SELECTION AND CHARACTERIZATION OF BACTERIAL
ISOLATES FORMONOCYCLIC AROMATIC DEGRADATION
BY
DWI
SURYANTO
GRADUATE PROGRAM
To my dearest children:
ABSTRACT
A number of anoxygenic photosynthetic bacteria (APB) were isolated from Moluccas, Central Kalimantan, West Kalimantan, West Java, and Yogyakana. Approximately, 65.8% of the APB isolates were able to utilize benzoate as their sole carbon source when grow photosynthetically. The ability of these bacteria to grow in gentisate was firstly reported. No growth was demonstrated in the presence of phenol, cathecol and salysilate as sole carbon sources. In addition, we also isolated five aerobic bacterial isolates that could grow at least in three different monocyclic aromatic compounds. One of the aerobic isolates could grow in atrazine.
The three APB, DS-1, DS-4 and Cas-13 as well as aerobic isolate DS-8 were Gram negative, motile, non-halophilic, non alkali- and acidophilic. The APB were rod-shape cells with swollen terminal. DS-8 was ovale-rod cell, has excellent swarming activity and produce extracellular protease and chitinase. Growth of the selected APB isolates were optimum at NaCl concentration of 0.5% (w/v) and initial p H of 7.5, respectively, while optimum NaCl concentration and initial pH for DS-8 growth, one of the aerobic isolates, were 1-1.5% (w/v) and 7-8.5, respectively. Each of the isolates could grow in benzoate up to 10 rnM.
APB isolates could grow and utilize cassamino acid and dextrin as their sole carbon sources. However, DS-8 could utilize either cassamino acid, glutamate, glucose, acetate, potato starch, or ethanol as the only carbon sources. Macrorestriction Fragment Length Polymorphisms (MFLP) employing Pulsed-Field Gel Electrophoresis (PFGE) of the APB isolates indicated that DS-1 was closely related to Cas-13 rather than to DS-4. Physiological or metabolic identification using Microbact (Medvet Science Pty. Ltd., Adelaide, Australia) indicated that DS-8 was closely related to Klebsiella ozaenae (49.85%) and Smatiu lzquefaciens (24.42%). Sequence analysis of 16s rRNA genes showed that the APB isolates were strains of R h o d o p ~ m o n u s p c t l ~ , while DS-8 was S. marcexens. A complete 16s rRNA sequence analysis, however, is needed to give more definitive information about the taxonomic position of the isolates.
STATEMENT O F RESEARCH ORIGINALITY
This is to verify that the dissertation entitled:
SELECTION AND CHARACTERIZATION O F BACTERIAL ISOLATES FOR MONOCYCLIC AROMATIC DEGRADATION
is my own work and has never previously been published. All of the incorporated
data and information are validated and stated clearly.
Bogor, 10 October 2001
SELECTION AND CHARACTERIZATION OF BACTERIAL
ISOLATES FOR MONOCYCLIC AROMATIC DEGRADATION
BY
DWI SURYANTO
A DISSERTATION
Submitted to the Bogor Agricultural University in partial fulfillment of the requirements for
the Doctorate Degree (Dr.)
GRADUATE PROGRAM
BOGOR AGRICULTURAL UNIVERSITY
200 1
This is to certify that the dissertation
Title SELECTION AND CHARACTERIZATION OF
BACTERIAL ISOLATES FOR MONOCYCLIC AROMATIC DEGRADATION
Name DWI SURYANTO
Student number 965080 BIO
Study Program/ Biology/Microbiology Sub Program
has been accepted toward fulfillment of the requirements for Doctorate degree in Biology/Microbiology
1. Committee members
Dr. Antonius Suwanto Dr. Ania Meryandini
Chairman
Prof. Dr. Bibiana W. Lay
J '
w
Prof. Dr. Muhammad Sri Saeni
2. Head of Study Program
4
Dr. Dede Setiadi
BIOGRAPHY
Dwi Suryanto. Born in Sungailiat, April 9, 1964. The second son of
father, Muhammad Sahuri and mother, Sujatmi. Graduated from Elementary
School (SD UPTB Pemali), Junior High School (SMP UPTB Pemali), and Senior
High School (SMA Negeri Sungailiat) in 1976, 1979, and 1982, respectively.
Continuing education at Faculty of Biology, Gadjah Mada University, Yogyakarta
in 1982, and obtaining bachelor degree majoring in Ecology in 1987. In 1991,
master degree w a s started in Department of Entomology, College of Natural
Sciences, Michigan State University, East Lansing, USA, under a USAID
scholarship program, and granted in 1993. In 1996, w a s admitted as a doctorate
student at Study Program of Biology, Sub-program of Microbiology, Bogor
Agricultural University, Bogor.
Appointed as a lecturer at Department of Biology, Faculty of Mathematics
and Natural Sciences, North Sumatra University, Medan. Married in 1992, and
have two dearest children, Muhammad Aditya Haryawan and Nindya Laksita
AKNOWLEDGMENTS
I would like to express my deepest appreciation and sincere thanks to my
major advisor, Dr. Antonius Suwanto, for his guidance, patience and support
throughout my doctorate degree, and for giving an opportunity to learn more
about the art of microbiology.
I would also like to express my gratitude to other committee members,
Dr. Anja Meryandini, Prof. Dr. Maggy T. Suhartono, Prof. Dr. Bibiana W. Lay,
and Prof. Dr. Muhammad Sri Saeni for their unfailing support, encouragement,
and guidance during the course of my work.
I have enjoyed the interaction and aid from the marvelous students,
friends, and technicians in Laboratory of Microbiology and Biochemistry of
Research Center for Biotechnology, Artini Pangastuti, Dr. Budiasih Wahyuntari,
Diana E. Waturangi, Etty Pratiwi, Irawan Tan, Munti Yuhana, Dr. Nisa
Rachmania, Nurhaemi Haris, Rina Martini, Temmy Desiliyarni, Dr. Wibowo
Mangunwardoyo, Widanarni, Witri Djasmasari, Dr. Yusminah Hala, Stefani
Adijuwana, Eni Sumartini and Ika Malikah, and the many others too numerous to
be named, but will remain dear to my heart.
To my dearest children, Muhammad Aditya Haryawan and Nindya Laksita
Laras, thank you both for being the light of my life.
Special thanks must go to Center for Microbial Diversity, FMIPA, IPB for
funding this research. Finally, I would also like to thank to my government, the
North Sumatra University, and PT Timah Tbk. for their support and confidence.
TABLE OF CONTENTS
ABSTRACT
...
i STATEMENT OF RESEARCH ORIGINALITY...
ll.
.
BIOGRAPHY...
v AKNO WLEDGEMENTS...
vi...
TABLE OF CONTENTS...
vul LIST OF TABLES...
x LIST OF FIGURES...
xiCHAPTER 1
.
GENERAL INTRODUCTION
Aerobic Degradation of Monocydic Aromatic Hydrocarbon Compounds
...
3 Anaerobic Catabolism of Monocyclic Aromatic Hydrocarbons...
7Genetic and Biochemistry of Catabolism of Aromatic Hydrocarbon
...
10 The General Objectives of The Research...
13CHAPTER 2
.
MATERIALS
AND
METHODSIsolation and screening of benzoate-utilizing bacteria
...
14Aromatic hydrocarbon utilization test
...
15 Benzoate and other C source utilization test...
15 Growth condition. measurement of growth.. . .
and quantitation of benzoate utrllzatron...
17 Examination of cell morphology and physiological property...
18 Total genomic DNA preparation...
19Total genomic analysis
...
20 Spectral analysis...
20 Amplification and sequencing of part of 16s rRNA genes...
21 Construction of ph~logenic tree...
21 Strain and plasmids...
22Di- and triparental mating
...
