Vol9, No
j,
July-
September 2000 p53 immunostaining in nasopharyngealcarcinoma
209
p53
protein
overexpression
in
nasopharyngeal carcinoma
in
Indonesian
patients
A.N.Kurniavan*, Anthony
S-Y
Leong$Abstrak
p53, baik gen maupun proteinnya, merupaknn salah satu unsur genetikyang paling banyak diteliti pada penyakit kanker. OIeh karena prevalensi Karsinoma nasofaring (KNF) hanya tinggi pada daerah lertentu saja, terutama
di
beberapa negara Asia, maka tidak banyak dijumpai tulisan mengenai ekspresi p53 pada KNF dalam kepustalcaan internasional. Tulisan ini membahas mengenai el<spresi protein p53 dengan pewamaan imunohistokimia pada 48 pasien KNF Indonesia dan menghubungkannya dengan 2 klasifiknsi KNF yaitu menurut WHO dan FORMUI"A,SI KERJA. Ekspresi protein p53 ditemulcan positif pada karsinomn sel skuamosa, Iarsinoma tipe A dan karsinoma tipe B berturut turut sebanyak 1007o , 82,6Vo dan 62,5Vo. Positivitas el<spresi p53 ini berhubungan bermakna dengan klasifikasi FORMUI-A,SI KERJA tersebut, sehingga membeilcan kesan adanya mabta prognostik p53 pada KNF.Abstract
p53 gene and its protein
is
oneof
the most widely studied genetic abnormalitiesin
cancer. Howeyer dueto the
restrictionof
nasopharyngeal carcinoma (NPC) to certain areas, mostly in Asia, scant attention has been paid to the over-expression of p53 in thehigh prevalence
of
NPCin
the intemational literature. This study examined the immunohistological expressionof p53
in
48 Indonesian NPC patients and correlatedit with
two NPC histological classifications. p53 protein over-expression were respectivelyfound
in
100%, 82.6Vo, and 62.5Vo of squamous cell carcinoma, typeA
carcinoma and type B carcinomnof
Working Formulation classification. This statistically significant correlation gives the impression that p53 may have prognostic relevance in NPC. Key w ord s : p 5 3 p ro t ein, nas op haryn g e a I c arcinoma, immuno lab e lli n g, g radin gINTRODUCTION
p53, a 373 amino acid
nuclear phosphoprotein
wasfirst identified
in 1979.r
Although initially
thought to
be an oncogene,it
has since been recogniZed that p53functions
as
a
tumor
suppressor
gene.2 Numeroussubsequent
reports
have
shown
that p53
abnor-malities occur frequently
in
a
very wide range of
tumors. Indeed,
abnormalities of
p53 appear to be the most common genetic changein
human cancer.3Nasopharyngeal
carcinoma (NPC) is
highly
prevalent
in
several
Asian
countries
and
is
rare
in
mostWestem countries. Because
of
this
difference
in
prevalence, scant attention has béen paid to theover-*
Department of Anatomic Pathology, Faculty of Medicine, University of Indonesia, Jakarta, Indonesia
s Hunte, Area PathoLogy Services, Discipline of Anatomical
PathoLogy, University of New Castle, New Castle, NSW, Australia
expression
of
p53
in
NPC,
particularly its
correlation
with
histological
subtypes.Based
on
pathology-based cancer
registry
statistics,NPC, mostly of the undifferentiated type,
was rankedas
the fourth most
common neoplasm
in
Indonesia(Department
of
Health,
Indonesian
Association
of
Pathologists, Indonesian Cancer
Society.
Cancer in
Indonesia 1994.
Unpublished histopathologic
data).This
study examined
the
over-expression
of
p53
in
48
cases
Indonesian
NPC patients,
employing
animmunohistochemical method.
MATERIALS
AND METHODS
The biopsy
specimens from
48
NPC
cases at
theDepartment
of
Anatomic
Pathology,
Faculty
of
Medicine, University
of
Indonesia, Jakarta,
wereexamined. Paraffin-embedded specimens
were
sentto the Institute
of
Medical
and
Veterinary
Science,210
Kumiawan and Leongwere stained.
Table
l. Vy'orking
formulation classificationMed J Indones
RESULTS
Positive nuclear staining
for
p53 protein
was found
in
38 (79Vo)of
the48
cases, thedistribution
shownin
Table
1. Sixteen
(33Vo)tumors revealed
l+
staining
and-another eight (l7Vo)
showed2+
staining. Eleven
(23Vo)
rumors showed
3+ staining
and
onl!
