DIVERSITY OF CULTURABLE ENDOPHYTIC FUNGI
IN CINCHONA CALISAYA WEDD.: MOLECULAR
PHYLOGENY AND ALKALOID PROFILE
Nani Radiastuti
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
STATEMENT OF ORIGINALITY
I hereby certify that my Doctor dissertation entitled “Diversity of Culturable Endophytic Fungi of Cinchona calisaya Wedd.: Molecular Phylogeny and Alkaloid Profile” is my original work directed by the supervisory committee. It has not been and will not be submitted for award of any degree to any other university or institution. I declare that the intellectual content of this dissertation is the product of my own work. All data, tables, figures and text citations reproduced from any other source have been explicitly acknowledged. I hereby transfer the copyright of this writing to Graduate School of Bogor Agricultural University.
Bogor, August 2015
SUMMARY
NANI RADIASTUTI. Diversity of Culturable Endophytic Fungi in Cinchona calisaya Wedd.: Molecular Phylogeny and Alkaloid Profile. Under supervision of GAYUH RAHAYU, IMAN HIDAYAT, IZUMI OKANE and SUMINAR SETIATI ACHMADI
Quina tree (Cinchona spp.) has been known as a medicinal plant with anti-malaria properties due to its alkaloid metabolites called quinine. However, quinine production in Indonesia is currently limited and insufficient to support industry demand because of reduction of quina plantation area. Therefore, alternative sources for quinine production are urgently needed. Fungal endophyte has been known for their potential to produce similar metabolites yielded by their host. Therefore, in this study, diversity of fungal endophytes from C. calisaya and their potential in producing cinchona alkaloids such as quinine, quinidine, cinchonine and cinchonidine, were examined. The community structure of fungal endophytes living in the different organs of quina was also elucidated.
Fungal endophytes were isolated from healthy twigs, leaves, roots, barks, petioles, fruits, and flowers. Samples were collected from 5 quina trees of C. calisaya at the quina plantation managed by the Research Institute for Tea and Cinchona, Gambung, Ciwidey, West Java. Identification of the endophytic fungal isolates involving combination of molecular phylogenetic analysis based on ITS rDNA region and morphology characteristics. Multigene phylogenetic analysis was carried out to determine dominant isolates. Detection cinchona alkaloids production by the endophytic fungi was determined through HPLC analysis.
About 687 endophytic fungal strains were isolated and were grouped into 96 morphotypes. All strains belong to three classes, 11 families, 18 genera, and 37 species of Ascomycota. The largest class was Sodariomycetes (Five families, viz, Nectriaceae, Melanconiaceae, Hypocreaceae, Chaetosphaeriaceae, Diaporthaceae), followed by Dothidiomycetes (Five families, viz, Didymellaceae, Leptosphariaceae, Botryosphaeriaceae, Davidiellaceae, Mycosphaerellaceae), and Eurotiomycetes (A single family, Trichocomaceae). The identified species found from C. calisaya include Aspegillus sydowii, A. versicolor, Cladosporium oxysporum, Colletotrichum acutatum, Col. aenigma, Col. arxii, Col. boninense, Col. brasilliense, Col. crassipes, Col. gloeosporioides, Diaporthe beckhausii, D. endophytica, D. eucalyptorum, D. ganjae, D. helianthi, D. hongkongensis, D. infecunda, D. litchicola, D. phaseolorum, D. pseudomangiferae, D. psoraleae-pinnatae, Fusarium incarnatum, F. oxysporum, F. solani, Gliocladiopsis tenuis, Ilyonectria anthuricola, Leptosphaerina chartarum, Neofusicoccum parvum, Penicillium citrinum, Pestalotiopsis adusta, Phomopsis palmicola, Pho. tersa, Phyllosticta capitalensis, Pyrigemmula aurantiaca, Peyronellaea coffeae-arabicae, Trichoderma hamatum, and T. atroviridae.Some of the species were newly reported as endophytes in Cinchona.
psoraleae-pinnatae in petiole. This data suggested that the endophytic fungi have an ability to adapt with different microhabitat.
The community structure analyses revealed that the twig was colonized by large numbers of fungi. Shannon–Wiener diversity index indicates leaves and fruits hosted the most diverse species. Diaporthe spp. appeared as the dominant taxa and commonly found mostly in the twigs, even though all organs, except flower, can be occupied by the member of Diaporthe. Endophytes community occupied plant organs above the ground were more abundant and more varies than those inhabited the organs below the ground. Fungal endophytes in root formed distinct community from flower and other above ground organs. These findings provide important information on high diversity, distribution and species dominance of the endophytic fungi on medicinal plant, especially C. calisaya.
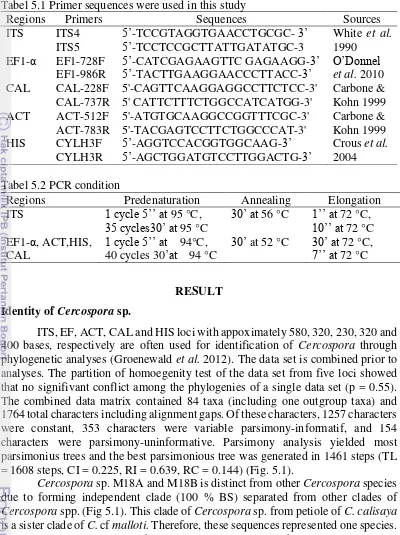
When the fungus was cultured on potato dextrose broth for 21 days in static condition, about 44 taxa were detected to produce quinine using HPLC analysis. These include Aspergillus (2 morphotypes), Cladosporium, Colletotrichum (3 morphotypes), Cercospora, Diaporthe (24 morphotypes), Fusarium (5 morphotypes), Leptosphaerulina (2 morphotypes), Neofusicoccum, Penicillium, Pestalotiopsis, Phomopsis, Phyllosticta, and Trichoderma. Quantitative analysis of quinine production indicated that Diaporthe sp. M13 and M70, and D. litchicola M21 appeared as the most promising strains in quinine production due to their highest quinine production. In addition, six morphotypes of Diaporthe (D. endophytica InaCC-F237, Diaporthe sp. InaCC-F235, InaCC-F236, InaCC-F238, InaCC-F239, InaCC-F2310) were capable in producing cinchonine and cinchonidine. Two morphotypes of Cercospora (M18a, M18b) and 5 strains of Fusarium (F. incarnatum M34, M66, M67; F. oxysporum M16; F. solani M93, M97) were found to produce quinine and cinchonine. The majority of these taxa are reported here as new taxa capable in producing quinine, cinchonine, and cinchonidine. This implied that endophytic fungi are potential as sources for quinine, quinidine and cinchonine and cinchonidine production.
Determination of several candidates new species were conducted using multi-gene approach based on sequence multi-generated from ITS rDNA, EF1-α, ACT, TUB, and HIS gene regions. Two strains of Cercospora indicated independent species but needed further morphological data to complete the identity of this candidate new species. Diaporthe spp. morphotype InaCC F-236, InaCC F-238, InaCC-F239, and InaCC F-2310 will also be proposed as candidates of new species based on combined analysis of ITS rDNA region and EF1-α gene. This study showed that multi-gene provides robust information and more accurate identity of endophytic fungi. When this approach failed for species identification, other genes should be included in phylogenetic analyses
Preliminary application of polyphasic approach in this study was limited to Diaporthe spp. The result indicated that alkaloids clustering did not correspond to the phylogenetic clustering, except for Diaporthe sp. M70–96 group. This means that cinchona alkaloids is rather strains dependent than species dependent.
RINGKASAN
NANI RADIASTUTI. Keanekaragaman Cendawan Endofit yang dapat Dikulturkan asal Cinchona calisaya Wedd.: Filogeni Molekular dan Profil Alkaloidnya. Dibimbing oleh GAYUH RAHAYU, IMAN HIDAYAT, IZUMI OKANE dan SUMINAR SETIATI ACHMADI
Pohon kina (Cinchona spp.) dikenal sebagai tanaman obat anti-malaria karena mengandung metabolit alkaloid yang disebut kuinina. Saat ini produksi kuinina di Indonesia jumlahnya terbatas dan tidak mencukupi kebutuhan industri disebabkan karena berkurangnya area perkebunan kina. Oleh karena itu, sumber alternatif produksi kuinina sangat diperlukan. Cendawan endofit diketahui memiliki potensi untuk memproduksi senyawa metabolit yang mirip atau sama dengan senyawa yang diproduksi oleh inangnya. Oleh karena itu, penelitian ini bertujuan untuk mengeksplorasi diversitas cendawan endofit dari Cinchona calisaya dan potensinya dalam memproduksi alkaloid sinkona seperti kuinina, kuinidina, sinkonina, dan sinkonidina. Analisis struktur komunitas cendawan endofit yang hidup di berbagai organ kina yang berbeda juga dilakukan.
Cendawan endofit diisolasi dari ranting, daun, akar, kulit kayu, buah, dan bunga yang sehat. Sampel dikoleksi dari 5 pohon kina C. calisaya yang tumbuh di perkebunan kina milik Pusat Penelitian dan Perkebunan Teh dan Kina, Gambung, Ciwidey, Jawa Barat. Identifikasi isolat cendawan endofit melibatkan kombinasi analisis molekular filogenetik berdasarkan daerah ITS rDNA dan karakteristik morfologi. Analisis filogenetik multigen dilakukan untuk mengidentifikasi isolat-isolat dominan. Deteksi produksi alkaloid sinkona oleh cendawan endofit ditentukan melalui analisis HPLC.