22Transformation of flanking DNA
...
23 Plasmid preparation...
24Southern hybridization
...
24...
Amplification using
b d
primer 25CHAPTER 3
.
SELECTION AND ISOLATION OF BACTERIA FOR BENZOATE DEGRADATION
ABSTRACT
...
26 INTRODUCTION...
27...
RESULTS
AND
DISCUSSION 28...
...
CONCLUSIONS
:
35CHAPTER 4
.
ISOLATION AND CHARACTERIZATION OF A NOVEL BENZO ATE-UTILIZING Serratia marcescens
...
ABSTRACT 37
INTRODUCTION ... 38 RESULTS AND DISCUSSION
...
40 CONCLUSIONS...
51CHAPTER 5
.
CHARACTERIZATION OF BENZOATE DEGRADING ANOXYGEMC PHOTOSYNTHETIC BACTERIA ISOLATED
FROM ENVIRONMENT
...
ABSTRACT 53
INTRODUCTION
...
54 RESULTS AND DISCUSSION...
56 CONCLUSIONS...
70CHAPTER 6
.
IDENTIFICATION O F RESPONSIBLE GENE FOR BENZOATE DEGRADATION IN
Serratia murcescens DS-8 AND Rhodopseudomonas palustris DS-4 ABSTRACT
...
72 INTRODUCTION...
73...
RESULTS AND DISCUSSION 74
CONCLUSIONS
...
77...
GENERAL CONCLUSIONS 78
REFERENCES
...
80 APPENDICES...
89...
APPENDIX 1.
ClustalW Analysis of DS-8 and Its Relatives 89 APPENDIX 2.
ClustalW Analysis of DS.1. DS.4. and Cas-13 and Their...
Relatives 91
APPENDIX 3
.
Sequences of 16s rRNA genes...
92 APPENDIX 4.
Sequence of benA of Acinetobacter calcoaceticus and P d o m o n a sput& for designing PCR primer
...
93...
APPENDIX 5.
Test of Microbact of DS-8 95LIST
O F
TABLES
CHAPTER 2.
MATERIALS
AND
METHODSTable 1. Bacterial strains and plasmid used in this study
...
2 1CHAPTER 3.
[image:128.511.31.464.33.743.2]SELECTION AND ISOLATION OF BACTERIA FOR BENZOATE DEGRADATION
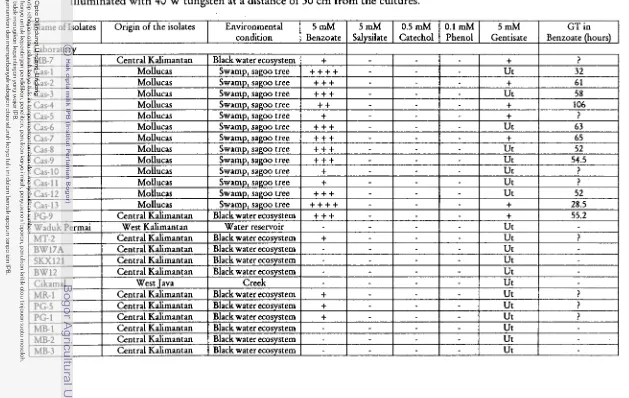
Table 1. The ability of anoxygenic photosynthetic bacteria to grow in
[image:128.511.37.461.217.736.2]monocyclic aromatic compounds in anaerobic condition illuminated
...
with 40 W tungsten at a distance of 30 cm from cultures 30 Table 2. The ability of bacterium to grow in monocyclic aromatic compoundsin aerobic condition
...
34CHAPTER 4.
ISOLATION AND CHARACTERIZATION OF A NOVEL BENZOATE-UTILIZING Serratia marcescens
Table 1. The appearance of DS-8 colonies when grown on different media
...
42 Table 2. Physiological and biochemical characterization of DS-8...
45 Table 3. Comparison of 16s rRNA sequence similarity of DS-8 anda number of closely related bacteria
...
45CHAPTER 5.
CHARACTERIZATION OF BENZOATE DEGRADING ANOXYGENIC PHOTOSYNTHETIC BACTERIA ISOLATED
FROM ENVIRONMENT
Table 1. Observation on cell morphology
...
56 Table 2. Peaks (nrn) and absorbances of cell extract ofRb.
sphaerozdes, DS-1,...
DS-4, and Cas-13 59
LIST OF
FIGURES
CHAPTER 1.
[image:129.511.40.467.40.667.2]GENERAL INTRODUCTION
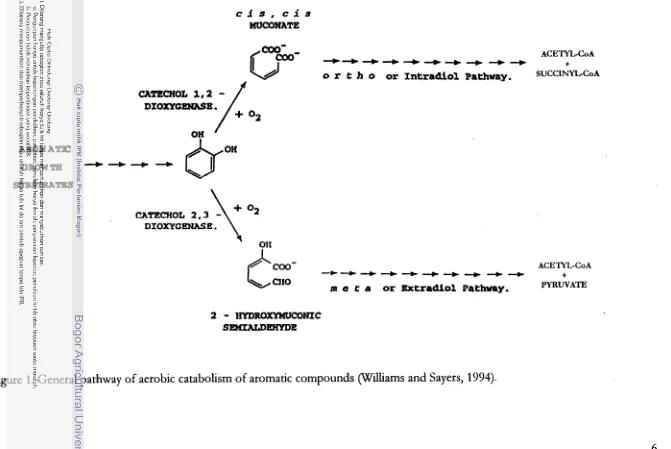
Figure 1. General pathway of aerobic catabolism of aromatic compounds
...
6Figure 2. General pathway of anaerobic catabohm of aromatic compounds
..
8*
CHAPTER 3.
SELECTION AND ISOLATION OF BACTERIA FOR
BENZOATE DEGRADATION
Figure 1. Photograph of growth of anoxygenic photosynthetic bacteria in modified Sistrom with 5 rnM benzoate as C source supplemented with vitamins
...
29 Figure 2. Growth of anoxygenic photosynthetic bacteria in modified Sistrom with 5 mM benzoate as sole C source supplemented with vitamins..
32Figure 3. Growth of aerobic bacteria in modified salt medium with 5 mM
...
benzoate as sole C source supplemented with vitamins 34
CHAPTER 4.
ISOLATION
AND
CHARACTERIZATION OF A NOVELBENZOATE-UTILIZING Serratia rnarcescens
Figure 1. Photograph of DS-8 cells ... 40
Figure 2. Photograph of colony expansion of DS-8 by its swarming activity after 12 hours of incubation time..
...
43Figure 3. Colony appearance of DS-8 on LB agar containing ampicillin,
...
streptomycin and spectinomycin, and gentamycin 43
Figure 4. Colony appearance of DS-8 on chitin agar (A) and skim milk agar (B)
...
43Figure 5. Dendrogram of DS-8 and its relatives
...
46~ i & r e 6 . ~ u h r e o f DS-8 on modified salt medium supplemented with 5 mM benzoate (A) without vitamin supplementation, (B) with vitamin supplementation
...
46Figure 7. Growth of DS-8 in benzoate supplemented with vitamins and without vitamins
...
47Figure 8. Profiles of growth and benzoate degradation of DS-8 in different benzoate concentration (A), NaCl concentration . . (B), . . and different initial pH (C)
...
49Figure 9. Growth of DS-8 in different C-sources
...
51CHAPTER 5.
CIlARACTERIZATION OF BENZOATE DEGRADING ANOXYGENIC PHOTOSYNTHETIC BACTERIA ISOLATED
FROM ENVIRONMENT
Figure 2. Colony appearance of (A) DS-1, (B) DS-4, and
(C)
cas-13 on [image:130.515.42.469.0.738.2]modified Sistrom with 5 mM succinate as their C-source
...
