PROTEASE FIBRINOLITIK DARI MIKROBA
PANGAN FERMENTASI ONCOM MERAH DAN TEMPE GEMBUS
DIANA NUR AFIFAH
SEKOLAH PASCASARJANA INSTITUT PERTANIAN BOGOR
PERNYATAAN MENGENAI DISERTASI DAN
SUMBER INFORMASI SERTA PELIMPAHAN HAK CIPTA
Dengan ini saya menyatakan bahwa disertasi berjudul Protease Fibrinolitik dari Mikroba Pangan Fermentasi Oncom Merah dan Tempe Gembus adalah benar karya saya dengan arahan dari komisi pembimbing dan belum diajukan dalam bentuk apa pun kepada perguruan tinggi mana pun. Sumber informasi yang berasal atau dikutip dari karya yang diterbitkan maupun tidak diterbitkan dari penulis lain telah disebutkan dalam teks dan dicantumkan dalam Daftar Pustaka di setiap bagian akhir Bab pada disertasi ini.
Dengan ini saya melimpahkan hak cipta dari karya tulis saya kepada Institut Pertanian Bogor.
Bogor, Desember 2014
Diana Nur Afifah
RINGKASAN
DIANA NUR AFIFAH. Protease Fibrinolitik dari Mikroba Pangan Fermentasi Oncom Merah dan Tempe Gembus. Dibimbing oleh MAGGY T. SUHARTONO, DAHRUL SYAH, dan YANTI.
Penyakit kardiovaskuler seperti penyakit jantung, tekanan darah tinggi, dan stroke adalah penyebab utama kematian di dunia, termasuk Indonesia. Dasar patofisiologi proses infark miokardial dan stroke adalah pembentukan bekuan fibrin atau trombus yang melekat pada dinding pembuluh darah yang terluka. Fibrin merupakan komponen protein utama dari pembekuan darah, yang terbentuk dari fibrinogen oleh trombin.
Enzim fibrinolitik dari mikroba pangan telah menarik perhatian untuk diteliti lebih jauh sebagai agen trombolitik. Genus Bacillus dari pangan fermentasi dapat menghasilkan enzim fibrinolitik kuat, seperti Bacillus natto dari natto, pangan fermentasi kedelai asal Jepang yang menghasilkan nattokinase (NK). Beberapa genus Bacillus lainnya dari berbagai pangan fermentasi juga telah ditemukan mampu menghasilkan enzim fibrinolitik kuat. Diantaranya adalah B. amyloliquefaciens DC-4 dari douchi, pangan fermentasi kedelai dari Cina,
Bacillus sp. CK dari chungkookjang, saus kedelai fermentasi dari Korea, Bacillus sp. strains DJ-2 dan DJ-4 dari doenjang, Korea, dan Bacillus sp. KA38 dari
jeotgal, ikan asin fermentasi dari Korea.
Penelitian ini berhasil mengisolasi bakteri penghasil protease fibrinolitik dari pangan fermentasi Indonesia, oncom merah dan tempe gembus. Empat puluh tiga isolat menunjukkan aktivitas proteolitik pada media skim milk agar (SMA), dan 38 diantaranya menunjukkan aktivitas fibrinolitik baik berdasarkan uji cakram fibrin maupun zimogram dengan substrat fibrinogen. Dua isolat terbaik, yaitu RO3 dari oncom merah dan 2.g dari tempe gembus, dipilih untuk diidentifikasi menggunakan kit API 50 CHB dan analisis 16S rRNA. Hasil uji biokimia menggunakan kit API 50 CHB khusus untuk Bacillus spp, menunjukkan bahwa isolat RO3 teridentifikasi sebagai B. licheniformis (99,9%) dan isolat 2.g sebagai
B. pumilus (99,7%). Identifikasi molekuler berdasarkan gen 16S rRNA telah dilakukan dan isolat RO3 teridentifikasi sebagai B. licheniformis (96%) dan isolat 2.g sebagai B. pumilus (97%). Urutan nukleotida telah didaftarkan di GenBank
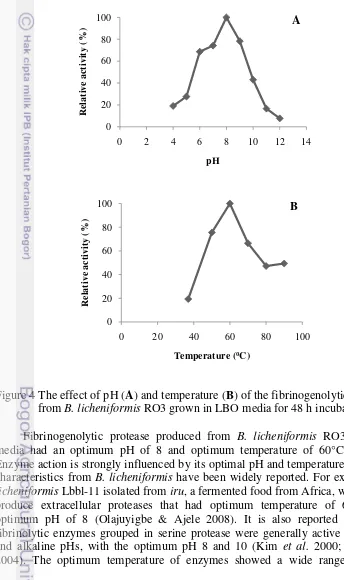
Protease fibrinogenolitik kasar yang dihasilkan dari B. licheniformis RO3 dengan media produksi LBO memiliki pH optimum 8 dan suhu optimum 60oC.
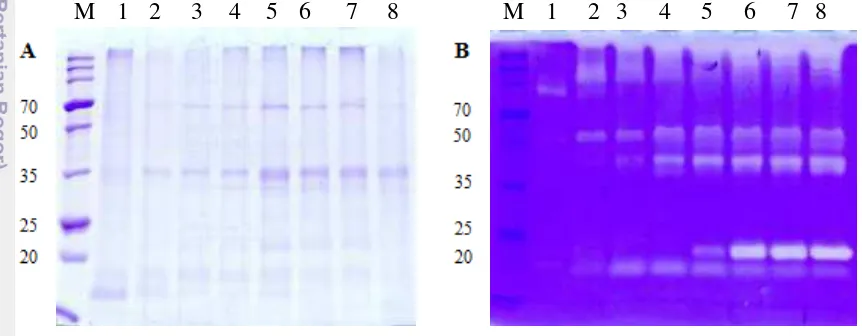
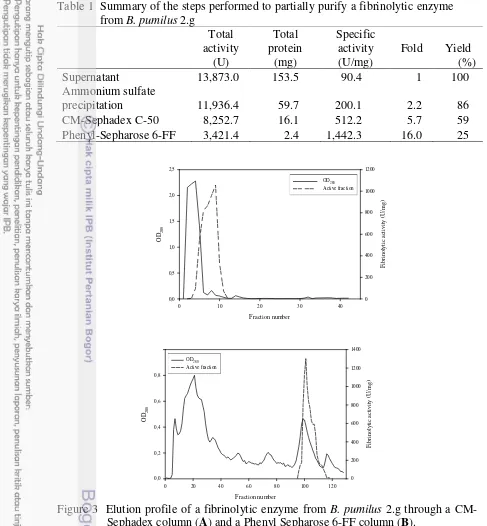
Bacillus pumilus 2.g menghasilkan beberapa fraksi protease yang memiliki aktivitas fibrinolitik kuat. Enzim fibrinolitik dengan berat molekul 20 kDa telah dimurnikan dari supernatan kultur B. pumilus 2.g dengan tahapan pengendapan amonium sulfat 80%, kromatografi penukar ion menggunakan matriks
CM-Sephadex C-50, dan kromatografi hidrofobik menggunakan matriks Phenyl Sepharose 6-FF. Kemurnian enzim meningkat 16 kali lipat dengan yield 25% pada tahap akhir pemurnian. Enzim fibrinolitik murni stabil antara pH 5 hingga pH 9 dan suhu kurang dari 60℃. Aktivitas fibrinolitik meningkat dengan adanya 5 mM MgCl2 dan 5 mM CaCl2 tetapi dihambat oleh 1 mM PMSF, 1 mM SDS, dan
1 mM EDTA. Enzim fibrinolitik murni dari B. pumilus 2.g mampu menghidrolisis substrat sintetik N-Succinyl-Ala-Ala-Pro-Phe-pNA, substrat untuk subtilisin dan kimotripsin, secara efisien. Hasil ini menunjukkan bahwa enzim fibrinolitik murni dari B. pumilus 2.g termasuk dalam golongan protease serin kelompok subtilisin. Enzim fibrinolitik murni dari B. pumilus β.g dapat mendegradasi rantai α dan β
dari fibrinogen dengan cepat tetapi tidak dapat mendegradasi rantai .
