Each of the Plasmodium copper transporter protein sequences was found to contain features common to the well-characterized copper transporters. 12 weeks..102 Figure 4.6 Affinity purification profiles for anti-CSD, -PMY and -LQD antibodies..103 Figure 4.7 Anti-peptide antibody detection of lactate dehydrogenase and the putative copper transport protein in P.
Introduction
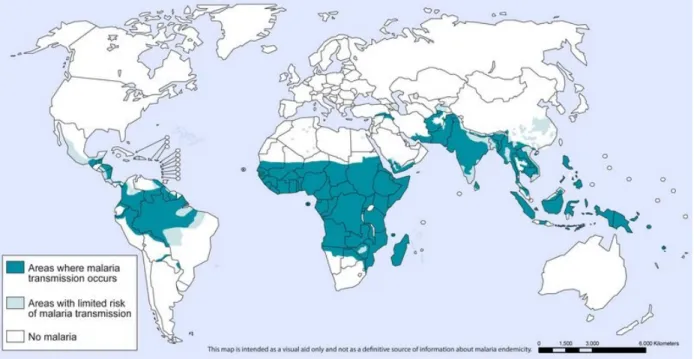
Overview of malaria
- Geographical distribution of malaria
- A brief history of malaria
- The Plasmodium parasite life cycle
- Treatment of malaria
- Plasmodium genomics and antimalarial drug discovery
This area was significantly reduced by the introduction of dichlorodiphenyltrichloroethane (DDT) (Maharaj et al., 2005). In fact, no new antimalarials have been introduced into clinical practice since 1996 (Gamo et al., 2010).
Biological and physiological importance of copper
- Acquisition of copper and the copper transport protein
- Structural requirements for copper uptake
This orientation was strongly supported by a tryptophan scan of human Ctr1 and yeast Ctr3 ( De Feo et al., 2010 ). In addition, it was suggested that the transport mechanism involves a conformational change of the protein during transport (De Feo et al., 2009).
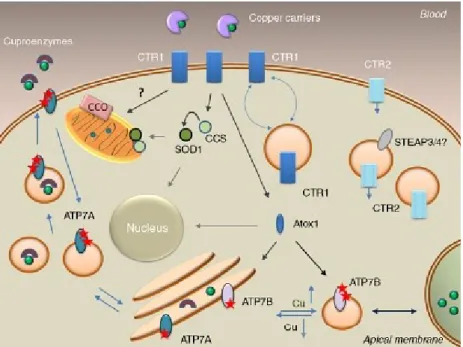
Intracellular copper trafficking
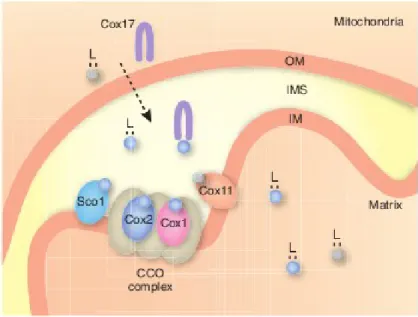
- Copper trafficking to the mitochondrion
- Structural features of Cox17
This therefore suggested that the function of Cox17 may be spatially restricted to the intermembrane space (Maxfield et al., 2004). This is achieved by the TOM complex through a Mia40/Erv1 disulfide-relief process ( Mesecke et al., 2005 ).
Outline of study
Apart from the identification of a parasite P-type ATPase efflux protein (Rasoloson et al., 2004), there are limited data on the acquisition and distribution of copper by the parasite. Significant amounts of purified protein were required to determine the copper-binding capacity of the parasitic copper transporter and metallochaperone.
Materials and methods
Introduction
Materials
Peroxidase-conjugated rabbit anti-IgY, peroxidase-conjugated rabbit anti-mouse IgG, and peroxidase-conjugated goat anti-rabbit IgG antibodies were from Jackson Immunochemicals (Pennsylvania, USA). Synthetic peptides for all three copper transporters were synthesized by Auspep (Melbourne, Australia), while those for the two Cox17 metallochaperones were synthesized by GL Biochem (Shanghai, China).