Sebanyak 687 strain cendawan endofit diisolasi dan dikelompokkan menjadi 96 morfotipe. Seluruh strain terbagi ke dalam 3 kelas, 11 famili, 18 genus, dan 37 spesies dari Ascomycota. Kelas terbesar adalah Sodariomycetes (5 famili: Nectriaceae, Melanconiaceae, Hypocreaceae, Chaetosphaeriaceae, Diaporthaceae), diikuti oleh Dothidiomycetes (5 famili: Didymellaceae, Leptosphariaceae, Botryosphaeriaceae, Davidiellaceae, Mycosphaerellaceae), dan Eurotiomycetes (1 famili: Trichocomaceae). Spesies yang teridentifikasi dari C. calisaya adalah Aspegillus sydowii, A. versicolor, Cladosporium oxysporum, Colletotrichum acutatum, Col. aenigma, Col. arxii, Col. boninense, Col. brasiliense, Col. crassipes, Col. gloeosporioides, Diaporthe beckhausii, D. endophytica, D. eucalyptorum, D. ganjae, D. helianthi, D. hongkongensis, D. infecunda, D. litchicola, D. phaseolorum, D. pseudomangiferae, D. psoraleae-pinnatae, Fusarium incarnatum, F. oxysporum, F. solani, Gliocladiopsis tenuis, Ilyonectria anthuricola, Leptosphaerina chartarum, Neofusicoccum parvum, Penicillium citrinum, Pestalotiopsis adusta, Phomopsis palmicola, Pho. tersa, Phyllosticta capitalensis, Pyrigemmula aurantiaca, Peyronellaea coffeae-arabicae, Trichoderma hamatum, and T. atroviridae. Beberapa spesies adalah catatan baru cendawan endofit yang berasal dari pohon kina.
di ranting, D. phaseolorum di buah, dan D. psoraleae-pinnatae di petiol. Data ini memberikan informasi bahwa cendawan memiliki kemampuan beradaptasi dengan mikrohabitat yang berbeda..
Analisis struktur komunitas menunjukkan bahwa ranting dikolonisasi lebih banyak jenis cendawan. Indeks diversitas Shannon-Wiener menunjukkan daun dan buah merupakan organ yang paling beragam spesies cendawan endofitiknya. Diaporthe spp. dominan hampir di seluruh organ kecuali bunga, dan banyak ditemukan di ranting. Komunitas cendawan endofit pada organ yang berada di atas tanah lebih melimpah dan lebih beragam daripada komunitas di bawah tanah. Komunitas cendawan endofitik pada akar sangat berbeda dari komunitas cendawan endofit pada organ lainnya yang berada di atas tanah. Hasil temuan ini memberikan kontribusi pada pengayaan informasi tentang keanekaragaman, distribusi, dan dominasi spesies dari cendawan endofit pada tanaman obat, khususnya C. calisaya. Hasil analisis HPLC menunjukkan bahwa sekitar 44 taxa terdeteksi memproduksi kuinina, terdiri dari:. Aspergillus (2 morfotipe), Cladosporium, Colletotrichum (3 morfotipe), Cercospora, Diaporthe (24 morfotipe), Fusarium (5 morfotipe), Leptosphaerulina (2 morfotipe), Neofusicoccum, Penicillium, Pestalotiopsis, Phomopsis, Phyllosticta, dan Trichoderma. Analisis kuantitatif menunjukkan bahwa Diaporthe sp. M13 and M70, dan D. litchicola M21 memproduksi kuinina dengan konsentrasi paling tinggi. Selain itu, enam morfotipe Diaporthe (D. endophytica InaCC-F237, Diaporthe sp. InaCC-F235, InaCC-F236, InaCC-F238, InaCC-F239, InaCC-F2310) mampu memproduksi kuinina, sinkonina dan sinkonidina. Dua morfotipe Cercospora (M18a, M18b) dan 5 strain Fusarium (F. incarnatum M34, M66, M67; F. oxysporum M16; F. solani M93, M97) mampu memproduksi kuinina dan sinkonina. Taksa pada studi ini merupakan catatan baru untuk cendawan yang mampu memproduksi kuinina, sinkonina, dan sinkonidina. Hal ini menunjukkan bahwa cendawan endofit dapat dijadikan sumber produksi alternatif untuk kuinina, kuinidina, sinkonina, dan sinkonidina.
Determinasi kandidat taksa baru dengan menggunakan pendekatan multi-gen berdasarkan sekuen daerah ITS rDNA, EF1-α, ACT, TUB, dan HIS menunjukkan bahwa dua strain Cercospora teridentifikasi secara molekuler sebagai independen spesies tetapi dibutuhkan data morfologi lebih lanjut untuk validasi kandidat spesies baru. Diaporthe spp. morfotipe InaCC F-236, InaCC F-238, InaCC-F239, dan InaCC F-2310 juga dipromosikan sebagai kandidat spesies baru berdasarkan analisis kombinasi daerah ITS rDNA dan gen EF1-α. Penelitian ini menunjukkan bahwa pendekatan multigen dapat memberikan informasi yang lebih tepat dan akurat. Ketika pendekatan ini gagal untuk identifikasi spesies, gen lain harus disertakan dalam analisis filogenetik.
Pendekatan awal aplikasi polifasik dalam penelitian ini terbatas pada Diaporthe spp. Hasil menunjukkan bahwa pengelompokkan alkaloid tidak mendukung pengelompokan filogenetik, kecuali untuk Diaporthe sp. M70–96. Hal ini berarti bahwa produksi alkaloid sinkona lebih tergantung pada strain daripada spesies.
© Copyright IPB, 2015
This work is under copyright of IPB 2015. No part of this work can be copied without citing the source. Citation is allowable solely for education, research, sciencetific paper writing, reporting, assay or review; and the citation will not cause liability to IPB
DIVERSITY OF CULTURABLE ENDOPHYTIC FUNGI
IN CINCHONA CALISAYA WEDD.: MOLECULAR
PHYLOGENY AND ALKALOID PROFILE
Nani Radiastuti
A dissertation submitted to the departement of Biology and the comitted on graduate school of Bogor Agriculture University in partial fulfillment of the requirements for
the degree of doctor
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
Examiner for internal examination :
1. Prof (R). Dr. Partomuan Simanjuntak M.Sc (Peneliti Utama Bioteknologi LIPI)
2. Dr. Wibowo Mangunwardoyo M.Sc (Dosen Departemen Biologi FMIPA UI)
Examiner for external examination :
1. Prof (R). Dr. Partomuan Simanjuntak M.Sc (Peneliti Utama Bioteknologi LIPI)
Dissertation Title : Diversity of Culturable Endophytic Fungi in Cinchona calisaya Wedd.: Molecular Phylogeny and Alkaloid Profile.
Name : Nani Radiastuti
NIM : G361100061
Study Program : Microbiology
Approved by
Dr. Ir. Gayuh Rahayu Principal Advisor
Dr. Iman Hidayat Izumi Okane Ph.D Prof. Ir. Suminar Setiati Achmadi Ph.D Advisor committee Advisor committee Advisor committee
Signed by
Head of Microbiology Study Program
Prof. Dr. Anja Meryandini MS Dr. Ir. Dahrul Syah M.Sc.Agr
Date of examination: 31st July 2015 Date of graduation:
Date of Promotion : 19th August 2015
ACKNOWLEDGEMENTS
First and foremost I want to thank God for blessing me to finish writing dissertation entitle: Diversity of Culturable Endophytic Fungi of Cinchona calisaya Wedd.: Molecular Phylogeny and Alkaloid Profile. The research work were started in September 2012.
I want to express my most sincerely gratitude to the advisory committe Dr Gayuh Rahayu, Dr Iman Hidayat, Izumi Okane Ph D, Prof Ir Suminar Setiati Achmadi Ph D for their contribution of time, and ideas to make my experience productive and stimulating.
I would like to extent my gratitude to all members of Division of Microbiology, centre for Biology. The Indonesian Institute of Science, Laboratory of Mycology (IPBCC) researchers, and my colleagues that have contributed immensely to my personal and professional time at Bogor Agricultural University during doctor course.
I would like to express my sincere gratefull to all the lecturers, administrative staffs of post graduate program of Bogor Agriculural University for their advisory, assistance and help during my study.
I gratefully acknowledge the funding sources that made my research doctor work possible. I was funded by the Indonesian ministry of education through post graduate study grant. This project was supported by The Japan Science and Technology Agency (JST), The Japan International Cooperation Agency (JICA), The Indonesian Ministry of Education, Directorate General of Higher Education (DIKTI), Directorate General of Islamic Higher Education (DIKTIS), and State Islamic University Syarif Hidayatullah Jakarta.
I would to thank my husband Amsal Bakhtiar and my children Reani Zulfa, Amelia Mirzanti, Daraswati Zakirah, my friends, and my students for their love, encouragement, and moral support.
I wish this study contribute to Indonesian megadiversity the database of science and to the technology development in Indonesia particularly on exploiting use of tropical microfungi.
