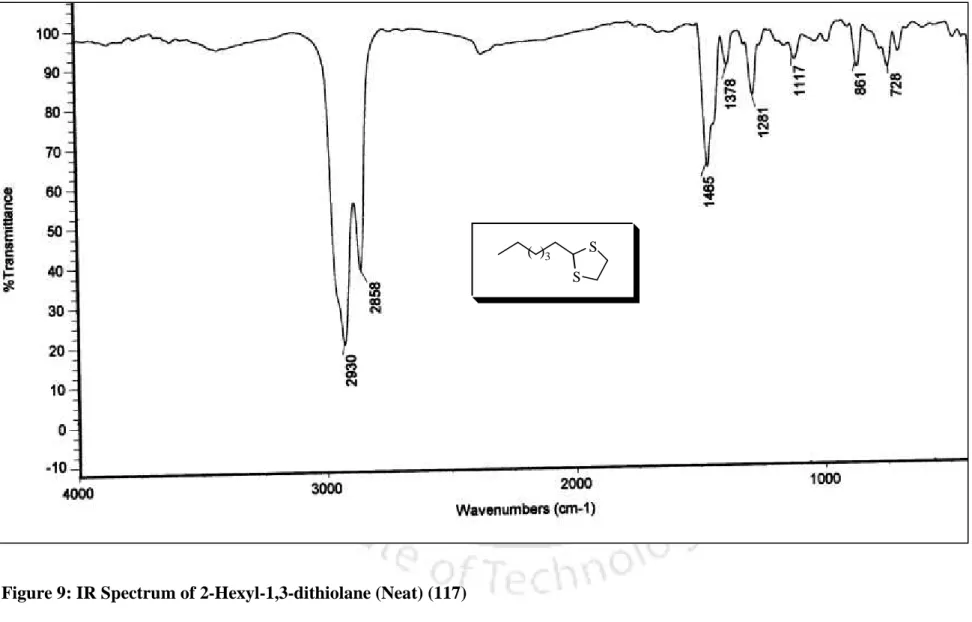
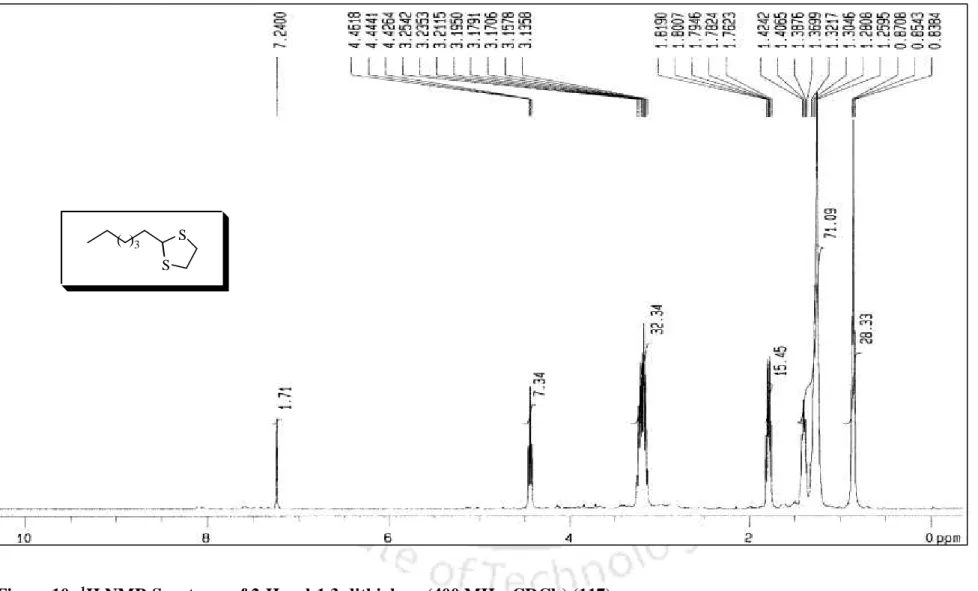
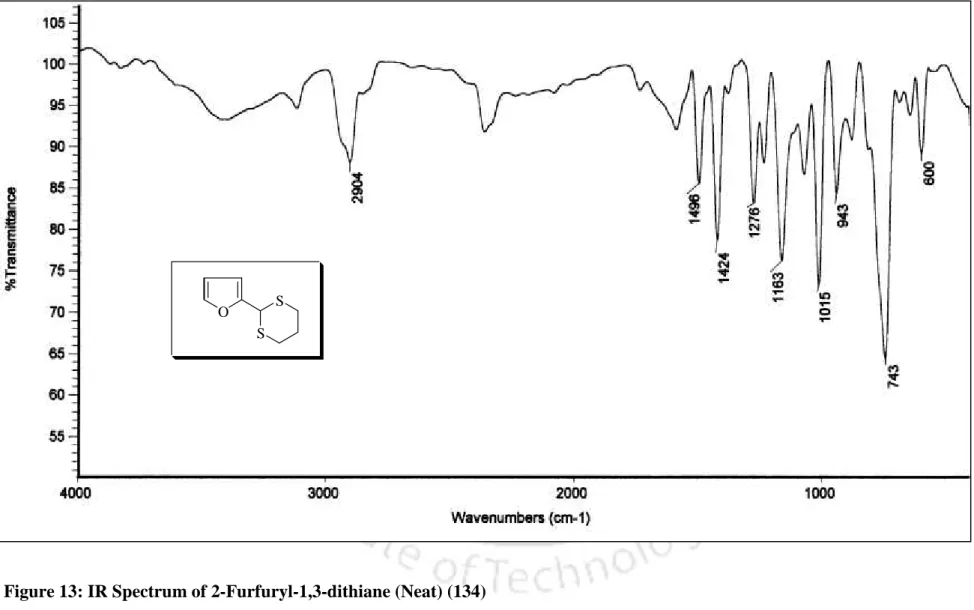
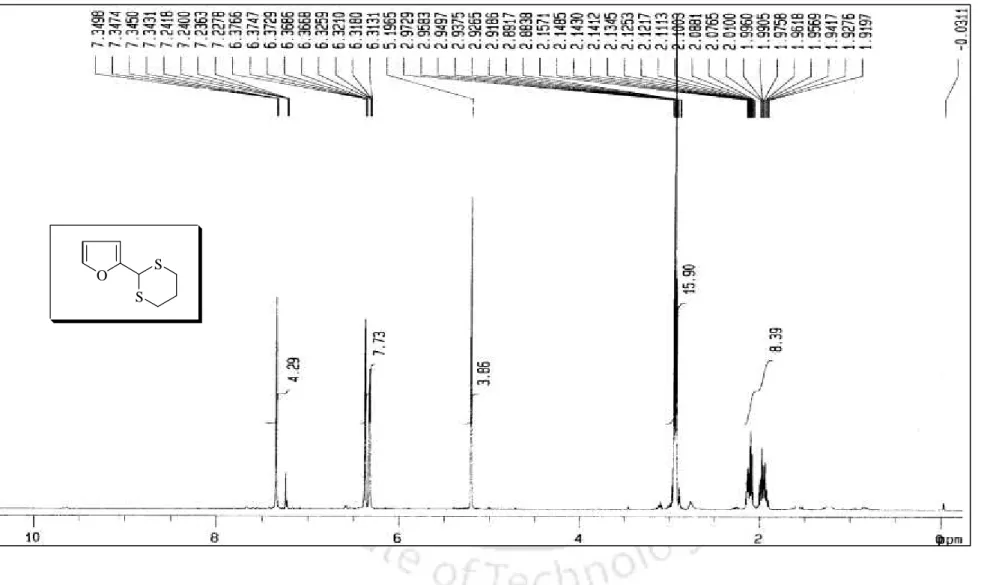
In addition, the melting point of the dithioacetal derivative was 131 56 C, which is comparable to the literature melting point [lit.37 m.p. The conversion of benzaldehyde to the corresponding 1,3-dithiolane derivative 98 was accomplished within 10 min. completed, which is much faster compared to the earlier reported procedure.40 In addition, 1,3-dithiolane derivative of benzaldehyde (98) was obtained. by distillation instead of troublesome chromatographic separation, while 1,3-dithiane from benzaldehyde (99) was obtained directly by recrystallization. More interestingly, highly acid sensitive substrates such as 2-furaldehyde and ester aldehyde (run 20 and 21) were also converted to the corresponding ones.
Next, our aim was to prepare dithioketals of the corresponding ketones by following the similar reaction conditions, which were unsuccessful by our earlier method. We also observed that dithioketals could be prepared from the corresponding carbonyl compounds in good yields in reasonable time (run 23-29) as shown in Table 2. It is important to emphasize that a highly hindered ketone benzophenone was smoothly converted to the corresponding 1,3-dithiane derivative of benzophenone 136 in 35 min, which is much faster than previously reported many methods.
Due to its operational simplicity, generality and efficiency, this method is expected to have much wider applicability for the conversion of various carbonyl compounds to the corresponding dithioacetals. After completion of the reaction, the product was obtained by direct recrystallization or by distillation under reduced pressure.