...
.. .
. .
. .
57 Figure 3. Colony appearance of (A) DS-1, (B) DS-4, and (C) Cas-13 onmodified Sistrom with 5 mM benzoate as their C-source
...
...
... . . .
58Figure 4. Absorption spectrum of cell extract of DS-1, DS-4, Cas-13, and
[image:130.515.41.467.44.569.2]Rb. sphaero2des 2.4.1.
. . .
.
. . .
.
. . .
.
. . .
.
.
.
. .
.
. . .
. .
. . . .
.
.
.
.
59 Figure 5.MFLP
profiles of total genome digested with A d . ... .. .
...
.
.. ...
.. .
.
60 Figure 6. Phylogenic tree of MFLP profiles after digestion with A d . .. .
. . .
60 Figure 7. Phylogenic tree of 165 rRNA gene sequences...
...
. .. ...
...
...
. . .
.. .
61 Figure 8. Growth of DS-1, DS4, and Cas-13 in 5 mM benzoate withvitamins (A) and with no vitamins (B)
... .. . . .. . .
...
.
.. . . .
.
. . .
63 Figure 9. Colony appearance of (A) DS-1, (B) DS4, and (C) Cas-13 onmodified Sistrom with 5 mM gentisate as their C-source
...
.
.. .
..
.
. .
64 Figure 10. Histogram of cell density and benzoate utilization of DS-1..
. . . .
..
65 Figure 1 1. Histogram of cell densityand
benzoate utilization of DS-4. . . ..
66 Figure 12. Histogram of cell density and benzoate utilization of Cas-13... . . .
67 Figure 13. Growth of DS-1, DS-4, Cas-13, and Rb. sphaerozdes 2.4.1. in 5 m M succinate(A) and acetate(B)
with vitamins... . .
. .
. . .
. . . .
. .
. . .
.
..
69 Figure 14. Histogram of cell density of other C-source utilization of DS-1,DS4, andCas-13
... ...
...
... ...
... ... ... ...
...
...
... ...
...
... ...
...
... ...
70CHAPTER
6.IDENTIFICATION OF RESPONSIBLE GENE INVOLVED IN
BENZOATE DEGRADATION OF
Swratia marcescens DS-8 AND Rhodopseudomonas palustris DS-4 Figure 1. Southern blot analysis of total cellular DNA of DS-8 and
-
CHAPTER
1.
GENERAL
INTRODUCTION
In the last few decades, many hydrocarbon compounds especially
aromatic hydrocarbons have been introduced in large quantity and accumulated in
soil, aquatic environment, anaerobic sediment, or even in deep-ground water
(Mohn and Kennedy, 1992; Kuo and Genthner, 1996; Laine and Laine and
Jergensen, 1996; Semple and Cain, 1996; Werwath et al., 1998). They become a
serious problem since they are toxic and carcinogenic (Shimao et al., 1989; Leahy
and Colwell, 1990; Dong et al., 1992; Valenzuela et al., 1997).
The persistence of the aromatic hydrocarbon compounds in environment
depends on the structure and the complexity of the compounds. Haloaromatic
and polycydic aromatic hydrocarbon in general are relatively recalcitrant (Leahy
and Colwell, 1990; Valenzuela et al., 1997). It is known that mineralization rate
(degradation of the compounds to C02 and H20) of higher-molecular-weight
complex aromatic hydrocarbon, such as resin, and asphalten is much slower than
degradation of lower-molecule-weight aromatic hydrocarbon such as monocydic
aromatic compounds (Leahy and Colwell, 1990). However, previous studies
showed that complex hydrocarbons were rapidly degraded in optimum condition
(Leahy and Colwell, 1990).
One of the important monocyclic aromatic introduced to environment is
benzoate. It is introduced through herbicide application or other industrial
practices (Werwath et al., 1998). It is also encountered as an important
intermediate in metabolic pathway of many aromatic hydrocarbon compounds
(Harwood and Gibson, 1988; Grund et al., 1990; Powlowski and Shingler, 1994;
Arendorf et al., 1995).
Metabolism of aromatic hydrocarbon compounds as well as other
hydrocarbons in nature depends on the catabolic reaction of microorganisms
(Semple and Cain, 1996). Degradation rate of the compound is affected by its
natural propeny, its concentration, and the microbial community in the
environment (Leahy and Colwell, 1990; Nicholson et al., 1992).
Biodegradation process are versatile and can be utilized at various stages
of treatment (Porcier, 1991). However, introduced microorganisms may
eventually not worked as they are supposed to (Nicholson et al., 1992). Lower
resistance, predation, competition, inhibition
by
other toxic chemicals or muchtoxic intermediate, and other microbial contaminants are responsible for
uncompleted biodegradation of organic compounds (Harwood et al., 1990;
Hiepieper et al., 1992; Miethling and Karlson, 1996; Blasco et al., 1997). Since
biotransformation products are not necessary safe, the complete mineralization of
toxic organic substances to C02 and H20 is the most desirable goal (Laine and
Jerrgensen, 1996; Blasco et a/., 1997).
Utilization of microorganisms as bioremediation agents of hydrocarbon
compounds has been plentifully reported (Blasco and Castillo, 1992; Lobos et al.,
1992; Nicholson et al., 1992; Blasco et al., 1997). Furthermore, industrial devoted
to the bioremediation of toxic organic pollutants are growing rapidly (Wyndham
et al., 1994). Biotechnological approach in biodegradation process of complex
hydrocarbons was established by choosing proper microorganisms or engineering
essential to assess risks at contaminated sites, implement biological treatment
processes, or design effective bioremediation strategies (Nicholson et al., 1992).
Selection and characterization of new prospecting aromatic-utilizing
microorganisms are still in need, while many workers are trying to optimize the
utilization of the available strains. For the former groups, many restricted their
works only on the ability of the bacteria to utilize the aromatic compounds. A
knowledge of genetic, physiological, and ecological characterization in the
screening and selection of bacteria are crucial in term of providing comprehensive
information of the strains in order to determine and to establish proper
technology for bioremediation (Leahy and Colwell, 1990; Nicholson etal., 1992;
Wyndham et al., 1994).
Aerobic Degradation of Monocyclic Aromatic Hydrocarbon Compounds
Study on biodegradation of aromatic hydrocarbon compounds has
primarily conducted for aerobic microorganisms (Genthner etal., 1989). Of these
microorganisms, bacteria like Achmonas, Acinetobacter, Akaligenes, Ahobacter,
3acillus, P d m w , and B u r k h o k spp. (ShLaao
etd,
1989; Leahy and Colwell,1990; Shen and Wang, 1995; Kuo and Genthner, 1996; Blasco et al., 1997), and
the fungi (Bugos et al., 1988; Bumpus, 1989; Spadaro et al., 1992; Gemble et al.,
1996) are degradation organisms that have been reported most successful in
catabolizing the aromatic compounds. Several other microbes (Grund etal., 1990;
Lenke and Knackmuss, 1992; Lenke etal., 1992; Lobos etal., 1992; Schmidt etal.,
1992; Behki et al., 1993; Allen et al., 1997; Miehling andKarlson, 1996) includmg
algae (Semple and Cain, 1996) have also shown their ability to aerobically
catabolize aromatic hydrocarbons.
Although a new oxidation pathway has been described via benzoyl-
Coenzyme A and 3 - h y d r o ~ y ~ ~ l - C o e n z ~ m e A in a denitnfylnglkabnzm sp.