Bioinformatics methods
- Sequence identification and analysis
- Homology modelling
- Phylogenetic tree construction
- Predict7™ antigenic peptide prediction
The included characterized sequences were for the copper transporters Homo sapiens (accession number NP_001850), Arabidopsis thaliana (GenBank: BAE98928) and Saccharomyces cerevisiae (GenBank: AAB68064). As with the copper transport proteins, the presence of potential signal sequences in each protein was determined using the TMHMM server and the SignalP 3.0 server (Center for Biological Sequence Analysis, Lyngby, Denmark).
Biochemical and immunochemical techniques
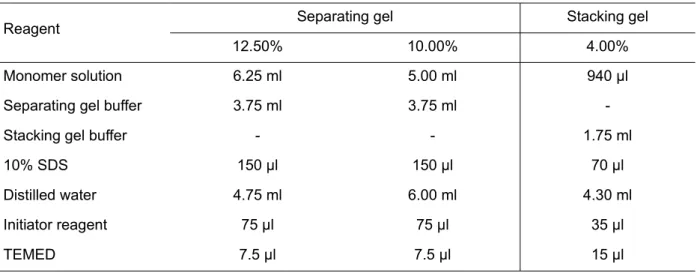
- Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- Materials
- Method
- Tricine-SDS-PAGE
- Materials
- Method
- Western blot
- Materials
- Method
- Bradford standard protein assay
- Materials
- Method
- Coupling synthetic peptides to rabbit albumin carrier for antibody production
- Materials
- Method
- Preparation of immunogen for immunisation in chickens
- Method
- Isolation of immunoglobulin Y (IgY) from chicken egg yolk
- Materials
- Method
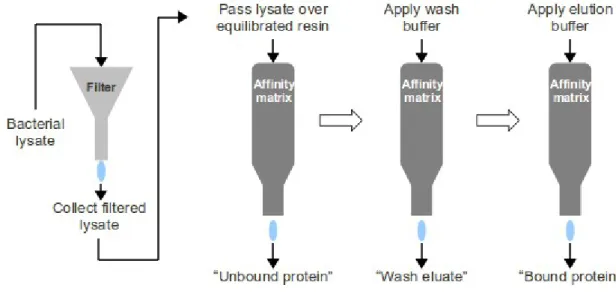
- Preparation of peptide-specific affinity matrices and purification of anti-peptide
- Materials
- Method
- Enzyme linked immunosorbent assay for anti-peptide IgY titre
- Materials
- Method
The solution was centrifuged (12000 g, 10 min, RT) to pellet the precipitate, with the resulting supernatant discarded. The reduced peptide solution was added to the equilibrated resin and the column was capped to seal the inlet and outlet.
Parasitological techniques
- Propagation of P. yoelii and P. berghei parasites in BALB/c mice and
- Materials
- Method
- Isolation of P. yoelii and P. berghei parasites from infected mouse red blood
- Materials
- Method
- Immunofluorescence microscopy
- Materials
- Method
A small volume of the cell suspension was added to each well (~20 µl) and the cells were allowed to settle for 30 min at room temperature. The secondary antibody solution was aspirated under vacuum and the wells were washed three times with PBS (in the dark).