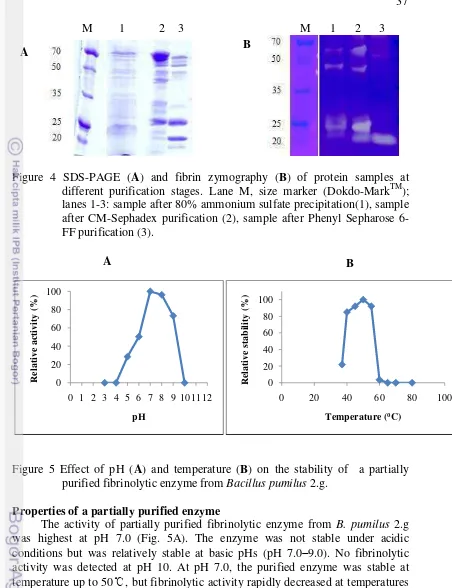
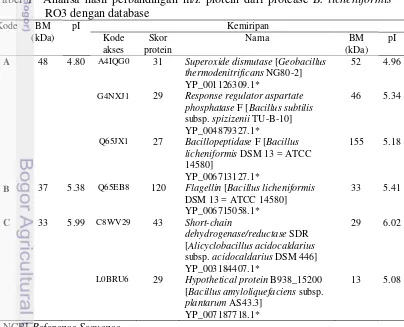
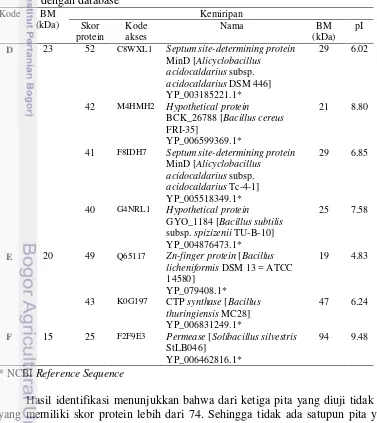
Bacillus licheniformis RO3 dan B. pumilus 2.g menghasilkan beberapa protease ekstraseluler. Dari tiga fraksi protease ekstraseluler B. licheniformis RO3 yang dipisahkan menggunakan elektroforesis 2D dan identifikasi menggunakan MALDI-TOF-MS, diperoleh satu fraksi dengan BM 48 kDa dan pI 4.80 yang memiliki kemiripan dengan bacillopeptidase F dari B. licheniformis DSM 13 dengan skor protein 27. Tiga fraksi dari B. pumilus 2.g yang dipisahkan menggunakan SDS-PAGE dan identifikasi menggunakan MALDI-TOF-MS, dengan BM masing-masing 23, 20, dan 15 kDa juga hanya memiliki kemiripan dengan protein database dengan skor protein ≤ 74. Kemungkinan beberapa fraksi protein tersebut adalah baru atau belum pernah dilaporkan sebelumnya.
SUMMARY
DIANA NUR AFIFAH. Fibrinolytic protease from fermented food red oncom and
tempeh gembus microorganisms. Supervised by MAGGY T. SUHARTONO, DAHRUL SYAH, and YANTI.
Cardiovascular diseases such as heart disease, high blood pressure, and stroke are the leading cause of death in the world, including Indonesia. Basic pathophysiology of myocardial infarction and stroke is the formation of fibrin clot or thrombus attached to an injured blood vessel walls. Fibrin is the major protein component of blood clots, which are formed from fibrinogen by thrombin.
Fibrinolytic enzyme from food microbes has attracted attention for thrombolytic agent. The genus Bacillus from fermented food can produce a strong fibrinolytic enzyme, such as Bacillus natto from natto, a fermented soy food from Japan that produce nattokinase (NK). The other Bacillus from various fermented food are B. amyloliquefaciens DC-4 from douchi, fermented soy food from China,
Bacillus sp. CK from chungkookjang, soy sauce fermentation of Korea, Bacillus
sp. DJ-2 strains and DJ-4 from doenjang, Korea, and Bacillus sp. KA38 from
jeotgal, fermented salted fish from Korea.
This study was able to isolate bacteria producing fibrinolytic proteases from Indonesian fermented food, red oncom and tempeh gembus. Forthy-three isolates showed proteolytic activities on skim milk agar, while thirthy-eight of them showed fibrinolytic activities both on fibrin plate and fibrinogen zymography. Two isolates, i.e. RO3 from red oncom and 2.g from tempeh gembus, were selected and identified using API CHB kit and 16S rRNA. The results of biochemical tests using API 50 CHB kit specifically for Bacillus spp, revealed that isolate RO3 was identified as B. licheniformis (99.9%) and isolate 2.g as B. pumilus (99.7%). Molecular identification based on 16SrRNAs gene was performed and isolate RO3 was confirmed as B. licheniformis (96%) and isolate 2.g was confirmed as B. pumilus (97%). The nucleotide sequences were deposited at GenBank with accession number AB968524 for isolate RO3 and AB968523 for isolate 2.g.
The high cost of enzyme production is one of the barriers to successful application of enzyme in the industry. Selection of media is a critical factor for the enzyme production. Bacillus licheniformis RO3 was screened from red oncom, an Indonesian fermented food. Three types of media were analyzed, i.e. Luria-bertani broth (LB), ½ LB + 1% skim milk (LBS), and ½ LB + 1% red oncom powder (LBO). In LB media, B. licheniformis RO3 was able to produce protease with activity of 0.024 U/ml or 0.157 U/mg at 36 h fermentation. In LBS media, the highest activity was 0.022 U/ml or 0.152 U/mg at 48 h fermentation. The best result was shown when B. licheniformis RO3 was grown on LBO media with the highest protease activity was 0.051 U/ml or 0.283 U/mg at 48 h. These results indicate that red oncom flour can be used as a good media for fibrinogenolytic protease production. Crude fibrinogenolytic protease enzyme from B. licheniformis RO3 had an optimum pH and temperature at 8.0 and 60°C.
application of ammonium sulfate precipitation 80%, ion-exchange chromatography by using CM-Sephadex C-50, and hydrophobic chromatography by using Phenyl-Sepharose 6-FF. After Phenyl-Sepharose chromatography step, the final purification fold was 16.0, and the yield was 25%. The partially purified enzyme was stable between pH 5 and pH 9 and temperature of less than 60℃. Fibrinolytic activity was increased by 5 mM MgCl2 and 5 mM CaCl2 but inhibited
by 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium dodecyl sulfate (SDS), and 1 mM ethylenediaminetetraacetic acid (EDTA). The partially purified enzyme quickly degrades the α and β chains of fibrinogen but cannot degrade the
chain.
Bacillus licheniformis RO3 and B. pumilus 2.g secretes several extracellular proteases. Three B. licheniformis RO3 extracellular protease fractions was separated by 2D electrophoresis and identified by MALDI-TOF-MS. One fraction with molecular weight of 48 kDa and pI 4.80 had similiarity to bacillopeptidase F from B. licheniformis DSM 13 in protein score, that is 27. The three fraction of B. pumilus 2.g was separated by MALDI-TOF-MS. Each fraction of it has molecule weight of 23, 20 and 15 kDa and protein score ≤ 74 compared protein database. The results showed that there are some new protein fraction found in both extracellular fibrinolytic proteases produced by Bacillus licheniformis RO3 and B. pumilus 2.g.