(Altenschmidt et al., 1993), the most common routes for aerobic degradation of
these compounds are usually through destabhtion of the aromatic ring to form
an intermediate catechol(1,2 dhydroxybenzene) (Grund et al., 1990; Blasco etal.,
1997). Protocatechuat (3,4 dihydroxybenzoate) and gentisate (2,5
dihydroxybenzoate) as intermediates were also postulated (Crowford, 1976;
Fuenmayor et al., 1998).
The conversion of the monocyclic aromatic to intermediate catechol
involves ring-dioxygenases. However, in some bacteria ring-monooxygenases are
the common enzymes for the conversion (Powlowski and Shingler, 1994;
Williams and Sayers, 1994; Shield etal., 1995). The catechols are substrates for the
second stage of catabolism which is performed by the actions of ring-cleavage
dioxygenases that break one of the carbon-carbon bonds of the ring by addition
of molecular oxygen. This reaction produces an unsaturated aliphatic acid
(William and Sayers, 1994). The ring cleavage usually occurs through ortho
cleavage (intradiol) which produced cis,cis muconic acid (or a derivative) and
through meta cleavage (extradiol) which produces Zhydro~~muconic
semialdehyde (or a derivative) (Williams and Sayers, 1994; Laine and Laine and
Jerrgensen, 1996; Blasco et al., 1997). The enzyme systems resemble each other,
even though many different metabolic pathways have been identified (Williams
and Sayers, 1994; Kudo et al., 1998).
The biochemistry of the two reaction sequences appears to be conserved
(Figure 1) in all eubacteria in which they are found. Therefore, aerobic aromatic
catabolism consists of a variety of pathways that converge on a common
intermediate (catechols) which are further assidated by a common pathway
(Williams
and Sayers, 1994). The pathway itself has undoubtedly been in existencefor a considerable period of evolutionary time (Williams and Sayer, 1994).
Phenol, benzoate, and their derivatives have generally been a subject of
intensive study on aerobic biodegradation of monocyclic aromatic
(Gurujeyalakshmi and Oriel, 1989; William and Sayer, 1994; Shen and Wang,
1995; Semple and Cain, 1996; Valenzuela et al., 1996). Phenol is found as natural
phenolic compound in plant materials as well as in the effluents of oil refineries,
petrochemical plants, pesticide application, and other industrial processes
(Gurujeyalakshmi and Oriel, 1989; Lenke et al., 1992; Werwath et al., 1998). It is
also found as an important intermediate in the anaerobic degradation of many
complex and simple aromatic compounds (Zhang and Wiegel, 1990). Like
phenol, benzoate and its derivatives are often encountered as intermediate of
complex aromatic hydrocarbon catabolism including biphenyl (Williams and
Sayers, 1994; Arendorf etal., 1995), chlorophenol (Williams and Sayers, 1994),
cinnamate, mandelate, 5-phenylvalerate, 3-phenyl propionate, and benzoylformate
(Harwood and Gibson, 1988). Thus, the factors that influence the rate and extent
of benzoate degradation may also influence biodegradation of other aromatic
compounds (Hopkins et al, 1995; Warikoo et al., 1996). It was also introduced
c i s , c i s
MUCONATE
ACETYLCoA
+
o r t: h o or IntradLol Pathway. SUCCINULCOA
ACE'I'YL-CoA
[image:136.753.29.700.40.489.2]+ la c c a ox XxEradiol Pathway. P Y R U V ~
Anaerobic Catabolism of Monocyclic Aromatic Hydrocarbons
Anaerobic degradation pathway of aromatic hydrocarbon (Figure 2) has
not been fully understood (Coschigano and Young, 1997; Harwood and Gibson,
1988; Pelletier and Harwood, 1998), but the process has been reported (Genthner
et al., 1989; Madsen and Licht, 1992; Nicholson et al., 1992; H o p b et al., 1995).
Madsen and Licht (1992) isolated and characterized an anaerobic
bacterium from municipal sludge. The bacterium, related to Closdum, was able
to dechlorinate chlorophenol. Anaerobic biodegradation of atrazine by the
facultatively anaerobic bacterium M9 1-3 has been studied (Crawford et al., 1998).
The isolate was capable to utilize atrazine as its sole
C
and N source underaerobic as well as anaerobic conditions (Radosevich et al., 1995). Nicholson et al.
(1992) showed that pentachlorophenol-acclimated methanogenic consortium
dechlorinated pentachlorophenol to ~chlorophenol, although not all routes
produce this intermediate. Kuo and Genthner (1996) isolated a new bacterium,
strain SB, that degrades benzoate only when coculture with an H2 or formate-
The effect of electron acceptors and electron donors availability in
anaerobic degradation of aromatic compounds might differ. Mohn and Kennedy
(1992) reported that addition of electron donors such as sucrose and some
potential products of sucrose fermentation, H2, formate, acetate and propionate
in a reactor had negligible effect on dehalogenation of chlorophenol. Added
elemental S, sulfate, nitrate, and ferric ions inhibited dehalogenation. However, Heindriksen et al. (1992) demonstrated that the addition of glucose in a glucose-
amended reactor stimulated dechlorination rate of pentachlorophenol. This might
be due to a higher concentration of the biomass. Hfggblom etal. (1993) showed
that the process depended on the availability of electron acceptor, and on the
position of the chlorine substituent. In anoxic sediments, nitrate, sulfate, or
carbonate may serve as terminal electron acceptors (Kohring et al., 1989;
Haggblom et al., 1993). When sulfate concentration tend to be low, such as in
anaerobic freshwater environment, carbonate reduction to methane serves as the
predominant electron sink. On the other hand, in marine system, sulfate
concentration tend to be high, and sulfate reduction or sulfidogenesis serves as
the major electron accepting process (Haggblom et al., 1993).
The effect of heavy metal ions like C d o ,
Cue,
C r o , or H g o onbiodegradation of 2-chlorophenol, 3-chlorobenzoate, phenol, and benzoate in
anaerobic bacterial consortia has been examinated. Although the effect of the
ions was different in different aromatic compounds, increasing degradation rate
was observed in benzoate with 0.01 ppm C r o , Cd(II) and Cu(II), in phenol
with 0.01 ppm C r o , and in 2-chlorophenol and 3-chlorobenzoate with 1.0 to
2.0 ppm Hg@) after an extended acclimation period (Kuo and Genthner, 1996).
Although previous works showed anaerobic degradation of many
monocyclic aromatic (Genthner etal., 1989; Madsen and Licht, 1992; Mohn and
Kennedy, 1992; Kuo and Genthner, 1996; Crawford et al., 1998), anaerobic
catabolism of benzoate has gotten more attention. Haggblom et al. (1993),
Hopkin et al. (1995), Kuo and Genthner (1996), and Warikoo et al. (1996)
observed anaerobic benzoate degradation in various bacteria. Harwood and
Gibson (1988), Kamal and Wyndham (1990), Wright and Madigan (1991), Blasco
and Castillo (1992), Gibson and Gibson (1992), Sasikala et al. (1994, and Shoreit
and Shaheb (1994) saw that anoxygenic phototrophic bacteria photo-
anaerobically catabolize benzoate and its derivatives or homologs. Since the
anoxygenic photosynthetic bacteria demonstrate biochemical versatility, they are
relatively easier to study rather than any other obligately anaerobic bacteria. A
complete pathway of the anaerobic degradation of aromatic compound has been
postulated from anoxygenic photosynthetic bacteria (Figure 2) (Pelletier and
Harwood, 1998).