3
gVo)
showed4+
staining.The distribution
of
p53
over_expression
in NpC
typed according
to
the
WHO classilication is
shownin_
Table
2-Positivity
was observed
in all
nine
casesof
squamous
cell
carcinoma
although
only
six
showed more than
3+ positivity
in
tumlor
cells.
Thetwo
casesof
differentiaæd non_keratinizing carcinomawere positive but
only
as
2+
or
less;
on
the
other
hand,
the
undifferentiated carcinoma
group
showeda variable
degree
of
p53 staining,
with
eight.casesshowing
more
than
2+
cell
positivity,
six
iases
Z+positivity, and
l3
cases Iessihan
l+-positivity.
Theremaining
10 casesdid not
stain atall
for
p53.Table2. p53 immunostaining in Nasopharyngeal Carcinoma
Percentage of positive tumor cells Number of cells (Vo)
0
l+
2+ 3+ 4+l0
(21)l6
(33) 8 (17) t1 (23) 3 (6)Total 48 (r00)
Keratinizing SCC
Type A carctnoma
Type B carclnoma
Table 3. p53 immunostaining in subtypes of NpC according to the WHO classification
Flatpavemented
Syncytial
SyncytialIntercellular
Large, owlæye Smaller, more bridgesand/or
like nuclei'
basophilickeratinization
nucleiHistologic pattern Tumor cells
Pleomorphism
Malignancy
High-grade5-year
2l%osurv.rate
p53 immunostaffi Histological Subtypes (WHO classification)
Hyperchrom
Spindlecell, atic,spindle
finecells
chromatinEvident
LirrleIntermediate
Low-grade3O-40Vo
6O-7OV"scc
NKC0 2 I 5 I 0
l+
2+ 3+ 4+0
I
I 0 0
l0
l3
6 6 2 Total
Vol 9, No 3, July
-
September 2000DISCUSSION
The
human
p53
gene
is
located
at
chromosomel7p13.l
and
encodes
a
373
amino acid
nuclear
phosphoprotein,
which
is involved
in
the
regulation
of
cell
proliferation.t'to Studies
in
human
cancershave revealed
that
p53
geneis
the most frequently
affected gene
in
a wide
range
of
tumors,
including
cancers
of
the colon, lung,
esophagus, breast,liver,
brain,
andhematolymphoid
malignancies. I r' I 3Chromosomal analysis
in
several established NPC
cell
lines have shown multiple genetic
aberrations,including chromosome
l7pl3.
There
are
several techniques available
for
thedetection
of
p53. Detection at the
chromosome
andgene
level
can beperformed
by
Southernblotting,
in
situ
hybridization or PCR;
whereas,identification of
the protein can be
done
with
immunohistochemical
techniques
which
show good correlation
with
p53.mutation.la'lu
Th"
monoclonal
antibody DO7
employed
in this
study
detectsboth mutant
andwild
type p53.
Wild
type
p53
hasa
short
half
life of
lessthan 30
minutes
and is loèatedin
the nucleus.Mutant
p53,
on
the
other hand, has a prolonged
half
life
of
several
hours, rendering
it
readily
detectable
by
immunolabelling
method.
It
has been
demonstrated thatp53 protein
maybind to cellular proteins
such asthe
mdm2
oncogeneproduct
and heat shock protein
70, as well as
to several
DNA viral
proteinsincluding
E6 HPVI6
protein,rT'r8
SV40T
antigen, andElb
protein from
adenovirus
type 5,
all
leadingto
its functional
inactivation
and
stabilization.'v
While
the
presence
of
irnmunostained
p53
proteindoes
not
necessarily
indicate
a
gene
mutation,
theover-expression
detectedby
immunostaining may
bea
useful indicator
of
altered
wild
type p53
function
resulting
from inactivation
and stabilization through
one
of
the
preceding mechanisms.
It is also
recognized
that
various other cellular insults
caninduce
wild
type p53
expression
in
tumor
cells.Within
apopulation
of
cells
in-vivo
thereis
different
sensitivity
fo genotoxic stimuli, yet to be
identified,
which
canstabilize
wild
type p53,leading to
elevatedprotein
levels.2O
Study
by
I-eung
et
al2r
in
EBV-associated
gastric
carcinoma
and
head-and
neck
carcinoma showed
a
weak
to
moderate
p53 expression,while
there was a statistically significant
difference
of
p53
expression
between
EBV-associated
gastric
carcinoma
and
EBV-negative
gastric carcinoma.