CONTENTS
LIST OF TABLES ii
LIST OF FIGURES iv
LIST OF APPENDICES v
1 GENERAL INTRODUCTION
Background 1
Objectives 3
Benefit 3
2 PHYLOGENETIC STUDY OF ENDOPHYTIC FUNGI OF CINCHONA CALISAYA
Introduction 5
Materials and methods 6
Result 8
Discussion 17
Conclusion 19
3 COMMUNITY STRUCTURES OF FUNGAL ENDOPHYTES IN CINCHONA CALISAYA
Introduction 20
Materials and methods 20
Result 21
Discussion 25
Conclusion 27
4 ALKALOID PROFILE OF ENDOPHYTIC FUNGI FROM CINCHONA CALISAYA
Introduction 28
Materials and methods 29
Result 30
Discussion 33
Conclusion 36
5 DETERMINATION OF NOVEL SPECIES CANDIDATES USING MULTIGENE–APPROACH
Introduction 38
Materials and methods 38
Result 39
Discussion 59
Conclusion 65
6 GENERAL DISCUSSION 66
7 GENERAL CONCLUSION 70
8 REFERENCES 71
10 APPENDICES
83
2.1 GeneBank ITS accession number, strains, and sources of fungal endophytes found in this study
8
2.2 GeneBank ITS accession number of reference strains used in this study
11
3.1 Endophytic Ascomycota isolated from Cinchona calisaya 21 4.1 Cinchona alkaloid production of endophytic Cercospora sp. 31 4.2 Cinchona alkaloid production of endophytic Diaporthe spp 34 4.3 Cinchona alkaloid production of endophytic Fusarium sp. 34 5.1 Primer sequences were used in this study 39
5.2 PCR condition 39
5.3 Thirty nine strains of endophytic Diaporthe spp. in C. calisaya 43 5.4 Comparison of morphological characters of Diaporthe spp. from C.
calisaya used in this study
51
5.5 Morphological comparison of the structures of three endophytic fungi Fusarium species associated with C. calisaya
58
LIST OF FIGURES
2.1 Maximum Parsimony phylogenetic tree showing the relationship between endophytic fungi from all of plant organ C. calisaya and related fungi and plants based on the sequences of 5.8S of rDNA.
16
3.1 Colonization rate of endophytic fungi in various organs of C. calisaya
22
3.2 The Shannon–Wiener diversity index of endophytic fungi in each plant organ
23
3.3 Frequency of occurrence of endophytic fungal species in each plant organs
24
3.4 Cluster fungal endophyte community in C. calisaya 25 4.1 Chemical structures of cinchona alkaloid 29 4.2 Quinine production of endophytic fungi in C. calisaya. 32 5.1 Phylogenetic tree based on combination of ITS, EF1–α, ACT, CAL
and HIS genes region representing placement of two sequences of Cercospora spp. from petiole organ of C. calisaya within Cercospora spp. of Groenewald et al. 2012.
41
5.2 Morphological characters of Cercospora sp. on C. calisaya 10– days culture on PDA.
42
5.3 Maximum-parsimony tree showing a relationship between endophytic fungi of Diaporthe spp. and references based on the sequences of combine between ITS5–5.8S–ITS4 of nuclear rDNA and EF1–α gene, and rooted with Diaporthella corylina CBS 121124.
45
5.4 Phylogenetic tree based on combination of ITS and partial EF1–α genes region representing placement of six sequences of Phomopsis spp. from different organ of C. calisaya within Diaporthe spp. of Gomes et al. 2013.
49
5.5 Morphological characters of Diaporthe cinchonae sp. nov. on C. calisaya for 7 days in PDA.
5.6 Morphological characters of Diaporthe endophytica on C. calisaya. for 7 days in PDA.
51
5.7 Morphlogical characters of Diaporthe sp. on C. calisaya for 7 days in PDA.
51
5.8 Maximum-parsimony tree showing the relationship between endophytic Fusarium sp. based on the sequnces of ITS of rDNA and Penicillium citrinum isolate AX4602 is taken as outgroup
53
5.9 Maximum-parsimony tree showing the relationship between endophytic Fusarium sp. based on the sequnces of EF1–α and Penicillium citrinum isolate AX4602 is taken as outgroup
54
5.10 Morphological character of Fusarium oxysporum M16 on C. calisaya for 7 days in PDA.
55
5.11 Morphological characters of Fusarium incarnatum M34 on C. calisaya for 7 days in PDA.
55
5.12 Morphological characters of Fusarium incarnatum M66 on C. calisaya for 7 days in PDA.
56
5.13 Morphological characters of Fusarium incarnatum M67 on C. calisaya for 7 days in PDA.
56
5.14 Morphological characters of Fusarium solani M93 on C. calisaya for 7 days in PDA.
57
5.15 Morphological characters of Fusarium solani M97 on C. calisaya. for 7 days in PDA
57
6.1 Clusters of endophytic Diaporthe spp. based on the similarity on their alkaloid profile
68
LIST OF APPENDICES
1.1 Schematic diagram of the research 83
2.1 Cultural characteristics of the endophytic fungi from C. calisaya on PDA
83
2.2 Colony of the endophytic fungi from C. calisaya on PDA 87 4.1 Alkaloid profiles of endophytic Diaporthe spp.
Colletotrichum spp., and others endophytic fungi
93
4.2 Cinchona alkaloid production of endophytic fungi in C. calisaya
109
434 4.3
HPLC analysis of quinine, quinidine, cinchonine and cinchonidine standard
112
4.4 HPLC analysis of endophytic fungi Cercospora sp. on quinine, quinidine, cinchonine and cinchonidine product
113
4.5 HPLC analysis of endophytic fungi Diaporthe sp. of quinine, quinidine, cinchonine and cinchonidine product
113
4.6 HPLC analysis of endophytic fungi Fusarium spp. of quinine, quinidine, cinchonine, cinchonidine product
115
4.7 GeneBank ITS and EF accession number of Fusarium spp. included in this study
116
4.8 GeneBank ITS, EF, ACT, CAL, and HIS accession number of Cercospora spp. included in this study
4.9 GeneBank ITS and EF1–α accession number of Diaporthe sp., D. cinchonae, D. endophytica of fungal isolates included in this study
122
1
GENERAL INTRODUCTION
Quina tree (Cinchona spp., Rubiaceae) is a medicinal plant, native to the Andes forest of Western South America. Local people used this plant for fever treatment. In 1820, Pelletier and Caventou extracted an alkaloid substance called quinine, from the Cinchona bark (Achan et al. 2011). Untill now, quinine is used as a primary drug for the treatment of severe malarial illness caused by Plasmodium falciparum infection (WHO 2014) in about 80% of malaria endemic countries. Quinine is also used as a food colorant, beverage flavor and raw material for some other chemical industries (Santoso et al. 2004). Therefore, quinine is one of the world’s economically important substances from plant.
As the demand for the quina bark has increased, Cinchona was domesticated and cultivated out side South America. Cultivation was started in the British and Dutch colonial area in Eastern Africa, Indonesia, Ceylon, India, and the Philippines (Dawson 1991). Weddell in 1848, brought some seeds of Cinchona calisaya Wedd. [syn. C. ledgeriana (Howard) Bern. Moens ex Trimen], from Bolivia to Paris. These are the first quina tree grown in Europe, and later the seeds were also sent to Algiers and Java (Taylor 1975). In 1852, during Dutch colonization in Indonesia, Quina seeds were sent from Bolivia to Java. The seeds were first planted in two places, Cibodas Botanical Garden and Gambung, West Java, Indonesia (Tao & Taylor 2011). Quina trees grow well in the tropics. At present, the area of Quina plantation in Indonesia is approximately 3.886 hectares which previously planted 12.000 hectares (Susilo 2011) and the largest area is in Java.
The quinine industry in Indonesia has existed before the Second World War and Indonesia had been recognized as the world's largest supplier of quinine (Susilo 2011). The bark production from the plantation only field 30–50 % of demand of the industry (Susilo 2011). This supply shortage is filled with the imported bark flake. Even though, Quina plantation in Indonesia is now under replanting program, an alternative source for quinine production has to be investigated. Therefore, it is necessary to explore natural resources for quinine production other than the Quina tree. One of the promising resources is endophytic fungi.
Endophytes are microbes, that for at least one periode of their life cycle, inhabit the internal plant tissues without causing apparent harm to the host (Petrini 1991). A higher diversity of endophyte was found in tropical plants (Banerjee 2011). Endophytic fungi that have been examined to date are found in all major plant lineages such as trees, shurbs, grasses, and ferns (Arnold et al. 2001). Almost all plant species are inhabiting one or more endophytic organisms (Tan & Zou 2001). These colonize both vegetative (leaves, petioles, bark or stems) and reproductive parts of their hosts (Arnold et al. 2003, 2007, Qiu et al. 2008).
indica and A. lactiflora (Asteraceae) became the source of endophytic Alternaria, Colletotrichum, Phomopsis dan Xylaria (Huang et al. 2009) and Colletotrichum sp. endophyte of A. annua has the capability to produce secondary metabolites that act as anti–microbes (Lu 2000). Suwannarach (2013) found Col. gloeosporioides, Col. acutatum, Phomopsis spp., Guignardia mangiferae and xylariaceous taxa from Cinnamomm bejolghota. Phoma-like species endophytes of Cin. mollisimum showed cytotoxic and antifungal activities (Santiago et al. 2011). Orlandelli (2012) isolated the predominant Bipolaris from Piper hispidum and Garcia et al. 2012 obtained Cochliobolus, Alternaria, Curvularia, Diaporthe, Phomopsis and Phoma from the medicinal plant Sapindus saponari. Guignardia mangiferae, Fusarium proliferatum, and Col. gloeosporioides from Taxus media produce taxol, a potency anti–cancer drug (Xiong et al. 2013). Another fungus, Col. gloeosporioides from Justicia gandarusa was also found to be capable of producing taxol (Gangadevi & Muthumary 2008). Aspergillus, Cladosporium, Fusarium, Nectria, Penicillium and Verticillium were isolated from Panax ginseng and Fusarium spp. were reported to produce saponin as having an antimicrobial substance (Wu et al. 2013). Therefore, the exploration of endophytic fungi from Quina tree would provide the opportunity to get a potency strain for producing quinine.