Genetic and Biochemistry of Aromatic Hydrocarbon Catabolism
Genetic and biochemical analysis of aerobic degradation has been done
primarily in Psardornonas (Altenschdt et al., 1993; Dunaway-Mario and Bab bin,
1994; Powlowslu and Shingler, 1994; William and Sayers, 1994; Shield etal., 1991;
de Souza et al., 1995; Blasco et al., 1997; Fuenmayor etal., 1998). Degradation of
aromatic compound is encoded in plasmids or chromosome (Harayama et al.,
1991; Jeffrey eta/., 1992; Bremer eta/., 1993). Some transposable elements such as
Tn4651 and Tn4653, the toluene transposons, and Tn4655, the naphthalene
transposon also carry the degradative genes (Wyndham et al., 1994). Shield et al.
(1995) found that TOM plasmid, a 108 kb degradative plasmid, are responsible
for toluene and phenol catabolism. This plasmid possesses genes coding for
toluene ortho monooxygenase and catechol 2,3-dioxygenase. Large plasmid
collectively called the TOL plasmids carrying xyl gene for toluene/xylene has
been a subject of intensive study (Assinder and Williams, 1990). Several other
degradative genes have also been identified. These include bph, dmp, nab and tod
(Williams and Sayers, 1994), gtd (Werwath et al., 1998),
ben
(Jeffrey et al., 1992), andnag (Fuenmayor et a!., 1998).
Several study on homology of the degradative genes has been carried out.
Kim et al. (1996) has conducted homology study of degradative genes in
Sphingomonas. Harayama et al. (1991) observed that
xylXYZ
of Pdmonusputzdzand benABC of Acimbacter calcoaceticus shared a common ancestry. Bundy et al.
(1998) saw the similarity between
the
anul BC-encoded anthranilate dioxygenaseand the benABC-encoded benzoate dioxygenase of Acinetobacter sp. strain ADPI.
Substitution of antC of Acinetobacter mutants by benC during growing in
anthranilate suggesting relatively broad substrate specificity of the BenC
reductase. In contrast, the bem4 B genes did not substitute for anulB (Bundy et al.,
1998) indicating a narrow substrate specificity (Hara~arna et al., 1991; Bundy et al.,
1998). The genes responsible in conversion of naphthalene to gentisate, nag, from
Pseudomonas sp. strain
U2
isolated from oil-contaminated soil have beensequenced. Sequence comparisons suggested that the novel genes represented the
archetype for naphthalene strains which use the gentisate pathway rather than the
Comparative study on enzyme responsible for degradation of aromatic
compound were conducted by Dong et al., (1992) and Neidle et al. (1991).
Catechol2,3 dioxygenase of
B.
rtearothennqphilus was functionally the same as theenzyme encoded by xylE in
P.
putida, although their thermostability andhomology between the two genes were rather
different
(Dong etal., 1992). Neideet al. (1991) demonstrated that the comparison of the deduced amino acid
sequences of BenABC of A. calcoaceticus with relative sequences including those
for the multicomponent toluate, toluene, benzene, and naphthalene 1,2
dioxygenase indicated that the similar size of the hydroxylase component sub-
units were derived from a common ancestor.
Study on genetic of anaerobic catabolism of aromatic compounds was
almost limited on anoxygenic photosynthetic bacteria. Anaerobic catabolism of
benzoate by anoxygenic photosynthetic bacteria involves
bad
genes. For the ring-cleavage of benzoate,
badl
that codes for Bad, a 2-ketocyclohexanecarboxylCoenzyme-A hydrolase, are seemingly responsible (Palletier and Harwood, 1998).
Biochemical analysis of the anaerobic monocyclic aromatic hydrocarbon
catabolism showed the possible pathways (Pelletier and Harwood, 1998) with
cyclohex-1,s-diene-1 carboxyl-CoA and 3-hydro~~pimelil Co-A as a common
intermediate before separating to their specific pathway and entering TCA cycle,
respectively.
Cloning of degradative genes has been reported. Kim and Oriel (1995)
successfully clonedpheA andpheB from B. stearothemzophilus BR.219 to Eschaachiu
coli. The genes are coding for the conversion phenol to catechol and catechol to
2-hydroxymuconic semialdehyde, respectively. Cloning and mapping of phenol
degradative genes for me& pathway from B. stearothermophilus
FDTP-3
to E. coliwas also carried out by Dong et al. (1992). Springael et al. (1994) reported a
transfer of degradative genes into heavy metal resistant Alraligenes eutrophus strains.
Goyal and Zylstra (1996) cloned degradative genes that distinct from the classical
gene nah from Cornamonus testosteroni GZ39, capable of degradation of polycy&c
aromatic hydrocarbon. Cloning and parrial sequence of atrazine degradative gene
from Pseudomonas sp. strain ADP have been conducted (de Souza et al., 1995).
They observed that the gene was wide spread in nature and contribute to the
formation of hydroxyztrazine in soil.
Only limited studies on degradative genes of anaerobic degradation of
aromatic compounds has been conducted. Coschigano and Young (1997) carried
out cloning and sequencing of tut genes which involved in anaerobic toluene
degradation pathway of a denitrdying bacterium.
The General Objectives of The Research
The objective of t h s study is to select and characterize bacterial isolates
capable of utilizing monocyclic aromatic hydrocarbon as their C-source. The
study of examination of anaerobic degradation of aromatic compounds was
restricted only on anoxygenic photosynthetic bacteria. To achieve this aim, we
utilized a number molecular techniques including analysis of 16s-rRNA genes of
selected isolates, Macro Restricted Fragment Length Polymorphism (MFLP) for
DNA ~rofiling analysis and spectral analysis for the anoxygenic photosynthetic
bacteria, transposition and mutation by transconjugation, and Southern
hybridization. Bacterial idendication for selected aerobic bacteria was performed
using Microbact kit (Medvet Science PTY Ltd., Adelaide, Australia). Physiological
properties were determined by growing the isolates in different monocyclic
aromatic compounds, different concentrations of benzoate and NaC1, and
different initial pH of the medium as well as non-aromatic C-sources. Microscope
observation was employed to examine morphological properties includmg cell
CHAPTER 2.
MATERIAL AND METHODS
Isolation and Screening of Benzoate-utilizing Bacteria
Screening of anoxygenic photosynthetic bacteria (APB) was carried out by
growing the isolates of Laboratory of Microbiology and Biochemistry, Research
Center for Biotechnology, Bogor Agricultural University and of environment
samples from Central Kalimantan, West Kalimantan, West Java, Molucca and
Yogyakarta
(DIY)
in Sistrom modified medium in 10 ml completely filled screwcap culture tubes. The medium contains 5.44 g K.iPo4,0.39 g WCl, 1 g NaCl,
0.6 g MgS04.7H20, 0.0884 g CaCl2.2H20, 0.004 g FeS04.7H20, 40pl
N H -
molybdate 1%,0.2 ml trace element
O
solution (0.1765 mgA EDTA, 0.1540mg/l MnSO4.2H20,0.5 mg/l ZnSO4.7H20,0.0392 mg/l CuSO4.5H20,0.0248
mg/l Co(N03)2.6H20, and 0.01 1 mg/ml H3BO3) and 0.2 ml vitamins (1 pg/ml
nicotinic acid, 0.5 pg/ml thiamin, and 0.01 pg/ml biotin) in 2000 ml, with 1 m M
Na-benzoate, 0.1 mM phenol, 5 mM Na-salysilate (2-hydroxibenzoate), and 0.5 mM
catechol as C-source.