This
suggesteda
non-mutational
p53 immunostaining in nasopharyngeal
carcinoma
211mechanism
of
p53 upregulation.
Murono
et
al22have
investigated
the
association
of
EBV
with
status
of
p53
protein
expression
in
NPC,
examined
theexpression
of
EBV
gene
and
gene
product,
p53protein
andbcl-2 protein.
Significant
correlation
wasfound
between
the
expression
of
EBV
and
p53protein,
but
not
between
p53
protein and
LMPI.
They
suggestedthat
someEBV-encoded protein
mayplay
a role
for
nuclear accumulation
of p53
protein.
Interference
by
EBV
infection
may thus cause altered p53function through epigenetic influence.
There
havebeen few
previous
reportsexamining
theover-expression
of
p53
protein
in
NPC
usin-gimmunohistochemical methods.
Niedobitek
et
al2''detected
p53
over-expression
in
five
of
nine
EBV-negative
squamouscell
NPCs
andin
nine
of
thirteen
EBV-positive
casesof
undifferentiated carcinoma.
In
a
study
of
41
casesfrom Hong Kong,
Porter
et
al2afbund p53
over-expression
in
70Voof
cases,
I2Voshowing strong immunostaining
for
the
protein.
Our
study showed similar result, and strong positivity
wasfound
evenin
ahigher
percentage (29Voof
casesshowed 3+ and 4 +).
Sheu
et
al2-5using the antibody D07,
demonstratednuclear staining
for
p53
in 96
(957o)of
l0l
lesions.Positive staining
in
adjacent dysplastic
cells
wasfound
in
79Vo
of
carcinomas
with
associateddysplastic epithelium.
Basedon their findings,
theseauthors suggested
that p53 over-expression occurs
atan early
stagein
the
development
of
NPC:
Further
more,
by
observing
the
co-expression
of
bcl-2
andp53
in
77Voof
NPC
casesin
Taiwan,
Sheu
et
al26suggested
that
mutated
p53
or
altered function of
wildtype
p53 may contribute
to
the
pathogenesisof
NPC,
in
which bcl-2 and p53 may
play
a
crucial
synergistic
effect
in
the carcinogenesisof
NPC.Studies
of
p53
over-expression
in
breast carcinoma
have suggested
that
acorrelation
exists betweenhigh
levels
of
expression
and
advanced stage
of
diseaseand
metatastic
tumor
spread.27However,
elevatedlevels
of
p53
have
not
been shown
to
be
of
prognostic relevance
in
other
epithelial tumors
suchas
small cell
carcinoma and adenocarcinoma
of
thelung,
and colonic carcinoma.
None of
the
threeimmunohistochemical
studies
in
NPC found
acorrelation between p53
over-expression with
survival, potential
for
metastasis,
clinical
stage, or
histological
parameters- _ including grade and212 Kurniawan and Leong
(ct)
T
* t:
6k
u*ur
l,(b)
É
[image:4.595.88.572.61.739.2]è'
Figure
l.
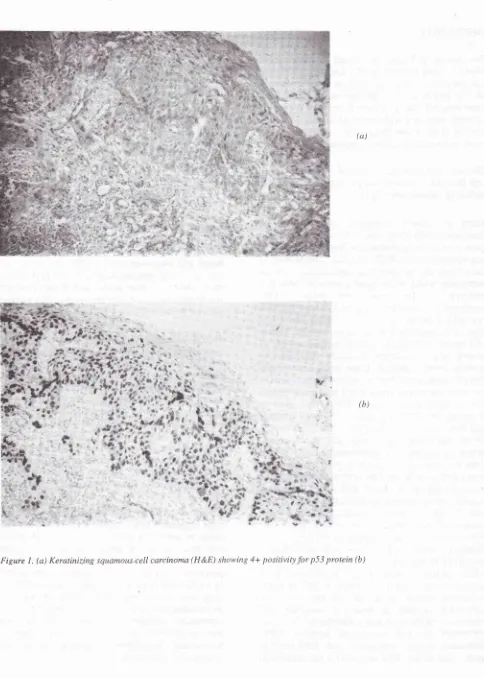
(a) Keratinizing squamous.cell carcinoma (H &E) showing 4+ positivity for p5j
protein (b)p5
j
immunostaining in nasopharyngeal carcinoma213
[image:5.595.76.574.97.635.2]Vol 9, No 3, July
-
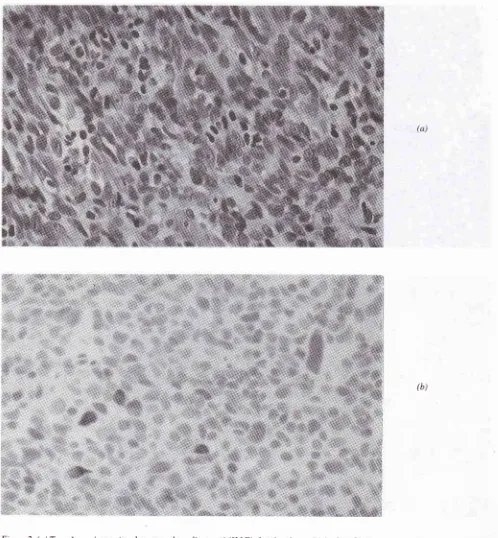
September 2000Figure 2. (a) Type A carcinoma (moderate grade malignancy) (H&E) showing
4+
positivityfor
p53 iimunostaining (b) The pleomorphic tumor cells display prominent nucleoli and spindledforms(a)
Vol 9, No 3, July
-