Molecular biology methods
- Agarose gel electrophoresis
- Materials
- Method
- Isolation of P. berghei total RNA
- Materials
- Method
- Isolation of P. yoelii and P. falciparum genomic DNA
- Primers for PCR-based amplification of PBANKA_130290, PY03823, PF10_0252,
- Polymerase chain reaction (PCR)
- Reverse transcriptase polymerase chain reaction for the analysis of
- Purification of DNA from agarose gel
- Ligation of purified cDNA fragments into a pGEM ® -T cloning vector
- cDNA ligation using T4 DNA ligase
- Materials
- Method
- Isolation of plasmid DNA
- Materials
- Method
- Expression plasmids used in the study
- Restriction endonuclease digestion of plasmids
- Materials
- Method
- E. coli hosts used for cloning and expression of recombinant proteins
- Transformation and culturing of E. coli transformants
- Materials
- Method
- Antibiotics used for positive selection and maintenance of E. coli transformants. .62
- Materials
- Method
- Purification of recombinant malaria parasite proteins
- Materials
- Method
- Fractionation of MBP-PbCtrNt by molecular exclusion chromatography
- Materials
- Method
- Copper binding studies and the bicinchoninic acid assay
- Materials
- Method
Primers were engineered with the appropriate restriction sites used for cloning and expression of the desired PCR products. The amplicon should be used for cloning and recombinant expression of the putative amino-terminal domain. For preparation of expression vectors, shrimp alkaline phosphatase (SAP) (1 µl) was added to the digestion mixture and incubated immediately (37°C, 30 min).
The final volume of the solution was adjusted to 50 ml with sterile (autoclaved) double distilled water.
Bioinformatic studies and antigenic peptide selection for the putative
Introduction
Flexibility is of particular importance because this property increases the likelihood that the selected peptide will adopt a spatial orientation similar to that found in the native protein. A BLASTp search of PlasmoDB, for a putative copper transport protein, identified coding domains for two potential transporters in most of the available parasite genomes. Suitable antigenic epitopes were identified in five of the identified parasite proteins using the Predict7TM epitope prediction algorithm (Cármenes et al., 1989).
For each of the copper transport proteins, peptide sequences were selected from the extracellular amino-terminal domain.
Results
- Putative copper requiring proteins of P. falciparum
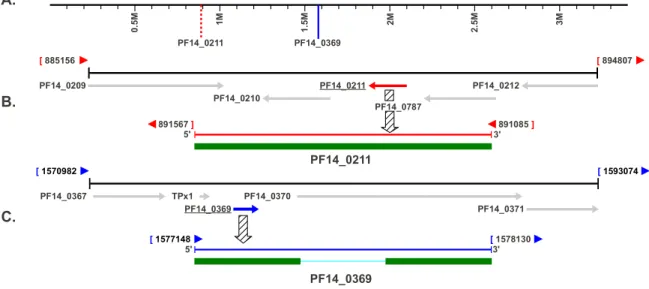
- Identification of a putative Plasmodium spp. copper transport protein
- Analysis of the coding sequences for two putative P. falciparum copper transport
- Identification of a putative Plasmodium spp. Cox17 copper metallochaperone
- Analysis of the putative P. falciparum Cox17 metallochaperone coding
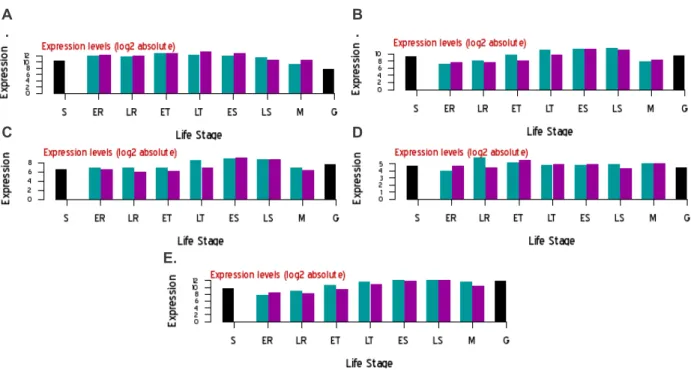
- Transcription levels of a P. falciparum copper transporter and metallochaperone.84
- Selection of immunogenic peptides for antibody production in chickens
Each of the Plasmodium sequences was analyzed using the transmembrane domain prediction servers TMHMM ( Krogh et al., 2001 ) and HMMTOP ( Tusnády and Simon, 2001 ). The identification of a putative copper transport protein sequence, within the Plasmodium proteome, required a preliminary characterization of the protein sequence. Similar evolutionary relationships were previously observed by phylogenetic analysis of cytochrome b and small rRNA subunit sequences (Rathore et al., 2001).