Quina tree including C. calisaya may have endophytic fungi. The diversity of endophytic fungi on Cinchona plant has not been intensively studied. The information available is scattered and limited to only that from bark and young stem (Simanjuntak et al. 2002, Mumpuni et al. 2004, Winarno 2006, Maehara 2010). Some of these endophytic fungi have been screened for secondary metabolites such as cinchona alkaloids (quinine, quinidine, cinchonine and cinchonidine). The endophytic Diaporthe from Cinchona bark can produce these substances (Maehara et al. 2012, 2013) since bark contains the highest quinine level besides other alkaloids such as quinidine, cinchonine, and cinchonidine (Taylor 1975).
Quinine is not only present in the bark of Cinchona, but also as reported by Simanjuntak et al. (2002) and Shibuya et al. (2003), that the bark of Cinchona contains the highest concentration of quinine, while root, stem, twig, leaf, and flower contain quinines in various concentrations. Whilest the information on fungal endophyte from bark and young stem is available, that from other organ is still lacking. Therefore, study on the community structure of fungal endophyte from all organs of Cinchona is carried out to get a more comprehensive information on its diversity and further to analyze its potency to produce quinine.
As the bio-prospect of the endophyte is attractive for industry, the fungal isolate as the agents of metabolites production should be obtained and identificated accurately. Therefore, the fungal endophytes community is estimated using a culture-dependent method. Limitations of this method have been reported such as sterilia mycelia of fungal cultures making the identification is difficult (Huang et al. 2008, Guo et al. 2000). This limitation is overcome to some extent by applying molecular approach on the bases of a single analysis, a multigene analyses (Zhang et al. 1998, Huang et al. 2009) or a polyphasic approach (Samson et al. 2007). However, Ganley et al. (2004) considered that the identification of endophytic fungi using available taxonomic method is often difficult as most of the endophytic fungi isolates tend to form complex species.
Bhagobaty & Joshi 2011; Frisvad 2015). Fungal chemotaxonomy based on secondary metabolites has been used successfully in ascomycetous fungi such as Alternaria, Aspergillus, Fusarium, Hypoxylon, Penicillium, Stachybotrys, and Xylaria (Frisvad et al. 2008). Yet, the presence of secondary metabolite, such as quinine substances in endophytic fungi from C. calisaya has not been known. Furthermore, the taxonomical value of that secondary metabolite has not been known either. Therefore, distribution of the secondary metabolite from endophytic fungi of Cinchona is studied. In this study, the secondary metabolites was investigated the presence of the cinchona alkaloid.
The information on the diversity of endophytic fungi from C. calisaya and its potential to produce cinchona alkaloids is still limited. A comprehensive study on the aspect of biodiversity of endophytes of C. calisaya and their potency for secondary metabolites necessary to be studied covering:
a. Phylogenetic study of culturable endophytic fungi using ITS rDNA region. b. Community structure of the fungal endophytes and their distribution within host
organs.
c. Discovery of the novel taxa that will contribute to the data of the fungal biodiversity of the potensial indigenous species.
d. Analyses of the potential endophytes to produce the cinchona alkaloid (quinine, quinidine, cinchonine and cinchonidine).
e. Evaluation of polyphasic approach for species delimitation of endophytic fungi by incorporating data.
The research was conducted by following schematic diagram (Appendix 1) that was designed for achieving the aims of this research, which are:
a. To get an inventory of the culturable endophytic fungi from the whole organs of C. calisaya with a correct scientific name. The culturable of endophytic in C. calisaya can be the first data of culture collection in Quina tree.
b. To investigate the diversity and community structure of fungal endophyte in C. calisaya. The community structure can provide an information about the diversity, composition, and dominance of endophytic fungi in C. calisaya. c. To analyse the capability of the endophytic fungi, in particular the dominant one
to produce alkaloid substances. The capability of the endophytic fungi can be use to produce cinchona alkaloid and replacing the bark of Quina tree.
By understanding the diversity of endophytes of C. calisaya and its potency to produce secondary metabolites. It is expected that knowledge on the endophyte-plant associations especially those on the roles of endophyte in secondary metabolite production of the host, will be improved and the opportunity to manipulate their potential for human benefit will be increased. This research is expected to have impacts on:
a. The increase of indigenous fungal culture collection Indonesia with accurate identity deposited in culture collection such as InaCC and IPBCC.
b. An overview on biodiversity of indigenous fungal endophytes indigenous Indonesia from C. calisaya.
c. Information on alkaloid profiles of endophytic fungi from C. calisaya.
d. Potential strains for further investigation in the purpose of sustainable production of quinine and other related substances.
publication (submitted).
2 PHYLOGENETIC STUDY OF ENDOPHYTIC FUNGI OF
CINCHONA CALISAYA
INTRODUCTION
Quina (Cinchona spp.) is an important medicinal plant that have been used since the 16th century. Quina tree species that is widely cultivated in Indonesia is Cinchona calisaya. Currently, C. calisaya is used as a section stock for multiplication of commercial Quina clone for quinine production.
Studies on endophytic fungi from Cinchona plant have been reported by several researchers in Indonesia. All the research were mainly dealt with those from bark and young stem (Simanjuntak et al. 2002, Shibuya et al. 2003, Mumpuni et al. 2004 and Winarno 2006). However, fungal endophyte may occupy leaves, stems, petioles, barks, and roots from many Angiosperm taxa of tropical plants (Benerjee 2011). Some researches on endophytic fungi from medicinal plants such as leaves, stems and inflorescences of Artemisia capillaris, A. indica, A lactiflora (Huang et al. 2009), and Annona squamosa (Lin et al. 2010), Piper hispidum leaves (Orlandelli 2012), bark and leaves of Taxus media (Xiong et al. 2013), root of Panax ginseng (Wu et al. 2013) have been reported. Therefore, all organs of medicinal plants can be source of endophytic fungi. Previous study on fungal endophytes of Cinchona spp. is restricted on those from certain part of the tree, and none of them concerned with fungal endophyte from all part of the tree. Exploration of fungal endophyte C. calisaya from the whole part of the tree was conducted in this study.
Most of the isolate have not been identified into species and when identification was made they mainly used morphological approach or combined with BLAST search. Thus, none of those research is on based phylogenetic. A phylogenetic based approach had strengthened our classification and identification. These study reports the diversity and phylogenetic relationship of endophytes from C. calisaya.
MATERIALS AND METHODS
Specimen collections
Specimens were collected from Quina tree grown in Quina germ plasm orchard managed by the Research Center for Tea and Quina, Gambung, West Java, Indonesia (29 September 2012). Sampling location 7°8'35.78"S, 107°30'59.55"E, 1400 m asl. Samples collected include flowers, leaves, petioles, stems, barks, and roots of C. calisaya. Five pieces of each healthy organs from 5 individual plants were taken and placed in zipped plastic bags. The plastic bags were sealed and labelled with the name of the host, collection site, date, and collectors. All specimens were kept in ice boxes prior to isolation in the laboratory.
Isolation
The isolation protocol of endophytic referred to the method described by Mostert et al. (2001) with modification. The samples were first washed in running tap water, than surface-sterilized using 70 % ethanol for 1 minute, followed by soaking in sodium hypochlorite 3 % for 2 minutes, and 70 % ethanol for 20 seconds. The samples were rinsed 3 times in sterile distilled water, and dried with sterile paper for at least 6 hours. The sterile distilled water of the final rinse was poured onto the agar medium as a quality control of sterilization process. After drying, samples were cut into segments approximately 1 × 1 cm and placed on the surface of malt extract agar (MEA) (Difco, USA) (4 segments/petri dish). All petri dish were incubated at room temperature (27 oC). Three replications were made for each sample. The growth of endophytic fungi mycelium were observed every day, for about 30 days. The growing colonies were purified using hyphal tip isolation method to get a pure culture.The working cultures were kept in potato dextrose agar (PDA Difco, USA) agar slant. The culture stock were preserved in glycerol-trehalose, kept in –80 °C in Institut Pertanian Bogor Culture Collection.
Microscopic observation
Colony characters of each isolate was determined from 14 days old culture grown on PDA.The colony characteristics observed include diameter, color of the surface and reverse, margin, texture and zonation. Microscopic structures, such as conidia, conidiophores were examined by using Olympus BX53 light microscope (OLYMPUS, Japan) under 1000× magnification using immersion oil (Barnett & Hunter 1998).
DNA extraction
250 µL chloroform, 250 µL phenol, and then centrifuged at 10.000 rpm for 10 minutes. Isopropanol (in concentration of half volume of the supernatant) was added into supernatant, and centrifuged at 10.000 rpm for 10 minutes. The supernatant discarded. The pellet was added by 100 µL ethanol 99 % and then centrifuged at 10.000 rpm for 10 minutes. The supernatant was discarded and dried for 30 minutes. The pellet was added by 50 µ L nuclease free water.