September 2000Our findings
of
79Vopositivity in 48
casesof
NPC,
of
which
46Vo stained more than lOVoof
tumor
cells, isin
concordancewith
theseprevious reports. We did
not
find
a correlation
of
p53
over-expression
with
subtypes
of
NPC when classified according to
theWHO
system.However,
this
wasnot
unexpected asthe WHO classification
is
not related primarily
totumor grade, but rather
to
the morphology
of
thetumor cells.
On the
other
hand,
the
Working
Formulation
as
proposed
by
Hsu
et
al6 has
beenproven
to
be
of
prognostic correlation.
Employing
the alternative Working Formulation
on which
theIndonesian classification
is
based,'
we found
that there was astatistically significant correlation
in
this
study.
All
cases
of
keratinizing squamous cell
carcinoma
(high
grade malignancy)stained for
p53,while positivity
in
Type
A
carcinoma (intermediate
grade malignancy)
was
82.6Vo
and
in
Type
B
carcinoma
(low
grade malignancy)
was 62.5Vo.IT isof
interest that these three
subtypesof NPC
showedsignificant
different rates
of
survival,'
therefore suggesting that p53 may have prognostic relevancein
NPC.
Masuda
et
al28
who
made a
clinicopathologic
studycorrelating p53,
bcl-2 ,I<-67,
sensitivity to radiation,
incidence
of
distant
metastases and survival, reportedthat
NPC
patients
who
were positive
for
p53 tended
to
be
resistant
to
radiotherapy
and
to
havesignificantly
poorer prognosis.
They
concluded
that the enhanced expressionof
p53 may be
aprognostic
factor
in
NPC
patients whose
tumor
is
resistant to
DNA-damaging
therapy.The
role of p53 as an
independent
prognostic
parameter should
be
tested through
multivariate
analysis
in
conjunction
with
other
prognostic
variables and
further
investigations
are necessary toidentify
appropriate
cut-off
values
of
p53 positivity
as suggested
by Dowell
and
Hall.l5
Furthermore,
it
may be
necessaryto
examine
the
relevance
of
not
only the
percentageof positive
staining
tumor
cellsbut
also
to employ
somequantitative method which
incorporates the intensity
of
immunostaining
such ascan
be
performed
with
image andlysis.REFERENCES
l.
Lane DP, CrawfordLV. T
antigenis
boundto
a hostprotein
in
SV 40 tranformed cells. Nature l9?9; 278:261-3.
2.
Dowell SP, Hall PA. The clinical relevanceof
the p53tumour-suppressor gene. Cytopathology 1994, 5: 133-45.
6.
p53 immunostaining in nasopharyngeal
carcinoma
215
Levine AJ,' Momand
J,
Finlay
CA.
The p53
tumoursuppressor gene. Nature
l99l;
351: 453-6.Leong A S-Y, Millios J. An assessment of the efficacy
of
the microwave antigen-retrieval procedure on arrange oftissue antigens. Appl Immunohistochem 1993; 1: 267-74.
Shanmugaratnam
K,
SobinLH.
Histological typingof
tumoursof
the upper respiratory tract and ear.2d
ed.Berlin, Heidelberg : Springer Verlag; 1991.
Hsu HC, Chen CL, Hsu MW, Lynn TC, Tu SM, Huang
SC. Pathology ofnasopharyngeal carcinoma. Proposal
of
a new histologic classification correlated with prognosis.
Cancer 1987; 59: 945 - 51.
Kumiawan
AN, Syatril
A,
SusworoR.