© Hak Cipta Milik IPB, Tahun 2014
Hak Cipta Dilindungi Undang-Undang
Dilarang mengutip sebagian atau seluruh karya tulis ini tanpa mencantumkan atau menyebutkan sumbernya. Pengutipan hanya untuk kepentingan pendidikan, penelitian, penulisan karya ilmiah, penyusunan laporan, penulisan kritik, atau tinjauan suatu masalah; dan pengutipan tersebut tidak merugikan kepentingan IPB
Disertasi
sebagai salah satu syarat untuk memperoleh gelar Doktor
pada
Program Studi Ilmu Pangan
PROTEASE FIBRINOLITIK DARI MIKROBA
PANGAN FERMENTASI ONCOM MERAH DAN TEMPE GEMBUS
SEKOLAH PASCASARJANA INSTITUT PERTANIAN BOGOR
BOGOR 2014
Penguji pada Ujian Tertutup: Prof Dr Ir Made Astawan, MSc
(Staf Pengajar Departemen Ilmu dan Teknologi Pangan Fakultas Teknologi Pertanian IPB)
Dr Harsi D. Kusumaningrum
(Staf Pengajar Departemen Ilmu dan Teknologi Pangan Fakultas Teknologi Pertanian IPB)
Penguji pada Ujian Terbuka: Prof drh Dondin Sajuthi, MST, PhD (Kepala Rumah Sakit Hewan IPB) Raymond R Tjandrawinata, PhD
PRAKATA
Puji dan syukur kepada Allah SWT atas segala karunia-Nya sehingga
disertasi dengan judul “Protease Fibrinolitik dari Mikroba Pangan Fermentasi
Oncom Merah dan Tempe Gembus” ini berhasil diselesaikan. Disertasi ini merupakan salah satu syarat untuk mencapai gelar Doktor pada Program Studi Ilmu Pangan, Sekolah Pascasarjana Institut Pertanian Bogor.
Sebagian hasil penelitian dalam disertasi ini telah diajukan sebagai artikel ilmiah pada beberapa jurnal, yaitu 1) Proteolytic and fibrinolytic activities of several microorganisms screened from red oncom and tempeh gembus, Indonesian fermented soybean cakes, abstraknya telah dipresentasikan secara oral pada 4th Annual International Symposium on Wellness, Healthy Lifestyle and Nutrition di Yogyakarta pada 30 November – 1 Desember 2013, sedangkan artikel lengkapnya telah diterima untuk dipublikasikan pada Malaysian Journal of Microbiology, 2) The use of red oncom powder as potential production media for fibrinogenolytic protease derived from Bacillus licheniformis RO3, telah dipresentasikan secara oral pada International Symposium on Food and Agro-Biodiversity di Semarang pada 16 – 17 Sepetember 2014 dan artikel lengkapnya sedang dalam proses telaah untuk diterbitkan pada Proceedia Food Science Elsevier, 3) Purification and characterization of a fibrinolytic enzyme from Bacillus pumilus 2.g isolated from tempeh gembus, an Indonesian fermented food, telah dipublikasikan pada Preventive Nutrition and Food Science Journal (PNF) Vol 19(3): 213-219, (4) Studi proteomik protease fibrinolitik ekstraseluler dari
Bacillus licheniformis RO3 dan Bacillus pumilus 2.g yang diisolasi dari pangan fermentasi indonesia.
Terima kasih kepada Ibu Prof Dr Maggy Thenawidjaja Suhartono, Bapak Dr Ir Dahrul Syah, MScAgr, dan Ibu Yanti, PhD selaku pembimbing yang telah banyak memberikan motivasi, arahan, dan bimbingan hingga terselesaikannya disertasi ini. Terima kasih kepada Prof Jeong Hwan Kim selaku pembimbing penelitian di Gyeongsang National University, Jinju, Korea. Terima kasih kepada penguji pada ujian tertutup: Prof Dr Ir Made Astawan, MSc dan Dr Harsi D. Kusumaningrum, serta penguji pada ujian terbuka: Prof drh Dondin Sajuthi, MST, PhD dan Raymond R Tjandrawinata, PhD. Terima kasih kepada DIKTI, Kemendikbud, Republik Indonesia atas bantuan beasiswa BPPS tahun 2010-2014, bantuan program Sandwich-like tahun 2013, dan bantuan penelitian melalui program Hibah Bersaing atas nama Prof. dr. Muhammad Sulchan, M.Sc, DA Nutr, SpGK. Terima kasih kepada Universitas Diponegoro yang telah memberikan bantuan pendidikan dan bantuan penelitian melalui program Hibah Doktor dana PNBP Undip 2014. Terima kasih kepada teman-teman IPN 2010, 2011, 2012, atas kebersamaannya. Ungkapan terima kasih juga disampaikan kepada keluarga, kedua orang tua, mertua, suami, anak-anak, kakak, adek, dan semua pihak yang telah membantu baik dalam pelaksanaan kuliah, penelitian, hingga penyusunan disertasi ini. Semoga karya ilmiah ini bermanfaat untuk pengembangan ilmu dan pengetahuan di bidang Ilmu Pangan dan bidang terkait lainnya.
Bogor, Desember 2014
DAFTAR ISI
DAFTAR ISI
1 PENDAHULUAN 1
Latar Belakang 1
Perumusan Masalah 2
Tujuan Penelitian 3
Manfaat Penelitian 3
Hipotesis Penelitian 4
Daftar Pustaka 4
2 METODOLOGI PENELITIAN 6
Waktu dan Tempat Penelitian 6
Tahapan Penelitian 6
Penelitian Tahap I 6
Penelitian Tahap II 6
Penelitian Tahap III 7
Penelitian Tahap IV 7
3 PROTEOLYTIC AND FIBRINOLYTIC ACTIVITIES OF SEVERAL MICROORGANISMS SCREENED FROM RED ONCOM AND
TEMPEH GEMBUS, INDONESIAN FERMENTED SOYBEAN CAKES
10
Abstract 10
Introduction 10
Materials and Methods 11
Results 13
Discussion 17
Conclusion 18
References 18
4 THE USE OF RED ONCOM POWDER AS POTENTIAL
PRODUCTION MEDIA FOR FIBRINOGENOLYTIC PROTEASE DERIVED FROM Bacillus licheniformis RO3
21
Abstract 21
Introduction 21
Materials and Methods 22
Results and Discussion 24
Conclusion 29
References 29
5 PURIFICATION AND CHARACTERIZATION OF A FIBRINOLYTIC ENZYME FROM Bacillus pumilus 2.g ISOLATED FROM TEMPEHGEMBUS, AN INDONESIAN FERMENTED FOOD
31
Abstract 31
Introduction 31
Materials and Methods 32
Results and Discussion 34
6 STUDI PROTEOMIK PROTEASE FIBRINOLITIK
EKSTRASELULER DARI Bacillus licheniformis RO3 dan Bacillus pumilus 2.g YANG DIISOLASI DARI PANGAN FERMENTASI INDONESIA
42
Abstrak 42
Pendahuluan 42
Bahan dan Metode 43
Hasil dan Pembahasan 45
Simpulan 49
Daftar Pustaka 49
7 PEMBAHASAN UMUM 52
Isolasi dan Identifikasi Mikroba dari Pangan Fermentasi Oncom Merah dan Tempe Gembus yang dapat Memproduksi Protease Fibrinolitik
52
Pemanfaatan Tepung Oncom Merah sebagai Media Produksi Protease Fibrinogenolitik Mikroba
57
Pemurnian dan Karakterisasi Enzim Fibrinolitik 59 Studi Proteomik Enzim Protease Fibrinolitik
dari Mikroba Pangan Fermentasi
63
Daftar Pustaka 65
8 SIMPULAN DAN SARAN 69
Simpulan 69
Saran 69
LAMPIRAN RIWAYAT HIDUP
1 PENDAHULUAN
Latar Belakang
Penyakit kardiovaskular yang meliputi infark miokardial akut, penyakit jantung iskemik, penyakit jantung valvular, aritmia, tekanan darah tinggi, dan stroke adalah penyebab utama kematian di dunia, termasuk Indonesia. Pada tahun 2008, sekitar 17,3 juta orang meninggal karena penyakit kardiovaskular, atau 30% dari semua penyebab kematian global. Dari jumlah ini, 7,3 juta disebabkan oleh penyakit jantung koroner dan 6,2 juta disebabkan oleh stroke. Pada tahun 2030 diperkirakan jumlahnya akan meningkat hingga 23,6 juta orang (WHO 2011). Dasar patofisiologi proses infark miokardial dan stroke adalah pembentukan trombus atau bekuan fibrin yang melekat pada dinding pembuluh darah yang terluka. Akumulasi fibrin dalam pembuluh darah dapat mengganggu aliran darah dan merusak jaringan pada jantung, menyebabkan denyut jantung tidak teratur, serangan jantung, dan kematian.