PCR amplification and sequencing
Amplification was done using Polymerase Chain Reaction (PCR) method performed in a 25 µ L reaction volume as follow: 10 µ L nuclease free water, 12.5 µL DreamTaq® green master mix (Thermo scientific, USA), 0.5 µL of forward and reverse primer, 0.5 µ L DMSO, and 1 µ L DNA template. The primer used for all strains is ITS, and the second primer varies among fungal genera found.
The primer pairs of ITS5 (forward) (5’–TCCTCCGCTTATTGATATGC –3’) and ITS4 (reverse) (5'–TCCGTAGGTGAACCTGCGC–3') (White et al. 1990) were used to amplify the Internal Transcribed Spacer (ITS) region including 5.8S rDNA. The PCR condition was 90 seconds at 95 °C for pre-denaturation followed by 35 cycles of 30 seconds at 95 °C denaturation, 30 seconds at 55 °C for annealing, 90 seconds at 72 °C for extension, and 5 minutes at 72 °C for final extension.
All PCR reactions were conducted using T100 thermal cycler (Bio-Rad, USA). PCR products were electrophorised in a 1 % (w/v) agarose gel soaked in 1× TAE buffer at 100 Volt for 30 minutes. 1 kb DNA ladder was used as a marker during the electrophoresis. The gel was soaked in EtBr (ethidium bromide) for 30 minutes prior to UV light examination using Gel Doc XR system (Bio–Rad, USA). Purified PCR products were sent to 1stBASE (Malaysia) for sequencing.
Phylogenetic analysis
Nucleotide sequences obtained from the respective primer pairs (ITS5 and ITS4) were examined and refined by direct examination using Chromas Pro 1.41 software (Technelysium Pty Ltd., Australia). Newly ITS sequences of endophytic from C. calisaya were aligned with sequence from NCBI using MUSCLE (Edgar 2004) implemented in MEGA 6 (Tamura et al. 2013). Species was used as outgroup in analyses. Regions designated as ambiguously aligned were excluded from the analyses. GeneBank accession number, strain code, and taxon names used in this study.
homogeneity test (Farris et al. 1994) with 1000 replicates. TreeGraph 2 software (Stöver & Müller 2010) was used to refine the phylogenetic tree.
RESULTS
In total 687 endophytic fungal isolates consisting 123 isolates from leaves, 67 isolates from petioles, 239 isolates from twigs, 49 isolates from barks, 56 isolates from roots, 17 isolates from flowers, and 136 isolates from fruits were obtained from 700 segments of C. calisaya. These cultures are grouped into 96 morphotypes (Appendix 2.1; 2.2) on the basis of their colony morphology and growth rate. Most of these morphotypes are mycelia sterilia. Therefore, molecular techniques on the bases of ITS5–5.8S–ITS4 sequence is used as primary approach for identifying them to either generic or specific level. ITS5–5.8S–ITS4 sequences of 96 morphotype were compared to 73 corresponding sequences of reference fungal taxa extracted from the genbank database and was rooted to Saccharomyces cerevisiae strain ATCC 18824T and CBS 1171 (Table 2.1). In this non-coded dataset, there were 860 total variable characters that composed of 230 constant characters, 65 variable characters parsimony-uninformative and 565 variable parsimony– informative characters. Heuristic search resulted most parsimonious trees with length = 4541, consistency index (CI) = 0.300, retention index (RI) = 0.759, rescaled consistency index (RC) = 0.228, and homoplasy index (HI) = 0.700. One of the most parsimonious tree is shown (Fig. 2.2).
The phylogenetic analysis showed that all morphotypes are included in the Ascomycota. This analysis not only showed that the diversity of the endophytic fungi associated with C. calisaya but also illustrate the phylogenetic placement of these endophytes within Ascomycota tree. Sodariomycetes (78.2 %) represents the largest group, followed by Dothidiomycetes (14.1 %) and Eurotiomycetes (7.7 %).
The fungal endophytes from C. calisaya is distributed in 2 main clades. The first main clade is a monophyletic Pestalotiopsis (Sordariomycetes, Xylariales, Amphisphaeria-ceae) that supported with 100 % bootstrap (BS) value. Strain M80 is within this clade, thus this identified as Pestalotiopsis sp.
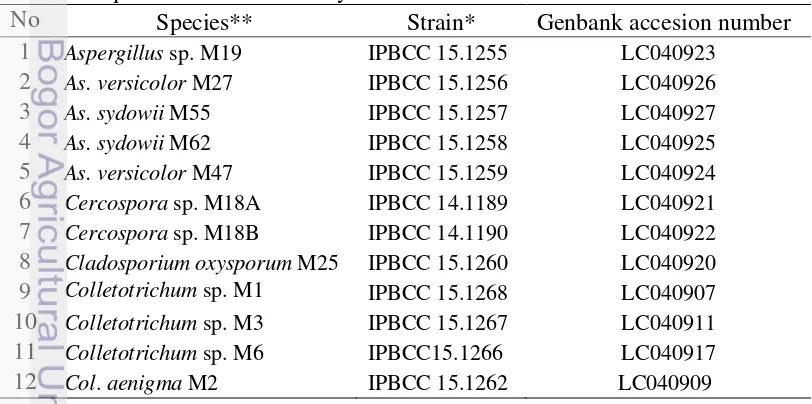
Table 2.1 GeneBank ITS accession numbers strains of fungal endophytes and sequence used in this study
No Species** Strain* Genbank accesion number 1 Aspergillus sp. M19 IPBCC 15.1255 LC040923
2 As. versicolor M27 IPBCC 15.1256 LC040926
3 As. sydowii M55 IPBCC 15.1257 LC040927
4 As. sydowii M62 IPBCC 15.1258 LC040925
5 As. versicolor M47 IPBCC 15.1259 LC040924
6 Cercospora sp. M18A IPBCC 14.1189 LC040921
7 Cercospora sp. M18B IPBCC 14.1190 LC040922
8 Cladosporium oxysporum M25 IPBCC 15.1260 LC040920
9 Colletotrichum sp. M1 IPBCC 15.1268 LC040907
10 Colletotrichum sp. M3 IPBCC 15.1267 LC040911
11 Colletotrichum sp. M6 IPBCC15.1266 LC040917
No Species** Strain* Genbank accesion number 13 Col. gloeosporioides M4 IPBCC 15.1269 LC040912
14 Col. gloeosporioides M7 IPBCC 15.1270 LC040919
15 Col. acutatum M57 IPBCC 15.1271 LC040908
16 Col. arxii M53 IPBCC 15.1272 LC040916
17 Col. boninense M28 IPBCC 15.1264 LC040914
18 Col. boninense M45 IPBCC 15.1264 LC040914
19 Col. brasiliense M76 IPBCC 15.1265 LC040913
20 Col. crassipes M30 IPBCC 15.1263 LC040918
21 Col. crassipes M82 IPBCC 15.1261 LC040910
22 Diaporthe sp. M9 IPBCC 15.1286 LC041055
23 Diaporthe sp. M12 IPBCC 15.1309 LC041062
24 Diaporthe sp. M13 IPBCC 15.1292 LC041020
25 Diaporthe sp. M14 IPBCC 15.1291 LC041028
26 Diaporthe sp. M15 IPBCC 15.1308 LC041049
27 Diaporthe sp. M22 IPBCC 15.1307 LC041030
28 Diaporthe sp. M23 IPBCC 15.1303 LC041042
29 Diaporthe sp. M33 IPBCC 15.1282 AB899787
30 Diaporthe sp. M38 IPBCC 15.1284 LC041019
31 Diaporthe sp. M39 IPBCC 15.1338 LC041060
32 Diaporthe sp. M41 IPBCC 15.1311 LC041054
33 Diaporthe sp. M42 IPBCC 15.1277 LC041035
34 Diaporthe sp. M43 IPBCC15.1276 AB899784
35 Diaporthe sp. M44 IPBCC 15.1293 LC041052
36 Diaporthe sp. M48 IPBCC 15.1310 LC041053
37 Diaporthe sp. M52 IPBCC 15.1300 LC041024
38 Diaporthe sp. M59 IPBCC 15.1284 LC041023
39 Diaporthe sp. M65 IPBCC 15.1290 LC041057
40 Diaporthe sp. M69 IPBCC 15.1287 LC041056
41 Diaporthe sp. M70 IPBCC 15.1304 LC041034
42 Diaporthe sp. M72 IPBCC 15.1340 LC041038
43 Diaporthe sp. M74 IPBCC 15.1306 LC041029
44 Diaporthe sp. M79 IPBCC 15.1289 LC041052
45 Diaporthe sp. M85 IPBCC 15.1284 LC041023
46 Diaporthe sp. M89 IPBCC 15.1288 LC041058
47 Diaporthe sp. M91 IPBCC 15.1283 LC041044
48 Diaporthe sp. M96 IPBCC 15.1305 LC041048
49 D. beckhausii M37 IPBCC 15.1275 LC041024
50 D. beckhausii M73 IPBCC 15.1273 LC041016
51 D. beckhausii M54 IPBCC 15.1274 LC041031
52 D. eucalyptorum M46 IPBCC15.1294 AB899785
53 D. eucalyptorum M81 IPBCC15.1295 LC041021
54 D. eucalyptorum M56 IPBCC 15.1296 LC041022
No Species** Strain* Genbank accesion number 56 D. endophytica M20 IPBCC 15.1315 LC041025
57 D. helianthi M21 IPBCC 15.1314 LC041026