Predict7TM analysis of the copper transporter protein sequences (Figure 3.10) identified the following peptides: CSDKQSGDDECKPILD for the P.
Discussion
- Identification of a putative Plasmodium spp. copper transport protein and
- Chromosomal location of the copper transporter and Cox17 coding domains
- Phylogenetic analysis of the Plasmodium copper transporter and Cox17 amino
- Peptide selection
Given the importance of trimer formation for protein function ( Aller et al., 2004 ), a similar formation is likely to exist for the Plasmodium copper transporters. Each sequence was systematically analyzed by SignalP, PlasmoAP, PlasMit (Bender et al., 2003), TMHMM and HMMTOP. Consequently, the rest of the chromosome is left relatively poorly defined (Gardner et al., 2002; Kooij et al., 2005).
A similar approach has been used to establish the orientation of human Ctr1 (Klomp et al., 2003).
The Plasmodium spp. putative copper transport protein: Native protein
Introduction
However, a drawback to this approach is the finding that heterologous expression of full-length Plasmodium membrane proteins is difficult (Krishna et al., 2001; Martin et al., 2009a; Vedadi et al., 2007). Recombinant expression of the amino-terminal copper-binding domain of the Plasmodium copper transporter was therefore chosen. This extracellular domain is predicted to be important for copper uptake as well as copper access to and from the pore of characterized copper transporters (De Feo et al., 2009; Jiang et al., 2005; Larson et al. , 2010).
However, it has been shown that the parasite requires intracellular copper for survival (Meshnick et al., 1990; Rasoloson et al., 2004).
Results
- Confirmation of a putative Plasmodium copper transporter coding domain
- Immunolocalisation of the murine and human malaria parasite putative copper
- Anti-peptide antibody production
- Detection and localisation of the malaria parasite copper transporters. 103
- Copper binding studies with MBP-PbCtrNt, MBP-PfCtr211Nt TD and
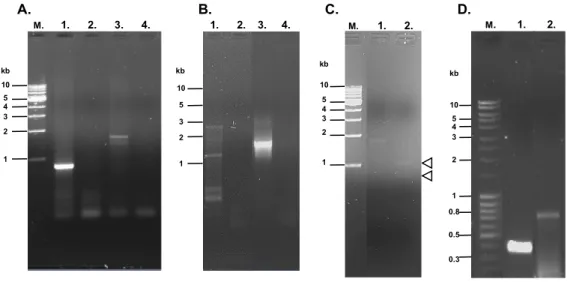
Detection of the native parasite copper transport protein used antibodies raised against the peptides designed in Section 3.2.8 (Figure 3.10). A prominent protein band was detected at approximately 32 kDa (Figure 4.7c), which is in good agreement with the predicted size of the putative transporter. This was supported by the omission of ascorbic acid from the BCA release assay, which still resulted in the formation of a purple BCA-Cu+ complex (Figure 4.20a, +, open row).
Thus, it is very likely that equation 2 is the rate-limiting step of the redox cycle (Figure 4.23).
Discussion
- Confirmation of a Plasmodium spp. copper transport protein coding sequence
- Identification of a native murine malaria parasite copper transport protein
- Recombinant expression of the amino terminus of a putative P. berghei copper
- Recombinant expression of the amino termini of two P. falciparum copper
- Copper binding to MBP-PbCtrNt, MBP-PfCtr211Nt TD and MBP-PfCtr369Nt TD
This result indicates that MBP-PfCtr211NtTD and MBP-PfCtr369NtTD chelate copper in solution, thus preventing its participation in the redox cycle (Figure 4.23). When expressed in the presence of copper, the final yield of MBP-PfCtr211NtTD and MBP-PfCtr369NtTD was significantly increased (Figure 4.22). Since copper is required in the periplasm, efficient regulatory mechanisms are essential (Macomber et al., 2007).
According to the dynamics of the redox cycle (Figure 4.23), this result suggested that each protein coordinates a Cu+ ion.