Fibrin merupakan komponen protein utama dari pembekuan darah, yang terbentuk dari fibrinogen oleh trombin (Voet & Voet 1990). Serat fibrin yang tidak larut dapat dihidrolisis menjadi produk degradasi fibrin oleh plasmin, yang dihasilkan dari plasminogen oleh aktivator plasminogen seperti aktivator plasminogen jaringan, aktivator plasminogen vaskular, aktivator plasminogen darah, urokinase, faktor Hageman, dan kompleks plasminogen-streptokinase (Collen & Lijnen 1991).
Terapi trombolitik melalui suntikan maupun oral oleh agen trombolitik untuk mendegradasi trombus dalam darah telah banyak diteliti dan dipraktekkan (Goldhaber & Bounameaux 2001; Tough 2005). Berdasarkan mekanisme kerjanya, agen trombolitik diklasifikasikan menjadi dua jenis. Pertama adalah aktivator plasminogen, seperti aktivator plasminogen jaringan (t-PA) (Collen & Lijnen 2004) dan urokinase (Duffy 2002), yang mengaktifkan plasminogen menjadi plasmin aktif untuk mendegradasi fibrin. Jenis lainnya adalah plasmin-like protein, yang langsung mendegradasi fibrin dalam pembekuan darah, sehingga dapat melarutkan trombus dengan cepat dan sempurna. Lumbrokinase dari cacing tanah dan fibrolase dari bisa ular dikenal sebagai plasmin-like protein (Chen et al. 1991; Mihara et al. 1991). Meskipun t-PA dan urokinase masih banyak digunakan dalam terapi trombolitik, harganya yang mahal (100 mg t-PA adalah $2,750) dan adanya efek samping yang tidak diinginkan, seperti risiko pendarahan dalam usus ketika dikonsumsi secara oral (Peng et al. 2005), mendorong berbagai penelitian untuk mencari sumber agen trombolitik yang lebih murah dan lebih aman.
Enzim fibrinolitik atau dikenal sebagai protease fibrinolitik dari mikroba telah menarik perhatian untuk diteliti lebih jauh sebagai agen trombolitik. Streptokinase dari Streptococcus hemolyticus dan stafilokinase dari
2
Genus Bacillus dari pangan fermentasi ternyata juga dapat menghasilkan enzim fibrinolitik kuat, seperti Bacillus natto dari natto, pangan fermentasi kedelai asal Jepang yang menghasilkan nattokinase (NK). Pemberian natto atau enzimnya secara oral tidak hanya dapat mendegradasi trombus secara langsung namun juga dapat meningkatkan pelepasan aktivator plasminogen endogen pada hewan percobaan dan subjek manusia (Sumi et al. 1990). Beberapa genus Bacillus
lainnya dari berbagai pangan fermentasi juga telah ditemukan mampu menghasilkan enzim fibrinolitik kuat. Diantaranya adalah B. amyloliquefaciens
DC-4 dari douchi, pangan fermentasi kedelai dari Cina (Peng & Zhang 2002),
Bacillus sp. CK dari chungkookjang, saus kedelai fermentasi dari Korea (Kim et al. 1996), Bacillus sp. strains DJ-2 dan DJ-4 dari doenjang, Korea (Choi et al.
2005; Kim & Choi 2000), dan Bacillus sp. KA38 dari jeotgal,ikan asin fermentasi dari Korea (Kim et al. 1997). Yoon et al. (2002) melakukan skrining secara sistematis terhadap strain penghasil enzim fibrinolitik dari berbagai pangan fermentasi komersial maupun buatan sendiri termasuk natto, chungkook-jang,
doen-jang, jeot-gal, dan tempe, pangan fermentasi dari Indonesia dan berhasil mengisolasi Enterococcus faecalis yang mampu memproduksi enzim dengan aktivitas fibrinolitik yang tinggi.
Berbagai temuan menarik tersebut menyiratkan bahwa dengan mengonsumsi makanan fermentasi yang mengandung protein tinggi dapat mencegah penyakit kardiovaskular. Mikroba penghasil enzim fibrinolitik yang ditemukan pada pangan fermentasi selain Bacillus, diantaranya adalah Fusarium
sp. BLB dari tempe (Sugimoto et al. 2007) dan Rhizopus chinensis 12 dari arak beras, Cina (Xiao-lan et al. 2005). Bahan pangan lain yang terbukti memiliki aktivitas fibrinolitik diantaranya adalah jamur pangan. Sejumlah peneliti telah melaporkan bahwa kandungan senyawa aktif dalam berbagai jamur pangan,
Cordyceps militaris (Kim et al. 2006; Choi et al. 2011), Schizophyllum commune
(Lu et al. 2010), Pleurotus eryngii (Cha et al. 2010), Volvariela volvaceae
(Sajuthi et al. 2010) yang diduga dapat mempengaruhi sirkulasi darah adalah protease yang memiliki aktivitas fibrinolitik.
Adanya manfaat biologis yang menjanjikan dari mengonsumsi makanan sumber enzim fibrinolitik, terbuka kesempatan luas untuk mengeksplorasi sumber enzim fibrinolitik baru dari pangan fermentasi khas Indonesia. Oncom merah dan tempe gembus merupakan salah satu pangan fermentasi khas Indonesia yang banyak ditemukan di daerah Jawa Barat dan Jawa Tengah yang terbuat dari fermentasi ampas kedelai. Hingga saat ini belum ada penelitian mengenai enzim fibrinolitik yang ada di oncom merah dan tempe gembus, baik penelitian isolasi bakteri penghasil enzim fibrinolitiknya maupun ekstraksi enzim fibrinolitik yang ada dalam oncom merah dan tempe gembus.
Perumusan masalah
Kacang-kacangan seperti kedelai dan hasil perikanan yang difermentasi ternyata memiliki aktivitas fibrinolitik yang kuat. Pangan fermentasi kedelai yang terkenal di Indonesia adalah tempe, oncom, dan tempe gembus. Berbeda dengan tempe, oncom dan tempe gembus merupakan pangan fermentasi ampas kedelai. Ampas kedelai atau lebih dikenal sebagai ampas tahu adalah limbah hasil pembuatan tahu. Aktivitas fibrinolitik kuat dari mikroba yang diisolasi dari tempe telah dilaporkan oleh beberapa peneliti. Namun penelitian mengenai enzim fibrinolitik yang ada di oncom merah dan tempe gembus, terutama mengenai isolasi mikroba penghasil enzim fibrinolitik maupun ekstraksi enzim fibrinolitik yang ada dalam oncom merah dan tempe gembus belum pernah dilaporkan.
Dalam aplikasi khusus enzim pemecah fibrin, temuan mikroba fibrinolitik lokal adalah tahap awal yang harus diikuti tahap berikutnya yaitu mencari media produksi yang efektif dan efisien. Tingginya biaya produksi enzim merupakan salah satu hambatan suksesnya aplikasi enzim di industri. Pemilihan media produksi merupakan faktor kritis untuk fermentasi enzim fibrinolitik.
Karakteristik enzim fibrinolitik mikroba pangan yang sudah terbukti aman dan efektivitasnya sebagai agen trombolitik perlu diketahui dengan baik. Karakteristik ini dapat diketahui menggunakan analisis biokimia. Informasi karakter enzim fibrinolitik mikroba dari pangan fermentasi asal Indonesia sangat diperlukan dalam aplikasi sebagai pangan fungsional maupun pengembangan selanjutnya sebagai obat trombolitik yang aman.
Dari latar belakang yang telah dikemukakan, dirumuskan beberapa masalah yang dikaji lebih lanjut dalam penelitian, yaitu: apakah di dalam oncom merah dan tempe gembus ditemukan adanya mikroba penghasil protease yang bersifat fibrinolitik, apakah tepung oncom merah dapat digunakan sebagai media untuk menumbuhkan mikroba penghasil protease yang bersifat fibrinogenolitik, bagaimanakah karakteristik dari enzim fibrinolitik yang dihasilkan oleh mikroba terpilih setelah dilakukan pemurnian?