Screening of aerobic bacteria was done by growing the isolate of the
environment from Central Kalimantan and West Java in modified salt medtum. The
medium contains 0.5 g K2HPO4,5 g NaC1,l g NH4C1,l g MgS04.7H20,20 pl
NH4-molybdate l0/o, 0.2 ml
TE
solution, and 0.05 ml vitamins in 1000 ml, with 5Aromatic Hydrocarbon Utilization Test
In order to obtain single colony, one oose of selected bacterial solution
was stricken into modified salt medium agar or modified Sistrom agar with 5 mM
benzoate as C source and incubated in appropriate growth condition. The ability
of aerobic isolates to grow in 5 mM Nagentisate, 1 mM phenol, 5 mM Na-
sal~silate, and 1 mM atrazine (2-chloro-4-ethylamino-6-is~pro~ilamino-l,3,5-
triazine (9040 purity) was examined by striking single colony in modified salt
medium solidified with 1.5% agar with tested aromatic compounds. Similar test
was done for APB with 5 rnM gentisate. For 0.1 mM phenol, 5 mM Na-salysilate
(2-hydroxibenzoate), and 0.5 m M catechol as C-source, liquid medium was used.
Benzoate and Other C-Source Utilization Test
Three isolates of anoxygenic photosynthetic bacteria, DS-1, DS-4 and
Cas-13, and one aerobic bacteria, DS-8, were chosen for further study. The two
formers of APB strains were isolated from Java, and the last was isolated from
Molucca. DS-8 was isolated from sewage in Bogor.
Benzoate utilization of DS-8 was determined by growing the isolate in a
modified salt medium supplemented with or without vitamins with 5 m M Na-
benzoate as a carbon source. Escherichia coli TOP10 was used as a control. For
testing DS-1, DS-4, and Cas-13 growth on aromatic compounds and the ability to
degrade aromatic compounds, the isolates were grown in modified Sistrom
by
omitting a l l carbon sources including nitrilo-triacetic acid, with 5 mM benzoate as
C-source supplemented with or without vitamins in 100 ml completely filled
supplemented with vitamins with 5 mM succinate and 5 mM acetate as their
carbon sources.
To examine degrading ability of three isolates of anoxygenic
photosynthetic bacteria, DS-1, DS-4 and Cas-13, and one aerobic bacteria, DS-8,
in different conditions, the isolates were grown either in modified salt or modified
Sistrom medium with different NaCl concentrations (0.5,1,1.5,2,2.5, and 3O/0),
benzoate concentrations (2.5,5,7.5,10 mM) and 5 mM benzoate without vitamin
supplementation, and at different initial pH (4,4.5,5,5.5,6,6.5,7,7.5,8,8.5, and
9).
Growth in other C sources was performed in modified salt medium
supplemented with vitamins with either 5 mM succinate, 5 m M glucose, 190
cassamino acid, 5 mM citrate, 5 mM glutarnate, 5 mM acetate, 3% ethanol, or 1%
potato starch as carbon sources for DS-8, and in modified Sistrom with either 5
mM mannitol, 5 mM glucose, 5 mM glutarnate, 3% cassamino acid, 5 mM Na-
tartrate, 1% dextrin, 3% ethanol, 3% glycerol added with 1°/o CaCO3, and 5 mM Na-
citrate as sole carbon sources for the APB.
Growth Condition, Measurement of Growth, and Quantitation of Benzoate Utilization
Test of utilization of benzoate by aerobic bacteria was carried out in 250
ml flask filled with 50
ml
salt medium with 5 mM benzoate. The flask wereshaken with 200 rpm in temperature of 30°C. Anaerobic test of the isolate was
done in modified salt agar medium supplemented with vitamins with 5 mM
benzoate as carbon source in anaerobic jar with BBL GasPak Plus (Becton
Similar test for APB was done in 1M)
ml
completely filled tube with Sistrommedium with 5 mM benzoate. All cultures of APB were illuminated with 40 W of
tungsten bulb in a distance of 30 cm. Growth were turbidimetrically measured
every 24 hours but 12 hours for 5 mM succinate and 5 mM acetate at 660 nm.
The ability to grow in benzoate with different condition was measured at
120 hours of incubation time. Utilization of other C-sources was measured at 72
hours of inoculation.
For all inoculation, the seed cultures were taken from 2-days old culture of
aerobic bacteria or 3days old culture of APB of 5 mM Na-benzoate medium.
Other C-source inoculations were taken from 2 days old culture. The cultures
were grown with the initial cell concentration of approximately 5xlV cell/ml.
All
aromatic compounds, but phenol were filter-sterilized.
Cell density was measured as absorbance at 660 n m (Harwood and
Gibson, 1988; Kamal and Wyndham, 1990) using Novaspec 11 (Pharmacia,
Uppsala, Sweden) spectrophotometer. Benzoate concentration was measured at
its absorption maximum of 276 nm using Hitachi Model U-2010 UV/Vis
spenrophotometer Wtachi Intnunent, Inc. Japan) following the establtshrnent of
standard curve relating benzoate concentration to W absorbance (Shoreit and
Shaheb, 1994).
Unless it mentioned otherwise, all media was adjusted to pH 7.2.
Examination of Cell Morphology and Physiological Properties
Cell shape, motility, and Gram staining were evaluated using a Nikon YS2-
T
microscope. Physiological characteristics of DS-8 were analyzed usingMicrobact
kit
test wedvet Science Pty. Ltd., Adelaide, Australia). Test ofproduction of extracellular protease and chitinase were monitored on salt medtum
agar with 2% colloidal chitin and Luria Bertani (LB) agar with 2% skim milk. The
salt medium contains 0.1 g K m 0 4 , 0.1 g NaC1, 0.7 g W4)2S04, 0.01 g
MgS04.7H20, and 0.05 g yeast extract in 100
d.
Eosin methylene blue
(EMB)
agar was used for preliminary screening for Enterobacteriaceae isolates. The appearance of the colonies were observed in LBagar supplemented with either 50 p g / d ampicillin, 50 pg/ml spectinomicin and
streptomicin, 10 pg/ml trimethoprim, 10 p g / d tetracyclin, 20 p g / d
gentamicin, 5 rnM benzoate, 5 mM salysilate, 5 mM gentisate, or 5 mM phenol.
The ability to degrade catechol was conducted by spraying 0.03% catechol on 1-
day old colony on LB agar. Test of growth in different temperature was
monitored on LB agar.
Total Genomic DNA Preparation
Modified phenol-chloroform-isoamyIalcoho1 treatment and ethanol
precipitation were used to extract the genomic DNA. A 5-ml overnight culture
was centrifuged at 5000 rpm for 5 minutes, washed with 1 ml0.85% NaC1, and
resuspended with 500 pl IxTE. Solution was added with 100 pl lisoz~me (50
mg/ml) and incubated at 37°C for 1 h. Freeze-thaw was carried out 2-3 times.
Incubating by shakmg for 1 hour at 65°C was carried out following an addition of
100 ~ 1 1 0 % SDS into the solution. A 10 p1 Proteinase-K (10mg/ml) was added
into the microtube followed by incubation for 1 hour at 37°C. After treated with
100 pl NaCl and 100 p1 pre-heated CTAB/NaCl (65"C), the solution was
incubated at 65°C for 20 minuts. A 0.5 ml phenol: chloroform: isoamylalcohol
(25:24:1) was added and mixed gently by inverting the tube. The tube was
centrhged for 10 minutes at room temperature. Aqueous phase was transferred
by pipetting and precipitated with 0.6 volume of cold isopropanol at room
temperature for 30 minutes. The aqueous phase was discarded following a
centrifugation of the tube for 10 minutes. Pellet was washed with 70% ethanol,
and discard the ethanol. Air dry the pellet to remove residual ethanol. Add 25 pl
nuclease-free water or TE
M
buffer. If desired, the samples may be stored at-
20°C (Sambrook et
al.,
1989).Total Genomic Analysis
Gel inserts for total genomic analysis have been prepared as Smith and
Cantor (1987). DNA restriction used method as describe previously (Suwanto
and Kaplan, 1989). Pulsed-field gel electrophoresis to obtain Macro Restricted
Fragment Length Polymorphism profiles was utilized for DNA separation using
CHEF-DR@
II
(Bio-Rad, Richmond, CA).Spectral Analysis
Cell of DS-1, DS-4, Cas-13, and
Rb.