The Plasmodium spp. putative Cox17 copper metallochaperone
Introduction
However, this idea was challenged by the finding that cytochrome c oxidase deficiency in cox17Δ yeast cells could be functionally complemented by restricting the Cox17 location to the mitochondrial intermembrane space ( Maxfield et al., 2004 ). Subsequent studies identified that CuL was also abundant in the cytosol as a copper-free ligand (Cobine et al., 2006b). Catalytically active cytochrome c oxidase is assembled through numerous steps, requiring more than 30 accessory proteins ( Cobine et al., 2006a ).
The indispensability of Cox17 function is highlighted by embryonic lethality in COX17 null mice ( Takahashi et al., 2002 ).
Results
- Confirmation of a native Plasmodium Cox17 coding domain
- Anti-peptide antibody production for parasite Cox17 protein detection
- Recombinant expression of P. yoelii and P. falciparum Cox17
- Copper binding studies with His 6 -PyCox17 and GST-PfCox17
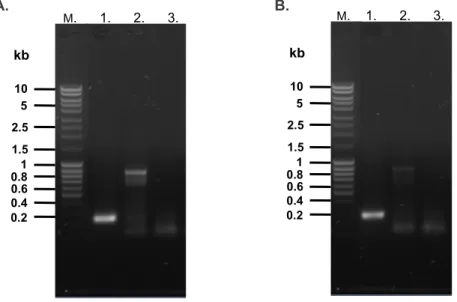
After cell lysis, each recombinant protein was affinity purified using Ni-NTA matrix for His6-PyCox17 (Figure 5.7) and glutathione-agarose for GST-PfCox17 (Figure 5.8). Similar to previous results (Chapter 4), both His6-PyCox17 and GST-PfCox17 could be reconstituted with Cu+ in vitro, as seen after incubation of the protein with CuCl2 in the presence of DTT and ascorbic acid (Figure 5.9a. The oxidation state of the bound copper is was determined by inclusion (Figure 5.9b, solid lines) or omission (Figure 5.9b, open lines) of ascorbic acid from the BCA release assay.
Interestingly, His6-PyCox17 and GST-PfCox17 give a very similar result for the ascorbate oxidation assay (Figure 5.10).
Discussion
- Confirmation of a putative Plasmodium Cox17 copper metallochaperone coding
- Recombinant expression of the putative P. yoelii and P. falciparum Cox17
- Copper binding to His 6 -PyCox17 and GST-PfCox17
The function of a hypothetical Plasmodium protein is determined using a thorough biochemical analysis (Birkholtz et al., 2008). The partially oxidized state binds a single Cu + ion, whereas the fully oxidized state cannot bind copper (Palumaa et al., 2004). This was suggested to be a result of the obligate dimeric state of GST (Heaton et al., 2001).
Based on previous findings, parasite Cox17 is believed to reside in the cytoplasm (Beers et al., 1997).
General discussion
Introduction
In yeast and mammalian cells, a copper transport protein functions to acquire extracellular copper and transport it into the cell (Kim et al., 2008; Lutsenko, 2010). The identification of a parasitic mechanism for copper excretion (Rasoloson et al., 2004) suggested that an opposite mechanism for copper acquisition is likely to exist. A yet-to-be-discussed mechanism for this process is copper acquisition via a dedicated copper transport protein.
Therefore, the main goal of this study was to identify and characterize a copper transport protein of the Plasmodium parasite and in addition, it attempted to identify and characterize a related copper metallochaperone.
Identification of Plasmodium parasite copper-requiring protein orthologs
In the present study, a Plasmodium ortholog for this protein was identified and found to possess characteristics suggestive of a role as a copper transport protein (Chapter 3). Identification of these copper-dependent protein orthologs supports the likelihood that the Plasmodium parasite requires intracellular copper for its metabolism. Within yeast cells, it was initially assumed that, after copper acquisition, intracellular copper ions were moved from the copper transport protein to mitochondrial cytochrome c oxidase via Cox17 (Beers et al., 1997; Glerum et al., 1996).