Tujuan Penelitian
Tujuan umum dari penelitian yang dilaksanakan adalah melakukan kajian terhadap protease fibrinolitik mikroba dari oncom merah dan tempe gembus. Tujuan khusus dari penelitian yang dilaksanakan adalah: (1) memilah dan mengidentifikasi mikroba dari pangan fermentasi oncom merah dan tempe gembus yang dapat memproduksi protease fibrinolitik, (2) memperoleh media yang baik untuk produksi enzim dengan pemanfaatan tepung oncom merah, (3) melakukan pemurnian dan karakterisasi enzim yang dihasilkan oleh mikroba terpilih, (4) melakukan studi proteomik enzim protease fibrinolitik dari mikroba pangan fermentasi.
Manfaat Penelitian
4
pengembangan pangan fungsional maupun pemanfaatan enzim sebagai obat trombolitik.
Hipotesis Penelitian
1. Di dalam oncom merah dan tempe gembus terdapat mikroba penghasil protease fibrinolitik.
2. Tepung oncom merah dapat digunakan sebagai media pertumbuhan mikroba penghasil protease fibrinogenolitik.
3. Enzim yang dihasilkan memiliki karakteristik tertentu uang selanjutnya dapat diaplikasikan dalam pengembangan pangan fungsional maupun dimanfaatkan sebagai obat untuk terapi penyakit kardiovaskular.
4. Terdapat beberapa fraksi protease fibrinolitik dari mikroba pangan fermentasi terpilih.
Daftar Pustaka
Banerjee A, Chisti Y, Banerjee UC. Streptokinase-a clinically useful thrombolytic agent. Biotechnology Advances 22:287–307.
Cha WS, Park SS, Kim SJ, Choi D. 2010. Biochemical and enzymatic properties of a fibrinolytic enzyme from Pleurotus eryngii cultivated under solid-state conditions using corn cob. Bioresource Technology 101:6475–6481.
Chen HM, Guan AL, Markland FS. 1991. Immunological properties of the fibrinolytic enzyme (fibrolase) from southern copperhead (Agkistrodon contortrix contortrix) venom and its purification by immunoaffinity chromatograph. Toxicon 29(6):683–694.
Choi D, Cha W, Park N, Kim H, Lee JH, Park JS, Park S.β011. Purification and
characterization of a novel fibrinolytic enzyme from fruiting bodies of Korean Cordyceps militaris.Bioresource Technology 102:3279–3285. Choi NS, Yoo KH, Hahm JH, Yoon KS, Chang KT, Hyun BH, Maeng PJ, Kim
SH. 2005. Purification and characterization of a new peptidase, bacillopeptidase DJ-2, having fibrinolytic activity: produced by Bacillus sp. DJ-2 from Doen-Jang. J Microbiol Biotechnol 15(1): 72–79.
Collen D, Lijnen HR. 2004. Tissue-type plasminogen activator: a historical perspective and personal account. J Thromb Haemost 2(4):541–546.
Collen D, Lijnen HR. 1994. Staphylokinase, a fibrin-specific plasminogen activator with therapeutic potential? Blood 84(3):680–686.
Collen D, Lijnen HR. 1991. Basic and clinical aspects of fibrinolysis and thrombosis. Blood 78:3114-3124.
Duffy MJ. 2002. Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies.
Clin Chem 48(8): 1194–1197.
Goldhaber SZ, Bounameaux H. 2001. Thrombolytic therapy in pulmonary embolism. Semin Vasc Med 1(2):213–220.
Kim SH, Choi NS. 2000. Purification and characterization of subtilisin DJ-4 secreted by Bacillus sp. strain DJ-4 screened from Doen-Jang. Biosci Biotechnol Biochem 64:1722–1725.
Kim HK, Kim GT, Kim DK, Choi WA, Park SH, Jeong YK, Kong IS. 1997. Purification and characterization of a novel fibrinolytic enzyme from Bacillus sp. KA38 originated from fermented fish. J Ferment Bioeng 84(4): 307–312.
Kim W, Choi K, Kim Y. 1996. Purification and characterization of a fibrinolytic enzyme produced from Bacillus sp. Strain CK 11-4 screened from Chungkook-Jang. Appl. En iron. Microbiol. 62:2482-2488.
Lu CL, Chen S, Chen SN. 2010. Purification and Characterization of a Novel Fibrinolytic Protease from Schizophyllum commune. Journal of Food and Drug Analysis 18(2):69-76.
Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikedo R, Seiki M, Maruyama M. 1991. A novel fibrinolytic enzyme extracted from the earthworm Lumbricus rubellus.Jpn J Physiol 41(3): 461–472
Peng Y, Yang X, Zhang Y. 2005. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo. Appl Microbiol Biotechnol 69:126–132.
Peng Y, Zhang YZ. 2002. Optimation of fermentation conditions of douchi fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4. Chin J Appl Environ Biol 8:285-289.
Sajuthi D, Suparto I, Yanti, Praira W. 2010. Purifikasi dan pencirian enzim protease fibrinolitik dari ekstrak jamur merang. Makara sains 14(2):145-150. Sugimoto S, Fujii T, Morimiya T, Johdo O, Nakamura T. 2007. The fibrinolytic
activity of a novel protease derived from tempeh producing fungus,
Fusarium sp. BLB. Biosci. Biotechnol. Biochem. 71(9):2184-2189.
Sumi H, Hamada H, Nakanishi K, Hiratani H. 1990. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 84:139-143.
Turpie AG, Chin BS, Lip GY. 2002. Venous thromboembolism: treatment strategies. British Medical Journal 325:948–950.
Tough J. 2005. Thrombolytic therapy in acute myocardial infarction. Nurs Stand
19(37):55–64.
Voet D, Voet JG. 1990. Biochemistry. Ed ke-2. New York: Wiley. hlm 1087-1095. WHO. 2011. Global status report on noncommunicable diseases 2010.
World Health Organization. Geneva.
Xiao-lan L, Lian-Xiang D, Fu-Ping L, Xi-Qun Z, Jing X. 2005. Purification and characterization of a novel fibrinolytic enzyme from Rhizopus chinensis 12.
Appl Microbiol Biotechnol 67(2): 209–214.
2 METODOLOGI PENELITIAN
Waktu dan Tempat Penelitian
Penelitian dilaksanakan pada bulan Februari 2012 sampai November 2013 di Laboratorium Mikrobiologi dan Biokimia Pusat Penelitian Sumberdaya Hayati, IPB, Laboratorium Mutu dan Keamanan Pangan, SEAFAST center IPB, Laboratorium Biokimia dan Teknologi Enzim, Universitas Katholik Atma Jaya Jakarta, dan Laboratorium Mikrobiologi, Gyeongsang National University, Jinju, Korea.
Tahapan Penelitian
Penelitian akan melalui tiga tahapan, yaitu (1) isolasi dan identifikasi mikroba dari pangan fermentasi oncom merah dan tempe gembus yang dapat memproduksi protease fibrinolitik, (2) pemanfaatan tepung oncom merah sebagai media produksi protease fibrinogenolitik mikroba, (3) pemurnian dan karakterisasi enzim yang dihasilkan oleh mikroba terpilih, (4) studi proteomik enzim protease fibrinolitik dari mikroba pangan fermentasi.
Penelitian Tahap I
Penelitian tahap pertama adalah isolasi mikroba penghasil protease fibrinolitik dengan menggunakan media SMA. Sampel yang digunakan adalah oncom merah segar, oncom merah yang telah dipanaskan 80oC selama 15 menit, tempe gembus segar, dan tempe gembus yang telah dipanaskan 80oC selama 15 menit. Mikroba yang mampu menghasilkan zona bening ditumbuhkan dalam media Luria Bertani broth (LB) agar dapat memproduksi protease fibrinolitik. Protease fibrinolitik yang dihasilkan diuji aktivitasnya secara kuantitatif menggunakan cakram fibrin (Hwang et al. 2007) dan kualitatif dengan teknik zimografi. Substrat yang digunakan untuk zimografi adalah fibrinogen. Mikroba terpilih adalah yang mampu menghasilkan enzim yang dapat mendegradasi substrat yang ditandai dengan adanya garis bening pada gel. Pada tahap isolasi diutamakan untuk mendapatkan mikroba yang aman dan dapat memproduksi protease fibrinolitik dengan berat molekul yang relatif rendah.