SECTION C)
Napthaldehyde (162)
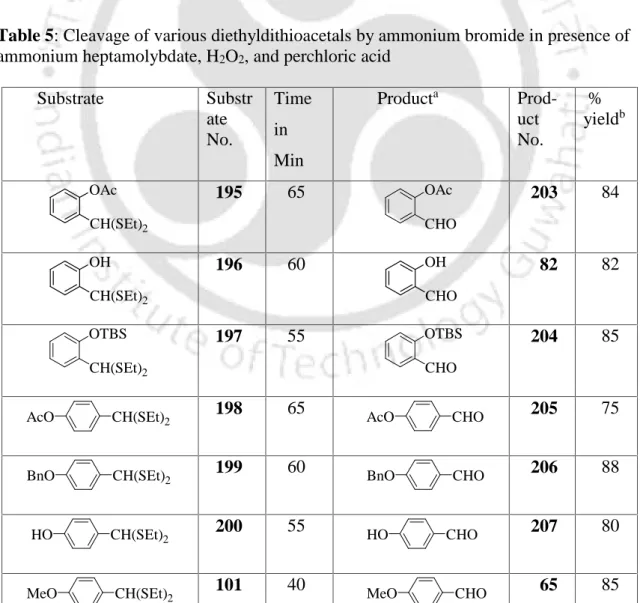
NEW SYNTHETIC PROTOCOL FOR HYDROLYSIS OF DITHIOACETALS USING OXIDATION OF AMMONIUM BROMIDE PROMOTED BY AMMONIUM HEPTAMOLYDATE AND HYDROGEN PEROXIDE. However, this method has several disadvantages such as i) they need to be prepared before use, although they are quite stable for a long time, ii) they are relatively expensive and iii) require a large amount of reagent by weight for deprotection per mmol reagent. the dithioacetals or dithioketals due to the high molecular weight of the organic ammonium tribromides. The characterization data of the hydrolyzed product are given in the experimental section, except those mentioned in the previous chapter.
The formation of the product can be explained by ammonium heptamolybdate reacting with H2O2 in the presence of a catalytic amount of perchloric acid to give diperoxomolybdate (VI), which oxidizes bromine (Br-) to Br+ (which can also exist as Br2 or Br3 -). NEW SYNTHETIC PROTOCOL FOR THE HYDROLYSIS OF DITHIOACETALS USING AMMONIUM BROMIDE OXIDATION PROMOTED BY AMMONIUM HEPTAMOLIBDATE AND HYDROGEN PEROXIDE. Some of the substrates and their corresponding carbonyl compound data are mentioned in the previous part A in the experimental section.
NEW SYNTHETIC PROTOCOL FOR DETHIOACETALIZATION INVOLVING VANADIUM PENTOXIDE-HYDROGEN PEROXIDE CATALYZED AMMONIUM BROMIDE OXIDATION. Although some of the shortcomings of the organic ammonium tribromide method were overcome by the molybdenum methodology, there are still some shortcomings, which give us further opportunities to find better methodologies. Taking data from the knowledge of the activity of vanadium bromoperoxidase (VBrPO),117 which catalyzes the bromination of marine natural products as well as our previous results114, we wanted to investigate whether vanadium peroxo complexes oxidize the bromine ion to the bromine ion it can be used. for defense or not.
Some of the analysis data of the starting dithioacetals are given in the experimental section. Next, we attempted to optimize the reaction conditions for deprotection of dithioacetals and ketals to obtain the desired carbonyl compounds. Some of the dithioacetals such as en 214 were prepared by following boron trifluoride etherate procedure as described in previous section A.
Some of the substrates and their corresponding carbonyl compound data are mentioned in the earlier section A and section B in the experimental part. The color of the solution was changed to bright dark brown-red after 25 minutes and ammonium bromide (0.1 g, 1.0 mmol) was added.
SECTION D)
In Chapter II of Part II in Section A, Section B and Section C, we showed that bromine ions can be used for dethioacetalization reactions. We have also developed for the first time a catalytic synthetic protocol for deprotecting dithioacetals involving bromonium ions by employing peroxovanadium oxidation of bromide ions to the bromonium ion. Although this procedure is quite effective compared to the other methods, we were still interested if another method could be devised using a combination of other reagents.