An
alternative classificationof
nasopharyngeal carcinoma as Working Formulation. Med J Univ Indon 1993; 2:37-46.Isobe
M,
Emanuel BS, Givol D, OrenM,
Croce CM. Localizationof
genefor
human p53 tumour antigen to bandl7pl3.
Nature 1986; 320:84-5.Pieteripol JA, Vogelstein
B.
No
room at the p53inn.-Nature 1993; 365: l7-18.
t0.
Levine AJ, Perry ME, Chang A , Silver A, Dittmer D,WuM
et al. The 1993 Walter Hubert l,ecture: the role of thep53
tumour-suppressor genein
tumorigenesis.Br
JCancer 1994; 69: 409-16.
I
l.
Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53mutations in human cancers. Science
l99l;253:49-53.
12.
Lane DP. p53, guardianof
the genome. Nature 1992;368: 15-16.
13.
Zambetti GP, Levine AJ. A comparison of the biological activitiesof
the wild+ype and mutant p53. FASEB J1993;7:855-6-5.
14.
Dei Tos AP, Doglioni
C, Laurino
L,
Barbarescki M, Fletcher CDM. p53 protein expression in non-neoplastic lesions and benign and malignant neoplasmsof
softtissue. Histopathology 1993; 22: 45-50.
15.
Dowell SP, Hall PA. The clinical relevanceof
the p53 suppressor gene. Cytopathology 1994; 5: 133-45.16.
BaasIO, Mulder
J-WR, Offerhaus GJA, Vogelstein B,Hamilton SR.
An
evaluationof
six
antibodies for immunohistochemistryof
mutant p53 gene product in archival colorectal neoplasms. J Path 1994; 172:5-12.17.
Lane S, WellsM.
Human papillomavirus. Editorial. p53, and cervical neoplasia. J Path I 994; 172: 299-300.18.
Vogelstein
B,
Kinzler
KW. p53
function
anddystunction. Cell 1992; 7O:523-26.
19.
Lambkin HA, Mothersill CM, Kelehan P. Variations in immunohistochemicaldetection
of p53
protein overexpressionin
cervical carcinomaswith
different , antibodies and methodsof
detection.I
Path 1994; 172'.l3-18.
20.
Hall PA, Lane DP. Editorial. p53in
tumour pathology: Can we trust immunohisto-chemistry?-revislted.J
Path1994:172:1-4.
21.
Leung SY, ChauKY,
Yuen ST, ChuKM,
Branicki FJ,Chung LP. p53 overexpression
is
differentin
Epstein-Barr virus
associated and Epstein-Barr virus negativecarcinoma. Histopathology, 1998; 33: 311 -7.
22.
Murono
S,
YoshizakiT,
Park CS,
Furukawa M. Associationof
Epstein-Barrvirus
infectionwith
p53216
23.
Kumiawan and Leong
Niedobitek
G,
AgathanggelouA,
BarberP,
SmallmanLA,
JonesEL,
Young
LS.
P53
overexpression andEpstein
-Barr
virus
infectionin
undifferentiated andsqu.rmous cell nasopharyngeal carcinomas. J Path 1993;
170:47
-
61.Porter
MJ, Field JK,
Lee JC, t eung SF,Lo D, van
HasseltCA, et
al. Detectionof
the tumour suppressorgene p53
in
nasopharyngeal carcinomain
Hong KongChinese. Anticancer Res, 1994; 14:1357-60.
Sheu LF, Chen A, Tseng HH, Leu FJ, Lin
IK,
HoKC et
al.
Asssessmentof
p53 expressionin
nasopharyngealcarcinoma. Hum Pathol 1995; 26: 380-6.
Sheu LF,Chen A, Meng CL, Ho KC,
Lin
FG, tæe WH.Analysis
of
bcl-2
expressionin
normal,
inflamed,Med J Indones
dysplastic nasopharyngeal epithelia, and nasopharyngeal
carcinoma: association with p53 expression. Hum Pdthol 1997:28:556-62.
Davidoff
AM,
Herndon JE, Glover NS, et al. Relation between p53 overexpression and established prognostic factors in breast cancer. Surgery I 99 1 ; Il0:
259-264.Masuda
M,
ShinokumaA,
HirakawaN,
Nakashima T, Komiyama S. Expressionof
bcl-2, p53, and Ki-57 andoutcome
of
patients
with
primary nasopharyngeal
carcinomas following DNA-damaging treatment. Head
Neck 1998; 2O:640-4. 27.
28
24
25.