58 D. ganjae M71 IPBCC 15.1313 LC041037
59 D. hongkongensis M31 IPBCC 15.1278 AB899786
60 D. hongkongensis M36 IPBCC 15.1279 LC041046
61 D. infecunda M63 IPBCC 15.1316 LC041032
62 63
D. infecunda M68
D. litchicola M78
IPBCC 15.1317 IPBCC 15.2997
LC041033 AB899788
64 D. lithicola M88 IPBCC 15.1298 LC041036
65 D. palmicola M11 IPBCC 15.1339 LC041017
66 D. phaseolorum M40 IPBCC 15.1318 LC041040
67 D. phaseolorum M10 IPBCC 15.1319 LC041043
68 D. pseudomangiferae M24 IPBCC 15.1299 LC041041
69 D. psoraleae-pinnatae M94 IPBCC 15.1320 LC041018
70 D. psoraleae-pinnatae M92 IPBCC 15.1321 LC041045
71 D. psoraleae-pinnatae M84 IPBCC 15.1322 LC041047
72 D. psoraleae-pinnatae M32 IPBCC 15.1323 LC041050
73 D. psoraleae-pinnatae M77 IPBCC 15.1324 LC041039
74 Fusarium incarnatum M34 IPBCC 15.1253 LC026132
75 F. incarnatum M66 IPBCC 15.1251 LC026133
76 F. incarnatum M67 IPBCC 15.1252 LC026134 77 F. solani M93 IPBCC 15.1248 LC026135 78 F. solani M97 IPBCC 15.1249 LC026136 79 F. solani M8 IPBCC 15.1247 LC026137 80 F. oxysporum M16 IPBCC 15.1250 LC026138 81 Gliocladiopsis tenuis M49 IPBCC 15.1325 LC040899 82 llyonectria sp.M64 IPBCC 15.1329 LC040895 83 Leptosphaerulina chartarum
M83 IPBCC 15.1331 LC040928
84 L. chartarum M87 IPBCC 15.1330 LC040929 85 Neofusicoccum chordaticola
M17 IPBCC 15.1332 LC040894
86 Penicillium citrinum M51 IPBCC 15.1333 LC040893 87 Pestalotiopsis sp. M80 IPBCC 15.1334 LC040892 88 Peyronellaea coffeae arabicae
M58 IPBCC 15.1336 LC040891
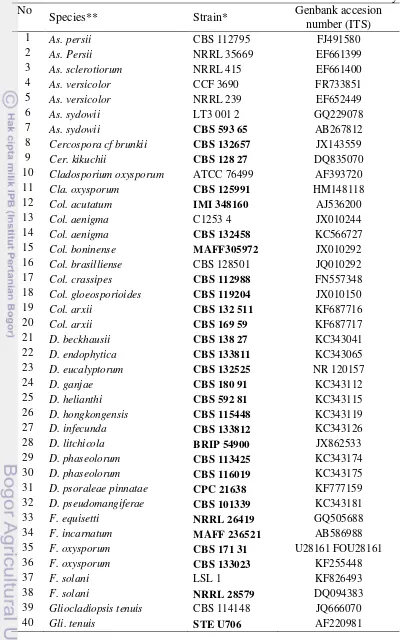
Table 2.2 GeneBank ITS accession number of reference strains used in this study No
Species** Strain* Genbank accesion
number (ITS)
1 As. persii CBS 112795 FJ491580 2 As. Persii NRRL 35669 EF661399 3 As. sclerotiorum NRRL 415 EF661400 4 As. versicolor CCF 3690 FR733851 5 As. versicolor NRRL 239 EF652449 6 As. sydowii LT3 001 2 GQ229078 7 As. sydowii CBS 593 65 AB267812 8 Cercospora cf brunkii CBS 132657 JX143559 9 Cer. kikuchii CBS 128 27 DQ835070 10 Cladosporium oxysporum ATCC 76499 AF393720 11 Cla. oxysporum CBS 125991 HM148118 12 Col. acutatum IMI 348160 AJ536200 13 Col. aenigma C1253 4 JX010244 14 Col. aenigma CBS 132458 KC566727 15 Col. boninense MAFF305972 JX010292 16 Col. brasilliense CBS 128501 JQ010292 17 Col. crassipes CBS 112988 FN557348 18 Col. gloeosporioides CBS 119204 JX010150 19 Col. arxii CBS 132 511 KF687716 20 Col. arxii CBS 169 59 KF687717 21 D. beckhausii CBS 138 27 KC343041 22 D. endophytica CBS 133811 KC343065 23 D. eucalyptorum CBS 132525 NR 120157 24 D. ganjae CBS 180 91 KC343112 25 D. helianthi CBS 592 81 KC343115 26 D. hongkongensis CBS 115448 KC343119 27 D. infecunda CBS 133812 KC343126 28 D. litchicola BRIP 54900 JX862533 29 D. phaseolorum CBS 113425 KC343174 30 D. phaseolorum CBS 116019 KC343175 31 D. psoraleae pinnatae CPC 21638 KF777159 32 D. pseudomangiferae CBS 101339 KC343181 33 F. equisetti NRRL 26419 GQ505688 34 F. incarnatum MAFF 236521 AB586988 35 F. oxysporum CBS 171 31 U28161 FOU28161 36 F. oxysporum CBS 133023 KF255448
37 F. solani LSL 1 KF826493
No
Species** Strain* Genbank accesion
number (ITS)
41 Gli. sagariensis CBS 199 55 JQ666063 42 Glomerella acutate IMI 117617 AF411700 43 Guignardia mangiferae CBS 123404 FJ538333 44 G. mangiferae UFMGC8 5025 JN159689 45 llyonectria anthuriicola CBS 564 95 NR 121494 46 l. vitis CBS 129082 JF735303 47 Leptosphaerulina chartarum TSS 152 KJ398148 48 Neofusicoccum chordaticola MUCC297 EU301020 49 N. chordaticola T NR 119487 50 Penicillium citrinum P 1637 JQ316514 51 P. citrinum NRRL 1841 NR 121224 52 Pestalotiopsis adusta ICMP 6088 NR 111788 53 Pes. microspora CBS 364 54 AF377292 54 Pes. neglecta LK29 DQ000992 55 Peyronellaea coffeae
arabicae CBS 123398 FJ426994
56 Pey. coffeae arabicae CBS 123380 FJ426993 57 Phoma glomerata XSD 41 EU273521 58 Pho. herbarum CBS276 37 JF810524 59 Pho. palmicola CP1 KF496903 60 Pho. tersa GRMP 42 JQ818195 61 Phy. capitalensis CBS 119720 FJ538340 62 Pyrigemmula aurantiaca CPC 18064 HM241693 63 Trichoderma atroviride CBS 142 95 F456917 64 T. atroviride NBRC 101776 NR 077207 65 T. hamatum CPK 2317 49
22 69
4200
66 T. hamatum Tri 612 4 KC 747811 67 T. hamatum DAOM 167 057 Z48816 68 Saccharomyces cereviseae ATCC 18824 KC881067 69 Sacch. cereviseae CBS 1171 ABO 18043
* Ex-type cultures are in bold **Abbreviation see appendix 4.10
other Fusarium clade there are 3 morphotypes of F. solani (M8, M94, and M97). Within Ilyonectria clade there is one morphotype of Ilyonectria anthuriicola M64 and within Gliocladiopsis clade there is one morphotypes of G. tenuis M49. Thirteen morphotypes of Colletotrichum representing 7 species i.e. Colletotrichum sp. (M1, M3 and M6) Col. acutatum M57, Col. aenigma (M2), Col. arxii M53, Col. boninense (M28 and M5), Col. brassiliense (M76), Col. crassipes (M30 and M82) and Col. gloeosporioides (M4 and M47) are within Colletotrichum clade. In the Trichoderma clade, there are one morphotype each of T. atroviridae M26 and T. hamatum M98. Pyrigemulla clade is represented by one morphotype of P. aurantiaca M69. Within Diaporthe clade, there are 27 morphotypes representing Diaporthe sp. (M9, M12, M13, M14, M15, M22, M23, M33, M38, M39, M41, M42, M43, M44, M48, M52, M59, M65, M69, M70,M72, M74, M79,M85, M89, M91, M96), D. beckhausii (M37, M54 and M73), D. endophytica (M90 and M20), D. eucalyptorum M46, M56, and M81, D. ganjae M71, D. helianthi M21, D. hongkongensis (M31 and M36), D. infecunda (M63 and M68), D. pseudo mangiferae M24, D. palmicola (M11 and M72), D. phaseolorum (M10 and M40), D. litchicola (M78 and M88) and D. psoraleae-pinnatae (M32, M77, M84, M92, and M94). Within Eurotiomycetes (Eurotiales, Trichocomaceae) clade (100 % BS) there are two sub-clades. Penicillium sub-clade (100 % BS) consisting P. citrinum M51 and Aspergillus sub-clade (57 % BS) consisting of Aspergillus sp. M19, As. sydowii M62, As. versicolor (M27, M47 and M55).
Dothideomycetes is a polyphyletic group composed of 4 sub-clades. In this group, Phoma sp. M50 (Pleosporales) is a sister clade of Phoma-Peyronelleae clade with 100 % BS. The strain is tentatively identification Phoma since the BLAST search result showed that this is the closest genus. Peyronelleae coffeae-arabicae M58 (Didymellaceae Pleosporales) and Leptosphaerulina chartarum (M83 and M87) (Leptosphariaceae, Pleosporales) form one sub-clade (81 % BS) with their reference strains. Neofusicoccum chordaticola M17 with their reference strains and Phyllosticta capitalensis M29, M35, and M61 with their reference strains (Botryosphaeriaceae, Botryosphaeriales) each forming one sub-clade with 100 % BS. Two other subclades are Cladosporium sub-clades (Davidiellaceae, Capnodiales) and Cercospora (Mycosphaerellaceae, Capnodiales) subclades with 100 % BS. Cladosporium oxysporum M25 and Cercospora sp. (M18A & B) forming clades with a strong BS (100 %).