Mikroba terpilih kemudian disimpan dalam gliserol sebagai stok bakteri untuk pengujian selanjutnya. Mikroba yang diharapkan adalah Bacillus yang aman dikonsumsi sehingga pemilihan isolat dilakukan dengan tahapan identifikasi isolat sebagai berikut: pewarnaan Gram yang dilanjutkan dengan pewarnaan spora, uji biokimiawi dengan kit API 50CHB dan APIWEBTMsoftware, dan identifikasi secara molekuler dengan analisis 16S-rRNA.
Penelitian Tahap II
(LB), ½ LB + susu skim 1% (b/v), dan ½ LB + tepung oncom merah 1% (b/v) pada suhu 37oC dalam inkubator goyang. Optical density (OD620), aktivitas
protease (Bergmeyer & Grassl 1983), aktivitas fibrinogenolitik secara kualitatif dengan zimografi, dan konsentrasi protein (Bradford 1976) diukur setiap 12 jam sekali selama 72 jam.
Penelitian Tahap III
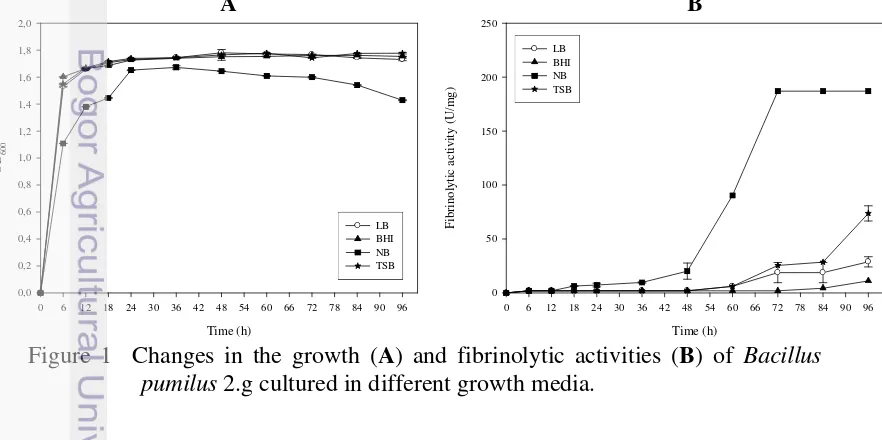
Penelitian tahap ketiga merupakan tahap pemurnian dan karakterisasi enzim yang dihasilkan oleh mikroba terpilih. Mikroba yang dipilih adalah B. pumilus 2.g yang diisolasi dari tempe gembus. Sebelum dilakukan pemurnian, pemilihan media produksi dilakukan pada 4 media yang berbeda, yaitu Luria Bertani broth (LB), tryptic soy broth (TSB), nutrient broth (NB), dan brain heart infusion (BHI). Pemurnian enzim dilakukan dengan pengendapan amonium sulfat, kromatografi penukar ion, dan kromatografi interaksi hidrofobik. Karakteristik yang diamati adalah berat molekul, stabilitas pH, stabilitas suhu, pengaruh inhibitor dan aktivator, spesifisitas substrat, dan degradasi fibrinogen.
Penelitian Tahap IV
9
Gambar 2 Diagram alir pelaksanaan penelitian tahap III dan IV Fermentasi pada 4
media yang berbeda
salting-out dengan amonium sulfat
Tahap III Pemurnian dan karakterisasi enzim fibrinolitik mikroba
Mikroba terpilih B. pumilus 2.g
Kondisi pertumbuhan terpilih
Produksi enzim
supernatan
Karakterisasi biokimia enzim
Enzim murni dialisis dan freeze dry
Kromatografi penukar ion, dialisis, freeze dry
pelet
Enzim kasar
Enzim semi murni
Kromatografi interaksi hidrofobik, dialisis, freeze
Tahap IV
Studi proteomik protease dari mikroba pangan fermentasi
Produksi enzim Mikroba terpilih B. licheniformis RO3
Supernatan
Elektroforesis satu dimensi (SDS-PAGE)
Elektroforesis dua dimensi (2D)
MALDI-TOF
Identifikasi
Mikroba terpilih B.pumilus 2.g
Produksi enzim
Enzim murni
Elektroforesis satu dimensi (SDS-PAGE)
MALDI-TOF
10
1
Abstraknya telah dipresentasikan secara oral pada 4th Annual International Symposium on Wellness, Healthy Lifestyle and Nutrition di Yogyakarta pada 30 November – 1 Desember 2013, sedangkan artikel lengkapnya telah diterima untuk dipublikasikan pada Malaysian Journal of Microbiology
3 PROTEOLYTIC AND FIBRINOLYTIC ACTIVITIES OF SEVERAL
MICROORGANISMS SCREENED FROM RED ONCOM AND
TEMPEH GEMBUS, INDONESIAN FERMENTED SOYBEAN CAKES1
Abstract
This study was to isolate, screen, and identify microorganisms from fermented food Red Oncom and Tempeh Gembus that can produce fibrinolytic proteases. Forthy-three isolates showed proteolytic activities on skim milk agar, while thirthy-eight of them showed fibrinolytic activities both on a fibrin plate and a fibrinogen zymography. The isolates that showed activity in fibrin plate and fibrinogen zymography with lower molecular weight and considered as safe were chosen and identified as Bacillus licheniformis and Bacillus pumilus by using API CHB kit and 16S rRNA. The novel fibrinolytic microorganisms were referred to as B. licheniformis RO3 and B. pumilus 2.g. Red Oncom and Tempeh Gembus are potential sources of microbial fibrinolytic protease. This is the first time that Red Oncom and Tempeh Gembus as Indonesian fermented foods based on soybean cake were shown having fibrinolytic microorganism. Microbial fibrinolytic enzymes from these fermented foods can be used for functional food formulation to prevent thrombosis and other related diseases.
Keywords: microbial fibrinolytic enzyme, red oncom, zymography,
B. licheniformis, B. pumilus
Introduction
Cardiovascular diseases are the leading cause of death in the world, including in Indonesia (WHO 2011). Accumulation of fibrin in the blood vessels usually results in thrombosis, leading to myocardial infarction and other cardiovascular diseases (Peng et al. 2005). Fibrin is the major protein component of blood clots, which are formed from fibrinogen by thrombin. Insoluble fibrin can be hydrolyzed to fibrin degradation products by plasmin, which is generated from plasminogen by plasminogen activators such as tissue plasminogen activator, vascular plasminogen activator, blood plasminogen activator, urokinase, Hageman factor and plasminogen-streptokinase complex (Collen and Lijnen 2004).
Thrombolytic therapy by injection and oral thrombolytic agents to degrade the thrombus in the blood have been widely studied and practiced (Tough 2005). Based on its mechanism of action, thrombolytic agents are classified into two types. The first is a plasminogen activator, such as tissue plasminogen activator (t-PA) and urokinase which activates plasminogen to plasmin and further hydrolyses fibrin (Collen and Lijnen 2004). Other type for thrombolytic therapy is using plasmin-like proteins, which directly degrade fibrin in the blood clotting, thus dissolve the thrombus quickly and perfectly. Lumbrokinase of earthworms and fibrolase of snake venom are known as plasmin-like protein (Mihara et al.
This encourages study to find the source of thrombolytic agent that is cheaper and safer.
Fibrinolytic enzyme originated from microbes has attracted the attention of many researchers to be applied as a thrombolytic agent. Streptokinase from
Streptococcus hemolyticus and Staphylokinase from Staphylococcus sp. are potential alternative plasminogen activator and had proven effective in thrombolytic therapy (Banarjee et al. 2004). However, Streptokinase is available in large quantities at high prices and during the production and purification is easily contaminated by other proteins that can cause antigenic effect. Furthermore, repeated use for a long time can induce allergies (Banerjee et al. 2004).