sphaemrdes
2.4.1 were harvested from 7days old anaerobphototrophic culture of modified Sistrom supplemented with
vitamins with 5 rnM succinate as sole carbon source and suspended in ICM buffer
(10 rnM phosphate buffer pH 7.0 and 1 rnM Na-EDTA pH 7.0). Sonication were
carried out using Soniprep 150 (MSE, UK) at amplitude of 2 1 for 2 minutes three
time with time interval of 1 minutes. Cell extractions were
centrifuged
at 3.000 rpmprotein concentration off 100 pg/ml. Protein concentration were determined
by
Pierce BCA* Protein Assay
Kit
(Rockford,El,
USA).Amplification and Sequencing of Part of 16s rRNA Genes
The 16s-rRNA genes were PCR-amplified using specific primers of 63f
and 1387r from genomic DNA (200 ng) using Ready-To-GO PCR Beads
(Pharmacia-Biotech, Uppsala, Sweden). These primers were successfully to work
with a broad range environmental samples (Marchesi et al., 1998). Phenol-
chloroform-isoamylalcohol(25:24:1) treatment, ethanol precipitation, and agarose
gel electrophoresis were used to purdy the genomic DNA. Total volume of PCR
reaction (25 pl) consisted of 1.5 U Taq DNA Polymerase, lOmM Tris-HC1 (pH 9
at room temperature), 50 mM KC1,l.S mM MgC12,200 ph4 of each dNTl?s and
stabilizer including BSA. The reaction was incubated in a Gene Amp PCR System
2,400 Thermocycler perkin-Elmer Cetus, Norwalk, Conn).
Part of 16s-rRNA gene was sequenced to infer the closest related
organism from Ribosomal Database Project (RDP) maintained in the University of
Illinois, Urbana-Champaign. The sequencing reactions were done by using the Big
Dye Ready Reaction Dye Deoxy Terminator kit, purify with ethanol-sodium
acetate ~recipitation. The reactions were run on an ABI PRISM 377 DNA
Sequencer (PE Applied Biosystems, Foster City, CA.).
Construction of Phylogenic Tree
Cluster analysis of 16s-rRNA gene was done by computer program from
European Bioinformatics Institute (http://www.ebi.ac.uk). Treecon computer
program (Yves Van de Peer of Department of Biochemistry, University of
their nucleotide sequences and MFLP profiles obtained from pulsed-field gel
electrophoresis.
Strains and Plasmids
Escherichia coli S17-1 was used to promote a transfer of plasmid pJFF350
(Omegon-Km) to DS-8. E. coli JM109 (pTnMod-OGm) and E. coli HBlOl
(pRK2013) were used for triparental mating of DS-4. E. coli DH5a used to
propagate and transfer DNA, was d o n n e d by the calcium chloride technique
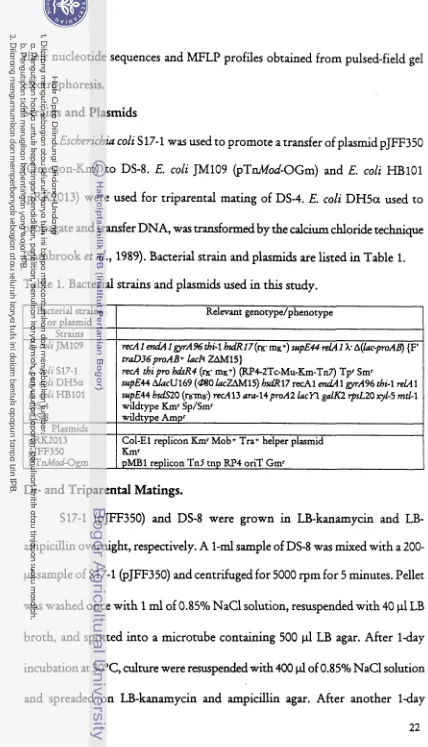
[image:152.540.45.473.27.774.2](Sambrook et al., 1989). Bacterial strain and plasmids are listed in Table 1.
Table 1. Bacterial strains and plasmids used in t h study.
Di-
and Triparental Matings.S17-1 (pJFF350) and DS-8 were grown in LB-kanamycin and LB-
ampicillin overnight, respectively. A 1-rnl sample of DS-8 was mixed with a 200-
pl sample of S17-1 (pJFF350) and centrduged for 5000 rpm for 5 minutes. Pellet
was washed once with 1 ml of 0.85% NaCl solution, resuspended with 40 pl LB Bacterial strains
or plasmid Strains El. coli JM109
E. coli S17-1
E. coli DHSa E. coli HBlOl DS-4 DS8 Plasmids pRK2013 pJFF350 PTnMod-Ogm
broth, and spotted into a microtube containing 500 p1
LB
agar. After 1-dayincubation at 30°C, culture were resuspended with 400 p l of 0.85% NaCl solution Relevant genotype/phenotype
recA 1 endA 1 gytA% thi-1 hsa'Rl7 (m- m ~ + ) supE44 & 1 k A(k-pAB) {F' traD36pmAB+ k I q ZAMlS)
r e d thi pro hdrR4 (K m ~ + ) (R.4-2Tc-Mu-Km-Tn7) Tpr Smr
supE44 AlacU169 (080 kZAM15) h d 1 7 r d l endA 1 gyrA96 thi-1 relA 1 supE44 hsdS20 (m-m~-) red13 ara-14poA2 lacYl galK2 rpsL20 xyl-5 mtl-1 wildtype Kmr Sp/Smr
wildtype Amp'
Col-El replicon Kmr Mob+ Tra+ helper plasmid ~ m r
pMBl replicon Tn5 tnp RP4 oriT Gmr
incubation, single transconjugant colonies were isolated on the same medium.
Negative selection were done on modified salt medium unsupplemented with
vitamins with 5 mM benzoate as its C source.
Similar technique were used for tripvental mating in which E. coli JM109
(pTnMod-OGm), E. coli HBlOl @RK2013), and DS-4 were mixed together, but
LB agar containing gentamicin, and streptomicin and spectinomicin was used
instead of LB-kanamycin and LB-ampicillin.
Transformation of Flanking DNA.
Since no mutant was obtained from D M , the study was restricted only on
DS-8 mutants.
Suspected colony of knocked down genes by transposition were grown in
LB kanamycin and ampicillin broth overnight in 30°C at 200 rpm. Modified
phenolchloroform-isoamyIalcoho1 treatment, and ethanol precipitation were used
to extract the genomic DNA as described previously. The DNA were digested
with Kpnl'and transformed to DH5a using method as described by Sambrook et
al. (1989).
A 1-ml overnight culture of DH5a was subcultured in LB broth for 3 h.
The culture was harvested by centrdugation at 5000 rpm for 2 minutes at 4.C.
The supernatant was discharge. Pellet was resuspended in 200 ml of ice-cold 50
mM CaC12
+
50 mM Tris and incubated on ice for 20 minutes; The cells werepelleted by centrdugation at 5000 rpm for 2 minutes at 40C. The supernatant was
discharged. Pellet was resuspended in 250 ml of ice-cold 0.1 M CaC12 and
reincubated on ice for 10 minutes. KpnI-digested DNA was put into the
seconds. The tubes was rapidly placed on ice to cool for 60 minutes. The cells
were transferred into 2 rnl of SOC broth. The culture was incubated for 45-60
minutes at 370C to allow the cell to recover. A 50-100
ml
of the transformationmix were plated onto LB-kanamycin agar and incubated overnight.