Figure 2.1 Maximum Parsimony phylogenetic tree showing the relationship between endophytic fungi from all of plant organ C. calisaya and related fungi and plants based on the sequences of 5.8S of rDNA. Tree was rooted with Saccharomyces cerevisae (KC881067 and AB018043). Bootstrap values > 50 % (1000 replicates) are shown at the branches.
Dothidiomycetes
[image:31.595.47.513.65.709.2]Cercospora sp., Cladosporium oxysporum, Colletotrichum spp., Col. acutatum, Col. aenigma, Col. arxii, Col. boninense, Col. brasiliense, Col. crassipes, Col. gloeosporioides, Diaporthe spp., D. beckhausii, D. endophytica, D. eucalyptorum, D. ganjae, D. helianthi, D. hongkongensis, D. infecunda, D. litchicola, D. phaseolorum , D. pseudomangiferae, D. psoraleae-pinnatae, Fusarium incarnatum, F. oxysporum, F. solani, Gliocladiopsis tenuis, Ilyonectria sp., Leptosphaerulina chartarum, Neofusicoccum chordaticola, Penicllium citrinum, Pestalotiopsis sp., Phoma sp., Phomopsis palmicola, Pho. tersa, Phyllosticta capitalensis, Pyrgemulla aurantiaca, Peyronellaea coffeae arabicae, Trichoderma hamatum, and T. atroviridae.
Some species of endophytic fungi can be found in almost all organs of plants, and some others were only found in one organ. There are 11 species found only one organ such as Col. arxii (M53) was isolated from the root, Col. acutatum M57 (fruit), Col. aegnima M2 (fruit), D. eucalyptorum M81 (bark), Diaporthe sp. M91 (bark), D. litchicola M88 (fruit), Ilyonectria sp. M64 (root), L. chartarum M87 (leaf), P. citrinum M51 (fruit), Pho. tersa M95 (twig), Pyr. aurantiaca M99 (root). These species are organ–specific. Phylogenetic study indicated that close related species might occur in different organs. The phylogenetic study describes distribution and adaptation of microhabitat specificity.
DISCUSSION
This study provides a complete representation of fungal diversity within a healthy tree of C. calisaya, since this study reports the diversity of fungal endophytes from all organs of the plant. In contrast, previous study were restricted on those from twig and bark only (Simanjuntak et al. 2002, Shibuya et al. 2003, Mumpuni et al. 2004, Maehara et al. 2010).
As most of the isolates are sterilia mycelia, molecular approach is chosen for identification. According to Hyde & Soytong (2007) problems associated with identification of mycelia sterilia could be solved using DNA-based analysis, even though molecular analysis alone has its own limitations. In this study, ITS region is used considering this region has been decided as the marker for fungi. The use of ITS sequences also has limitations in phylogenetic analysis, as this noncoding ITS sequence is fast evolving with many variable characters. It is usually difficult to achieve a perfect sequence alignment at high taxonomic levels. Meanwhile, it has been shown that some of sequences down loaded from GenBank for comparative analysis may not be accurate in the identification (Hyde & Soytong 2007). It is suggested that using different gene sequences can resolve this type of difficulties in the phylogenetic analysis of the fungi.
morphotyping has been useful in estimating fungal numbers and the species is the basic unit in biodiversity.
Endophytic fungi consists of Ascomycetes and Basidiomycetes. Although Ascomycota and Basidiomycota have endophytic members, most reports stated that endophytic fungi primarily belong to Ascomycetes and its anamorphs (Davis et al. 2003, Arnolds 2007, Khan et al. 2010). All fungal endophytes found in this study belongs to Ascomycota and no basidiomycetous strains were found. Nevertheless, the existence of basidiomycetous endophytes from Cinchona were reported by Mumpuni et al. (2004) and Maehara et al. (2011).
This study reported 18 genera of endophytic fungi from C. calisaya. of those genera found in this study, only Diaporthe, Phomopsis, and Penicillium which have been reported Maehara et al. (2010) in C. ledgeriana (syn. of C. calisaya). Therefore, Aspergillus, Cercospora, Cladosporium, Colletotrichum, Fusarium, Gliocladiopsis, Ilyonectria, Leptosphaerulina, Neofusicoccum, Peyronellaea, Pestalotiopsis, Phoma, Phyllosticta, Pyrigemmula and Trichoderma were new records of endophytic fungal genera in Cinchona.
In C. calisaya, genera Diaporthe is the dominant endophytes represented by 130 isolates, followed by genera Colletotrichum (117 isolates). Phomopsis (anamorph of Diaporthe) was reported as dominant in Cinchona ledgeriana (Maehara et al. 2010). Colletotrichum and Phomopsis were frequently identified as dominant endophytes in various plants (Cannon & Sommons 2002, Devarajan & Suryanarayanan 2006, Huang et al. 2008, Costa et al. 2012. The Coelomycetes such as Phoma, Phomopsis, and Phyllosticta are common endophytic fungi (Avekamp et al. 2008). Many species of fungi were commonly described as endophytes, but others could be found occasionally colonizing the host tissue and were isolated only once or twice in several samples (Siqueira et al. 2011). A single endophytic fungal species that form relationships with two related plant species or demonstrate a preference for one particular host is categorized as having host selectivity (Cohen 2006).
CONCLUSION
All plant organs of C. calisaya hosted endophytic fungi. In total, 687 isolates were obtained from 5 healthy plants. These isolates were divided into 96 morphotypes. Phylogenetic study showed that the morphotypes represented 18 genera and 42 species of Ascomycota. Of these genera, 15 genera are the first reported and mainly belong to Sordariomycetes. Phylogenetic taxa can inhabit different microhabitat.
INTRODUCTION
Endophytic fungi that widely distributes within the plant tissues are rich in species diversity (Qiu et al. 2008). Endophytes are considered as important component of biodiversity and distribution of endophytic fungi in each host plant are different. Diversity of endophyte was expected to be higher in tropical plants (Banerjee 2011) including those in medicinal plant. Medicinal plants are known to harbour endophytic fungi that associated with the production pharmaceutical substances (Zhang et al. 2006). Therefore, it is important to explore endophytic fungi in the medicinal plant such as Quina tree (Cinchona spp) in order to enable pharmaceutical substance production. While C. calisaya (syn. C. calisaya var. legderiana) is one of the best clone of cultivated Quina tree for quinine production in Indonesia. Recently, the plantation area has been reduced drastically (Susilo, 2011). Therefore, alternative agent for quinine and other active metabolites production should be investigated. Hence, the study of fungal endophyte community from all organs of C. calisaya is initiated.
Prior to this study, some fungal endophytes of Cinchona from Indonesia have been reported. However, most researches on endophytic fungi of Cinchona spp. focused on isolation from certain part of the plant and screening their potential for secondary metabolites production. Eventhough some of these endophytic fungi from bark has been studied for their metabolite products (Simanjuntak et al. 2002, Winarno 2006, Maehara et al. 2010), no comprehensive study covering the fungal endophytes community structures in the whole part of C. calisaya has been done. Information on fungal endophytes community in Cinchona is important as bases for understanding the endophyte roles in relation to secondary metabolic production of their host. Despite its limitation, culture based method was selected for studying the community structure of endophytic fungi as this method will also provide genetic resource material for secondary metabolites biotechnology. Therefore, this study was aimed to analyse the community structure of endophytic fungi in bark and other plants organs (leaf, petiole, twig, root, flower, and fruit) of C. calisaya to elucidate the diversity, species dominance and distribution endophytic fungi in different organs.
MATERIALS AND METHODS
Isolation and identification of the fungi
Specimen collection, fungal endophyte isolation and identification were done following protocol that was described in page 6.
Data analysis
Community structures were expressed as diversity, colonization rate and frequency of occurrence of the species in each plant organs. One colony is considered as one individual fungus. Shannon-Wiener diversity index (H’) was employed to evaluate and compare the diversity of fungal communities between different organ of C. calisaya plant. H’ was calculated according to the following formula:
H’ = - ∑pi × lnpi i=1
where k is the total of fungal species, and pi is the proportion of individuals that species i contributes to the total (Tao et al. 2012).
Colonization rate (%) was calculated as a percentage of the total number of segments colonized by the fungi divided by total number of segments observed. While frequency of occurence (%) of an endophytic species was calculated to estimate the species dominance and distribution of the fungi. The fungal occurrence in different organ was calculated using the following formula:
Number of strains in species-i
Frequency of occurrence of species-i (FO) = --- × 100 % Total number of strains found
Unweighted pair group method with arithmetic mean (UPGMA) cluster analysis was performed using Jaccard’s coefficient using Multi-Variate Statictical Package software (MVSP) version 3.13r. on the bases of diversity index, the presence of the fungi, and frequency of its occurence. Dendrogram of community relatedness was reconstructed on the bases of similarity distance of Jaccard’s coefficient.
RESULTS
Phylogenetic analyses using ITS4–5.8S–ITS5 region is able to identify 96 representative isolates of the morphological groups from the 687 fungal isolates. The community assemblages of the endophytic fungi belongs to Ascomycota (Table 3.1), in which mostly are members of Sordariomycetes.