Recently, many fibrinolytic enzymes have been identified from traditional fermented foods such as Japanese Natto (Fujita et al. 1993), Chinese Douchi
(Peng and Zhang 2002), Korean Doen-jang (Choi et al. 2005), Korean
Cheonggukjang (Jeong et al. 2007) and Korean Meju (Jo et al. 2011a). These interesting reports imply some fermented foods contain enzymes that can potentially prevent cardiovascular diseases. This fact opens opportunities to explore new sources of fibrinolytic enzyme from typical Indonesian fermented food. Red Oncom and Tempeh Gembus are two of the typical Indonesian fermented foods made from fermented soy pulp. Until now there has been no research on the fibrinolytic enzyme from Red Oncom and Tempeh Gembus, either isolation of bacteria producing enzymes or extraction of fibrinolytic enzyme present in Red Oncom and Tempeh Gembus. The purpose of this study was to isolate, screen, and identify microbes from fermented food Red Oncom and
Tempeh Gembus that can produce fibrinolytic proteases. Materials and Methods
Materials
Red oncom was obtained from the traditional markets in Bogor, West Java, whereas Tempeh Gembus was obtained from the traditional markets in Semarang, Central Java. Growth media including skim milk agar (SMA) was purchased from Difco. Luria-Bertani broth (LB) was made from yeast extract and tryptone were purchased from Oxoid. Fibrinogen from bovine plasma was purchased from Sigma. API 50CHB kit was purchased from bioMerieux.
Isolation of Proteolytic Bacterial Strain
Heat pretreatment at 80oC for 15 min was applied to some of the samples to avoid possible pathogen contamination. One-tenth gram of the sample was suspended in sterile physiological saline (0.85% NaCl). The suspension was plated on sterile skim milk agar (SMA), and then incubated at 37oC for 48 h. A clear zone of skim milk hydrolysis gave an indication of protease producing organisms. At the end of incubation period, the protease colonies were picked, purified and sent for further fibrinolytic screening.
Fibrinolytic protease production
12
incubation, the fermentation broth was centrifuged at 6000 g at 4oC for 15 min. The clear supernatant was collected as source of enzyme.
Analysis of protease activity and protein determination
Protease activity was measured according to the Bergmeyer method (Bergmeyer et al. 1λ83) with casein (1%) as the substrate. As much as 50 l
enzyme filtrate was mixed with 250 l substrate and incubated at 37o
C for 10 min. Trichloroacetic acid (TCA) 0.1 M was added and incubated at 37oC for 10 min, and centrifuged at 4000 g for 10 min. The supernatant was mixed with Na2CO3
0.4 M, followed by addition of Folin Ciocalteau reagent (1:2) and incubated further at 37°C for 20 min. The reaction products were measured at 578 nm. Substrate solution without enzyme was used as control. One unit (U) of enzyme activity was defined as enzyme which produces 1 mol of tyrosine per min.
Protein concentration was analysed by Bradford’s method (1λ76) using reagents consisted of 100 mg Coomassie brilliant blue (CBB) G-250 in 50 ml ethanol 95% and 100 ml phosphate acid 85% in 1 liter. Bovine serum albumin was used as the protein standard. Triplicate experiments were conducted for each measurement.
Screening of Fibrinolytic Bacterial Strain
Fibrinolytic bacterial strain was screened by fibrin plate and fibrinogen zymography (Hwang et al. 2007). 0.2% fibrinogen solution in 50 mM sodium phosphate buffer (pH 8) was mixed with 2% agarose solution along with 0.02 ml of a thrombin solution (100 NIH units). The solution was applied to a petri dish and left for 1 h at room temperature to form a fibrin clot layer. Twenty l of the enzymes was dropped onto a fibrin plate and incubated at 37oC for 5 h. The activity of fibrinolytic enzyme was estimated by measuring the dimensions of the clear zone on the fibrin plate. Fibrinogen zymography was carried out in 12% polyacrylamide gels containing 0.1% fibrinogen. The enzymes were diluted in 5x sample buffer, which consisted of 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, and 0.1% bromophenol blue. Electrophoresis was carried out at 70 V and 50 A for 4 h until the bromophenol blue reached the bottom of the gel. After electrophoresis, the gel was soaked in 2.5% Triton X-100, for 1 hour at room temperature for enzyme renaturation. The gel was washed with distilled water to remove Triton X-100, and then incubated with 50 mM phosphate buffer (pH 8) at 37oC for 12 hours. The gel was stained with Coomassie blue for 1 h and then destained. The clear bands, correspond to the areas where fibrinogen was digested. Identification of microorganisms
Identification of microorganisms followed three steps. First is microbiology analysis i.e. Gram staining, spore staining, and morphological examination. Second is biochemical tests with API 50CHB kit for Bacillus spp. followed by identification using Apiweb™ software. Third is molecular identification for the best fibrinolytic bacterial strain. The selected microorganisms were enriched, and its 16SrRNAs were analyzed in order to identify the microorganisms at the species level. Amplification of 16SrRNA was performed with universal primers,
63Fμ 5’-CAG GCC TAA CAC ATG CAA GTC-3’ and 1387Rμ 5’-GGG CGG
PCR products was isolated from the agarose gel and sequenced with a BidDye Terminator v3.1 cycle sequencing chemistry, genetic analyzer 3730XL (Applied Biosystems). A sequence similarity search was performed using BLAST in the NCBI database.
Results
Proteolytic activity of several microorganisms
Isolation of microbes from Red oncom and Tempeh Gembus revealed 43 isolates, 30 isolates from Red Oncom and 13 isolates from Tempeh Gembus that produce protease enzyme characterized by their ability to produce clear zones on the SMA plate. Figure 1 showed the clearing zone observed from the best proteolytic microorganism when grown in SMA. Isolates tested were: RO3 and 2.g.
Figure 1 Proteolytic activity of several microorganisms
Fibrinolytic and fibrinogenolytic activity of several microorganisms
Fibrinolytic activity on the fibrin plate showed that 40 isolates were able to produce a clear zone. The activity of fibrinolytic enzyme was estimated by measuring the dimensions of the clear zone on the fibrin plate (Table 1).