Identification of a putative Plasmodium parasite Cox17 metallochaperone sequence, as well as its associated co-metallochaperone, suggests a similar parasite mechanism for copper loading in cytochrome-c oxidase.
Production of anti-peptide antibodies used for the detection of a Plasmodium
An in silico analysis of these sequences (section 3.2.2) identified features characteristic of known copper transporters (Figures 3.1 and 3.2), thereby supporting the possibility that these proteins play a role in parasite copper acquisition. In an attempt to confirm native expression of the parasite copper transporter and Cox17 metallochaperone, anti-peptide antibodies were raised for use in immunofluorescence and western blotting studies. The antibodies raised in this study detected a murine malaria parasite copper transporter in parasite lysates as well as by immunofluorescence microscopy.
The discovery of a malaria parasite copper transporter in rodents confirms the native parasitic expression of this protein and supports the possibility that a dedicated parasitic mechanism for copper acquisition exists.
Recombinant expression of the amino terminal domain of the putative
This was thought to be due to the presence of a signal sequence in PbCtrNt, which has been reported to interfere with recombinant expression of Plasmodium parasite proteins (Mehlin et al., 2006; Vedadi et al., 2007). As with the amino-terminal domain of the copper transport protein, both the murine malaria parasite and P. The reasons for this were not clear, but the result seems to support the idea of using the "power of orthologs" for successful recombinant Apicomplexan expression. parasite proteins (Vedadi et al., 2007).
The use of synthetic, codon-optimized Plasmodium genes has thus far improved the quality and quantity of other heterologously expressed recombinant Plasmodium proteins (Chowdhury et al., 2009; Flick et al., 2004; Mehlin et al., 2006; Vedadi et al., 2007), suggesting that a similar result could be achievable for PfCox17.
Copper binding to a recombinant amino terminal domain of the putative
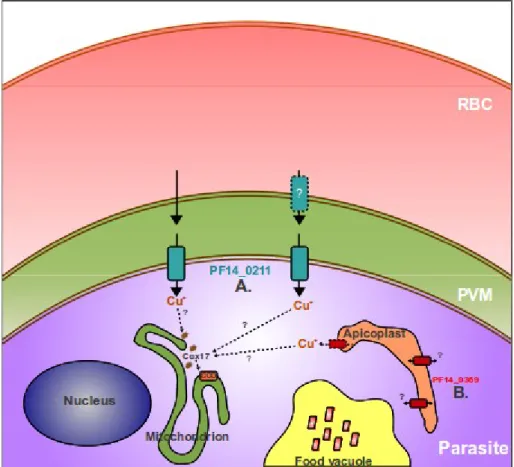
Proposed mechanism for Plasmodium parasite copper acquisition
This is a possibility as the signal/s for targeting membrane proteins to the parasitophorous vacuole membrane remains unknown (Martin et al., . 2009a). A striking feature of the apicoplast is its close association with the parasite mitochondrion (Bannister et al., 2000; van Dooren et al., 2006). However, the possibility exists that the parasite apicoplast acts as a site for copper storage, similar to the Chlamydomonas chloroplast (Merchant et al., 2006).
In yeast and mammalian cells, Cox17 is required for the delivery of copper to cytochrome-c oxidase for its activation in mitochondria (Kim et al., 2008; Lutsenko, 2010).
Conclusions and future direction
The role of mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. The role of the N-terminus of mammalian copper transporter 1 in the cellular accumulation of cisplatin. The 'permeome' of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum.
Identification and initial characterization of three novel cyclin-related proteins of the human malaria parasite Plasmodium falciparum.
![Table 2.6 Primer properties, MgCl 2 concentration and thermal cycling conditions used for PCR Primer name T m (°C) [Primer]](https://thumb-ap.123doks.com/thumbv2/pubpdfnet/10642027.0/69.892.129.815.652.1156/primer-properties-concentration-thermal-cycling-conditions-primer-primer.webp)