Table 3.1 Endophytic Ascomycota isolated from C. calisaya
Class Species name
Eurotiomycetes Aspergillus sp., As. sydowii, As. versicolor, P. citrinum Dothidiomycetes Cercospora sp, Cladosporium oxysporum, Phyllosticta
capitalensis, Leptosphaerulina chartarum, Neofusicoccum chordaticola, Phoma sp., Peyronellaea coffeae arabicae Sodariomycetes Colletotrichum spp., Col. acutatum, Col. aenigma, Col. arxii,
Col. boninense, Col. brasiliense, Col. crassipes, Col. gloeosporioides, Diaporthe spp., D. beckhausii, D.
eucalyptorum, D. endophytica, D. infecunda, D. ganjae, D. hongkongensis, D. helianthi, D. litchicola, D. phaseolorum, D. pseudomangiferae, D. psoraleae-pinnatae, Fusarium
incarnatum, F. oxysporum, F. solani, Gliocladiospsis tenuis, Ilyonectria sp., Pestalotiopsis sp., Phomopsis palmicola, Pho. tersa, Pyr. aurantiaca, Trichoderma hamatum, T. artroviride
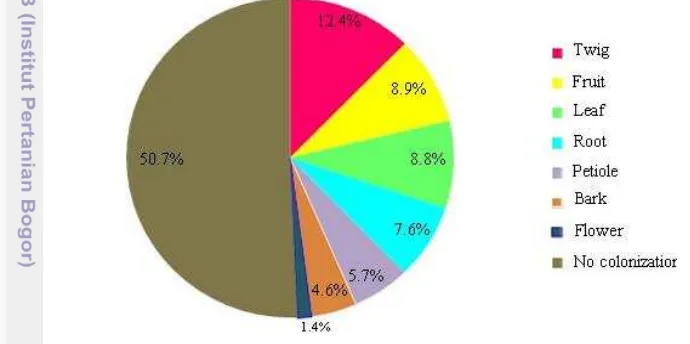
20 % of the total species. Only about 49.3 % of the total segments studied occupied by the endophytic fungi and the remaining about 50.7 % were free from fungal endophytes (Fig 3.1). Of those occupied by the fungi, the segments hosted either one fungus or more. This means that the fungus may not be grown continuously within the organ tissue. Of the organs studied, twig was the most preferred organ being colonized (12.4 %), followed by fruit (8.9 %), leaf (8.8 %) and root (7.6 %). The other organs were colonized less frequent (between 1.4 %–5.7 %).
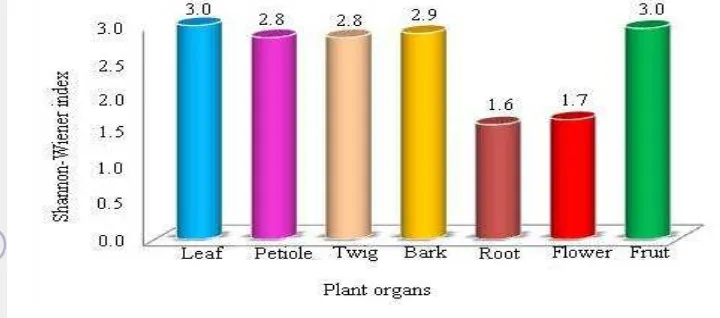
[image:37.595.103.448.315.487.2]Although endophytic fungi occurred in all plant organs,their diversity in each plant organ varied. Leaf and fruit (H’=3.0) bore the most diverse endophytic fungi, followed by bark (H’=2.9), twig and petiole (H’=2.8). While flower (H’=1.7) and root (H’=1.6) contained less diverse endophytic fungi (Fig. 3.2). The number of taxa within organs (leaf, fruit, bark, twig and petiole) with high diversity index was also different from the ones (flower and root) with low diversity index. Within high diversity index organs, about 25–36 taxa were found, while only 7–15 taxa occurred in low diversity index organs.
Figure 3.1 Colonization rate of endophytic fungi in various organs of C. calisaya
Figure 3.2 The Shannon-Wiener diversity index (H’) of endophytic fungi in each plant organ
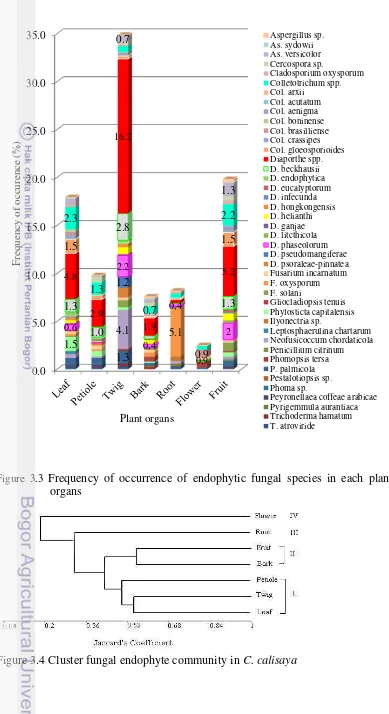
Distribution of fungal endophytes are different among organs (Figure 3.3). There are 11 species of Diaporthe and an unidentified group of Diaporthe called Diaporthe spp. Among the species found, Diaporthe spp. exhibits the widest distribution in the plant organs, and it was dominantly found in twig (16.2 %). In the other organs (fruit, leaf, petiole, bark, root and flower) the frequency of Diaporthe spp. occurrence were 5.2 %, 4.8 %, 2.9 %, 1.9 %, 0.4 %, 0.1 %, respectively. The plant organs harbored 1–9 species of Diaporthe, of which twig and fruit contain the most diverse species of Diaporthe spp. Other than Diaporthe spp., the most common species within some organs are unique. For instance, Neofussicoccum chordaticola was common fungi on the twig with FO about 4.1 %. In the leaf, Diaporthe spp. (4.8 %) and Colletotrichum spp. (2.3 %) were the most common taxa. In the flower, Col. brasiliense was the most common with 0.9 % FO. Fusarium oxysporum (5.1 %) was the most common fungal endophytes in the root. However, the most common fungal endophyte species in the petiole, fruit and bark was not unique in which Diaporthe spp. were the most common fungi with frequency of occurrence 2.9 %, 5.2 % and 1.9 %, respectively.
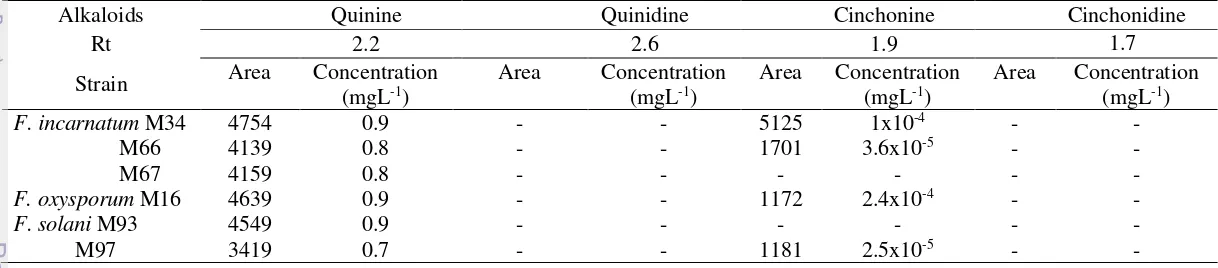
As the diversity, and the distribution of the fungal species is varies among organs, their assemblage forming different community structure in each plant organ. Based on UPGMA analyses, with similarity index < 0.5 as the cutting score, endophytic fungal communities can be divided into four clusters (Figure 3.4). The fungal community in twig was close to those in leaf and petiole with similarity index > 0,52. The fungal communities in bark and fruit were also closely similar. While those in root and flower from distinct communities. The close similarity among fungal endophytes community from leaf, petiole, and twig were possibly due to the presence of two predominant species, i.e. Colletotrichum spp. and Diaporthe spp. The flower and root were apparently separate due to differences in fungal endophytes composition, there were exclusively species i.e. only Col. gloeosporioides in flower and T. atroviride. Ilyonectria sp., G. tenuis in the root.
Figure 3.3 Frequency of occurrence of endophytic fungal species in each plant organs
Figure 3.4 Cluster fungal endophyte community in C. calisaya
0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 1.3 4.1 1.5 5.1 1.2 0.6 2.2 0,4 0,4 2 1.3 1.0 2.8 1.3 4.8 2.9 16.2 1.9 0,9 5.2 1.5 1.5 0.9 2.3 1.3 0.7 2.2 0.7 1.3 Fre quenc y of occ ur enc e (% ) Plant organs Aspergillus sp. As. sydowii As. versicolor Cercospora sp. Cladosporium oxysporum Colletotrichum spp. Col. arxii Col. acutatum Col. aenigma Col. boninense Col. brasilliense Col. crassipes Col. gloeosporioides Diaporthe spp. D. beckhausii D. endophytica D. eucalyptorum D. infecunda D. hongkongensis D. helianthi D. ganjae D. litcthicola D. phaseolorum D. pseudomangiferae D. psoraleae-pinnatea Fusarium incarnatum F. oxysporum F. solani Gliocladiopsis tenuis Phylosticta capitalensis Ilyonectria sp. Leptosphaerulina chartarum Neofusicoccum chordaticola Penicillium citrinum Phomopsis tersa P. palmicola Pestalotiopsis sp. Phoma sp.
[image:39.595.89.478.79.793.2]