The usual standard procedure for cleaving dithioacetals is primarily involving a suitable electrophile, which is captured by the nucleophilic soft sulfur atom followed by hydrolysis with water. Next, we understand whether electrophilic species such as NO+ can be used for the regeneration of carbonyl compounds from the corresponding protected compounds or not as we are mainly working to develop a new synthetic methodology. After going through the literature, we noticed that sodium nitrite in combination with trifluoroacetic acid,103 isoamyl nitrite118 and a mixture of nitrogen oxides119 have been used for the deprotection of dithioacetals to carbonyl compounds.
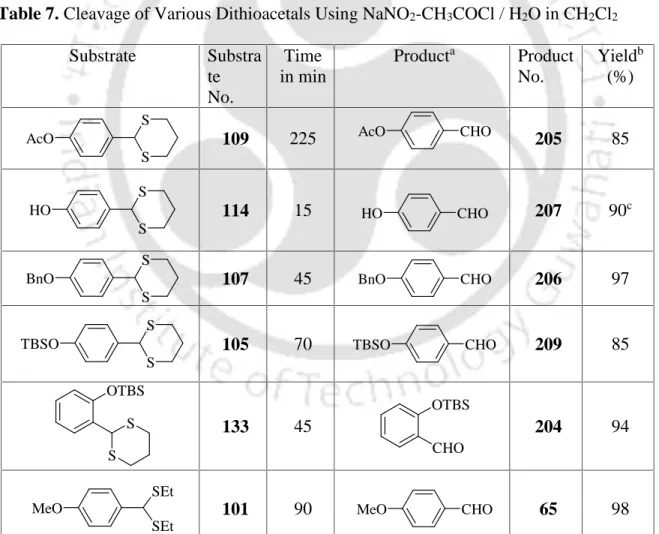
However, these procedures have some disadvantages, such as incompatibility with other protecting groups such as TBS ethers due to the large excess of trifluoroacetic acid, drastic reaction conditions and longer reaction times,118 and offer a low yield for enolizable ketone.119In this chapter we would like to discuss that sodium nitrite in combination with acetyl chloride is a useful reagent system for the cleavage of dithioacetals under mild reaction conditions as shown in Scheme 70. When compound 109 was treated with 2 equivalent amounts of NaNO2-CH3COCl (1:1) mixture at a temperature of 0-5oC it became was converted to the product 205 without difficulty and in good yields. Similarly, compound 111 was converted to compound 207 in good yields by following an identical procedure without acetylation of the hydroxyl group.
The starting ditoacteals 133 and their hydrolyzed products 204 were characterized by IR,1H NMR recording as shown in Figures 39-41. The formation of the parent carbonyl compounds from their corresponding dithiacetals can be rationalized as follows. In conclusion, we have demonstrated a mild and simple procedure for the deprotection of dithioacetals to the parent carbonyl compounds using a mixture of sodium nitrite and acetyl chloride, which is a useful addition to existing procedures.
A NEW SYNTHETIC PROCEDURE FOR THE REGENERATION OF CARBONYL COMPOUNDS FROM THEIR CORRESPONDING DITHIOACETALS USING A COMBINATION OF SODIUM NITRITE AND ACETYL CHLORIDE. Characterization data for all dithioacetals and their corresponding carbonyl compounds are mentioned in the previous Chapter I and Chapter II in the experimental section, except for the dithioacetal derivative 221 and its corresponding aldehyde 222.
![Figure 4: IR Spectrum of 2-[4-tert-Butyldimethylsilyloxyphenyl]-1,3-dithiane (KBr) (105)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10455715.0/105.1262.111.1083.141.726/figure-ir-spectrum-tert-butyldimethylsilyloxyphenyl-dithiane-kbr-105.webp)
![Figure 5: 1 H NMR Spectrum of 2-[4-tert-Butyldimethylsilyloxyphenyl]-1,3-dithiane (400 MHz, CDCl 3 ) (105)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10455715.0/106.1262.110.1087.140.705/figure-nmr-spectrum-tert-butyldimethylsilyloxyphenyl-dithiane-mhz-cdcl.webp)
![Figure 6: 13 C NMR Spectrum of 2-[4-tert-Butyldimethylsilyloxyphenyl]-1,3-dithiane (100 MHz, CDCl 3 ) (105)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10455715.0/107.1262.116.1087.142.703/figure-nmr-spectrum-tert-butyldimethylsilyloxyphenyl-dithiane-mhz-cdcl.webp)
![Figure 8: 13 C NMR Spectrum of 2-[4-Hydroxyphenyl]-1,3-dithiane (100 MHz, CDCl 3 ) (114)](https://thumb-ap.123doks.com/thumbv2/azpdfnet/10455715.0/109.1262.110.1088.130.715/figure-nmr-spectrum-hydroxyphenyl-dithiane-100-mhz-cdcl.webp)