Plasmid Preparation
In general, DNA plasmid minipreparation was done with Quantum
Prep" Plasmid Miniprep Kit (Bio-Rad, Hercules, CA). The preparation was
done as specified by the manufacturer.
Southern Hybridization
Total bacterial DNA was extracted as previously described (Sambrook et
al. 1989). After digested with KpnI, DNA were fractionated on 1.5O/0 agarose gel
in Ix TAE buffer. The gel was stained with EtBr and photographed under UV
illumination. DNA was denaturated by soaking the gel into denaturation solution
(1.5 N NaCl and 0.5 N NaOH) for 30 minutes at room temperature with
constant, gentle agitation and then rinsed briefly in deionized water.
Neutralization was done by soaking the gel for 15 minutes 2 times into the
neutralization solution p H 7.5 (1 M Tris and 1.5 N NaCl) at room temperature
with constant, gentle agitation.
DNA was transferred in 20x SSC to a nylon Zeta-Probe (Bio-Rad
Laboratories, CA) following NEBlot Phototope Kit protocol (New England
Biolabs, Inc. MA).
Hybridization of biotynilation labeled probes using to the blot was
performed as described in Phototope Detection Kit protocol. Random
biotynilated octamers were used to prime DNA synthesis in vitro from
denaturated double-stranded template DNA as described by NEBlot Phototope
Probe Labelling protocol.
Amplification Using
benA
PrimerPrimers for amplification of suspected benA gene from
S.
marcescens DS-8were designed based on available sequences of benA of Alcaligenes calcoaceticus and
Pseudornonasputzda obtained from GenBank database. Technique for ampldication
was described previously.
Genomic DNA (200 ng) of
S.
marcescens DS-8 were PCR-amplified usingb e d primers forward (5'-GTGCACTGGAACTA) and reverse (5'-
TCCTTCGTCTTC) using Ready-To-GO PCR Beads (Pharmacia-Biotech)
.
Agarose gel electrophoresis were used to purrfy the genomic DNA. Total volume
of PCR reaction (25 pl) consisted of 1.5 U
Taq
DNA Polymerase, 10mM Tris-HCl (pH 9 at room temperature), 50 mM KCI, 1.5 rnM MgCL, 200 pM of each
dNTPs and stabilizer including BSA. The reaction was incubated in a Gene Amp
CHAPTER
3.
SELECTION AND ISOLATION OF BACTERIA
FOR
BENZOATE DEGRADATION
ABSTRACT
Thuzy-four anoxygenic photosynthetic bacteria and 7 other environmental isolates from West Java and DXY were examined for their ability to grow anaerobically in light in monocydic aromatic compound, including benzoate, salysilate (2-hydro~~benzoate), phenol, gentisate (2,5-dihydroxybenzoate), and catechol (1,2-benzenediol). Five aerobic bacteria were tested for aerobic utilization of the monocyclic aromatic compounds. Twenty-seven out of 41 of isolated anoxygenic photosynthetic bacteria (65.8%) were able to grow in 5 rnM
benzoate. DS-1, DS-4, and Cas-13 of the purple non-sulfur bacteria and DS-8, MR1.2, and PG9.1 of the aerobic bacteria showed relatively short generation time. The ability of purple non-sulfur bacteria to grow in 5 rnM gentisate was firstly reported. MR1.2 was the only Gram positive isolate of the aerobic bacteria capable of growing in 1 mM atrazine (2chloro-4-ethylamino-6-isopropylamino-
1,3,5-triazine).
INTRODUCTION
Various bacteria show their ability to aerobically catabolize aromatic
hydrocarbon compounds (Shimao et al., 1989; Lenke et al., 1992; Miethling and
Karlson, 1996; Valenzuela et al., 1997), however Pseudomoms and Bacillus are the
most common bacteria that have gotten much attention for intensive study in
their aromatic degradation ability (Brenner et al., 1993; Shen and Wang 1995;
Molina et a/., 1998) and genetic properties (Orser and Lange, 1994; Powlowski
and Shingler, 1994; Williams and Sayers, 1994; Shields et al., 1995).
Unlike aerobic degradation of aromatic compounds, relatively less
information in anaerobic degradation of those compounds are available
(Genthner, 1989). However, previous works showed excellent ability of various
bacteria to anaerobically degrade the compounds. Anaerobic degradation of
benzoate and its derivates has mainly been concerned in anoxygenic photosynthetic
bacteria (APB) (Harwood and Gibson, 1988; Kamal and Wyndham, 1990; Wright
and Madigan, 199 1; Shoreit and Shabeb, 1994). The ability to utilize other aromatic
compounds has also been demonstrated. Blasco and Castillo (1992) observed that
Rhodobacter capsulatus E l F l degraded mononitrophenol and dinitrophenol with
acetate as
C
source. Rhodopseudomonaspalus~ was able to utilize various phenoliccompounds, hydroxylated and methoxylated aromatic, aromatic aldehyde, and
hydroaromatic acid (Harwood and Gibson, 1988). Rdp. palustris also showed the
ability to catabolism pyridine and pyrazine (Sasikala etal., 1994). The ability of APB
to grow in monocyclic aromatic both aerobically and anaerobically is one of the
(Harwood and Gibson, 1988; Kamal and Wyndham, 1990; Wright and Madigan,
1991; Shoreit and Shabeb, 1994).
The aim of this study is to screen and to isolate bacteria capable of uulivng
benzoate as their sole C-source aerobically and phototrophic anaerobically. Other
aromatic compounds includmg phenol, salysilate, and gentisate were also chosen as
substrates for their growth.
RESULTS
AND
DISCUSSIONThe result on ability of APB to grow in 5 rnM benzoate as their sole C
source showed that 15 isolates (36.6%) well grew, 12 isolates (29.3%) grew poorly,
and 14 isolates (34.1%) did not grow (Figure 1). Although some isolates grew
poorly, the number of exarninated isolates including environmental isolates that
capable of utilizing benzoate were moderately high (65.896) (Table 1). Thu
showed that the degradation of benzoate within the group were common in APB.
Degradative genes for the anaerobic benzoate utilization might be involved (Pelletier
and Harwood, 1998). Unlike the aerobic degradation of monocyclic aromatic
compound that yields catechol or its derivates as common intermediates before ring
cleavage (Williams and Sayers, 1994), the anaerobic degradation of this compound
produces 2-ketocyclohexane-lcarboxyl-CoA immediately before ring cleavage
(Pelletier and Harwood, 1998). Of the APB,
Rs*,
palustris were the most commonmember capable of u h g benzoate as its C source (Harwood and Gibson, 1988;
+ + + + excessive grow + + + well grow
+ + moderately grow
+
less grow not grow Ut untested? grow very slow, GT not calculated
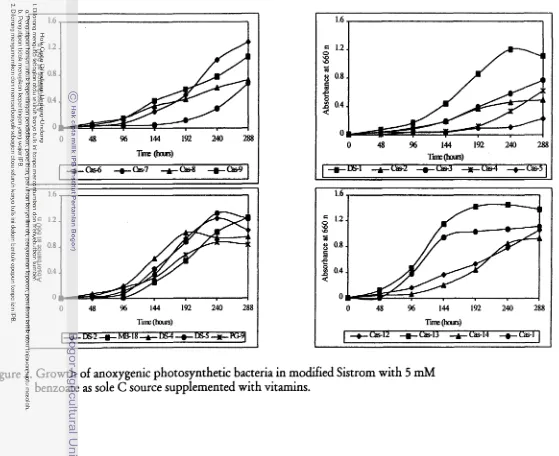
0 48 % 144 192 240 Tim (lntus)
Figure 2. Growth of anoxygenic photosynthetic bacteria in modified Sistrom with 5 mM