Table 1 Fibrinolytic activities on fibrin plate Isolate Fibrinolytic
activities (cm)
Isolate Fibrinolytic activities (cm)
Isolate Fibrinolytic activities (cm)
RO1 0.90±0.10 RO16 0.47±0.15 1.g 0.90±0.00
RO2 0.87±0.15 RO17 0.37±0.12 2.g 1.45±0.07
RO3 1.00±0.10 RO18 0.33±0.06 3.g 0.90±0.14
RO4 None RO19 0.30±0.00 4.g 1.00±0.00
RO5 1.23±0.25 ROa 0.50±0.00 5.g 0.70±0.14
RO6 0.97±0.12 ROb 0.37±0.12 6.g 1.00±0.00
RO7 1.23±0.15 ROc 0.33±0.06 7.g 0.45±0.07
RO8 1.13±0.32 ROd 0.50±0.10 a.g 0.50±0.00
RO9 0.67±0.15 ROe 0.30±0.00 b.g 0.50±0.00
RO10 0.47±0.15 ROf 0.30±0.00 c.g 0.35±0.07
RO11 1.35±0.35 ROg 0.80±0.14 d.g 0.30±0.00
RO12 None ROh 0.30±0.00 e.g 0.30±0.00
RO13 None ROi 0.33±0.14 f.g 0.35±0.07
RO14 0.87±0.21 ROj 0.35±0.07 -- --
RO15 1.17±0.06 ROk 0.35±0.07 -- --
Figure 2 Fibrinolytic activity analyzed by zymography
M, Spectra™ Multicolor Broad Range Protein Ladder (Fermentas); 1-19 refer to enzymes from isolates RO1-19; a-k refer to enzyme from isolates ROa-k (Red Oncom); 1.g-7.g and a.g-f.g refer to enzyme from isolates 1.g-7.g and a.g-f.g (Tempeh Gembus)
70 140 260
25 35 40
10 15 50 100
M 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
kDa 17 18 19 a b c d
35 70 140
10 25 260
40
15 50 100
f.g j k
h i M e f g
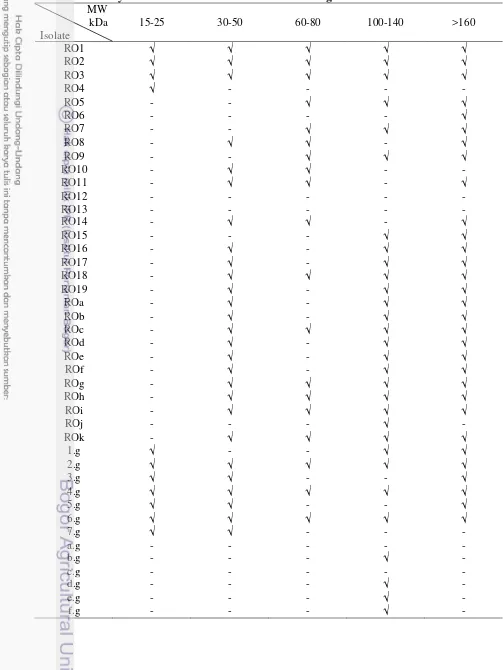
Table 2 Fibrinolytic fractions with various molecular weight
MW kDa Isolate
15-25 30-50 60-80 100-140 >160
RO1
RO2
RO3
RO4 - - - -
RO5 - -
RO6 - - - -
RO7 - -
RO8 - -
RO9 - -
RO10 - - -
RO11 - -
RO12 - - - - -
RO13 - - - - -
RO14 - -
RO15 - - -
RO16 - -
RO17 - -
RO18 -
RO19 - -
ROa - -
ROb - -
ROc -
ROd - -
ROe - -
ROf - -
ROg -
ROh -
ROi -
ROj - - - -
ROk -
1.g - -
2.g
3.g - -
4.g
5.g - -
6.g
7.g - - -
a.g - - - - -
b.g - - - -
c.g - - - - -
d.g - - - -
e.g - - - -
16
Fibrinogenolytic activity of a protease was also analyzed in situ with zymography method. The substrate used was fibrinogen 0.02%. Protease activity and protein concentration loaded into the gel were about 0.01-0.53 mU and 0.34-1.59 µg. Microbial protease RO1-19 were isolated from fresh Red Oncom while ROa-k were isolated from heated Red Oncom. Isolates 1-7.g were from fresh
Tempeh Gembus, while a-f.g were from heated Tempeh Gembus (Fig. 2). Of the 43 proteolytic isolates, we found that 38 isolates showed fibrinogenolytic activity and some of them have different pattern of fractions with various molecular weight (Table 2).
Microorganisms identification
The isolates with the best fibrinolytic activity were selected for microbial identification. Gram and spore staining identified that isolate RO1, RO2, RO3, RO16, RO17, RO18, RO19, ROa-k, 1-6.g and a-f.g, were Bacillus (gram positive and have spore). Isolates RO1, RO2, and RO3 show similar fibrinogenolytic pattern in situ. Therefore only isolate RO3 was selected for further identification. Similarly, similar fibrinogenolytic pattern was shown by isolates RO16, RO17, and RO19. Therefore for the further identification only isolate 19 was selected. Among ROa-k isolates, isolates ROg and ROj were selected for identification. Among various isolates from Tempeh Gembus, only 2.g was selected for further identification. The results of biochemical tests using API 50 CHB kit specifically for Bacillus spp, revealed that isolate RO3 was identified as B. licheniformis
(99.9%), isolate RO19 as B. cereus 1 (71.7%), isolates ROg as Brevibacillus laterosporus (99.3%), isolate ROj as B. cereus 1 (40.5%), and isolate 2.g as B. pumilus (99.7%).
Target of isolation of fibrinolytic bacteria was to find safe isolates. Therefore isolate RO3 and 2.g were used for further research. Molecular identification based on 16SrRNAs gen was performed and isolate RO3 was confirmed as B. licheniformis (96%) and isolate 2.g was confirmed as B. pumilus
(97%). The nucleotide sequences were deposited at GenBank with accession number AB968524 for isolate RO3 and AB968523 for isolate 2.g. The phylogenetic tree constructed on the basis of the sequences and presented in Figure 3.
Fibrinolytic activity on the fibrin plate (Fig. 4) showed that isolate B. licheniformis RO3 and B. pumilus 2.g were able to produce a clear zone. Plasmin 20 mU was used as a positive control.
Figure 4 Fibrinolytic activity on fibrin plate A. B. licheniformis RO3 on LB media; B. B. licheniformis RO3 on NB media; C. B. pumilus 2.g on LB media; D. B. pumilus 2.g on NB media; P: plasmin 20 mU
Discussion
The genus Bacillus from fermented foods was reported to produce strong fibrinolytic enzyme, such as Bacillus natto from Natto (Fujita et al. 1993), B. amyloliquefaciens DC-4 from Douchi (Peng and Zhang 2002), B. amyloliquefaciens MJ5-41 from Meju (Joet al. 2011a), B. amyloliquefaciens
LSSE-62 from Chinese soybean paste (Wei et al. 2011), Bacillus sp. DJ-2 from
Doen-jang (Choi et al. 2005), Bacillus licheniformis KJ-31 from Jeot-gal (Hwang
et al. 2007), and Bacillus coagulans form Terasi, Indonesian fermented fish (Prihantono et al. 2013).
18
and fibrinogen zymography with lower molecular weight and considered as safe were chosen and identified as Bacillus licheniformis and Bacillus pumilus. The two novel fibrinolytic microorganisms were referred to as B. licheniformis RO3 and B. pumilus 2.g.
Research conducted by Olajuyigbe and Ajele (2008) succeeded in isolating
B. licheniformis Lbbl-11 from “iru”, a traditionally fermented African locust bean condiment that can produce extracellular protease. Research conducted by Hwang
et al. (2007) succeeded in isolating B. licheniformis KJ-31 from Korean traditional Jeot-gal that can produce fibrinolytic enzymes. Bacillus strains such as
B. subtilis, B. pumilus, B. amyloliquefaciens, and B. licheniformis are the common bacilli species isolated from Cheonggukjang, a Korean soybean fermented food (Kim et al. 2003; Kwon et al. 2004; Kim et al. 2009; Joet al. 2011b).
Result of fibrinogen zymography showed that all fibrinogen degrading enzymes were produced in different patterns. Particularly, B. licheniformis RO3 and B. pumilus 2.g showed several fractions. Research on the B. licheniformis
CH3-17, this microorganism secreted six fibrinolytic proteins into the culture medium which can be observed by zymography conducted with the culture supernatant (Kim et al. 2009). B. subtilis secretes several proteases into the culture medium, including alkaline protease (subtilisin, encoded by apr), neutral protease (encoded by npr), bacillo-peptidase F (encoded by bpr), Epr (extracellular protease, encoded by epr), Mpr (extracellular metalloprotease, encoded by mpr), and Vpr (extracellular serine protease, encoded by vpr. Among them, subtilisin and neutral protease are the most important enzymes and are responsible for >90% of the total extracellular protease activity (Choi et al. 2004).
The genus Bacillus can easily be isolated from food and environment. Most of them are non toxic and have a good impact in human health such as B. pumilus
JB-1 that was isolated from Korean Cheonggukjang is an immuno-stimulating strain (Kwon et al. 2004). Presently we are conducting media optimation for producing fibrin degrading enzyme from B. licheniformis RO3 and B. pumilus 2.g.
Conclusion
Red oncom and Tempeh Gembus are potential sources of microbial fibrinolytic protease. We found 38 isolates that could produce fibrinolytic proteases. A few isolates were identified as Bacillus spp. Isolate RO3 was confirmed as B. licheniformis and isolate 2.g as B. pumilus.
Acknowledgment
This work was supported by Directorate General of Higher Education, Ministry of Education and Culture, Republic of Indonesia.
References
Bergmeyer HU, Grassl M, Walter HE. 1983. Enzymes; in Methods of Enzymatic Analysis (eds) HU Bergmeyer and M Grassl. Verlag Chemie. Weinheim. Germany. pp. 1007-1009.
Bradford MM. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principles of protein dye-binding. Analytical Biochemistry 72:234-254.
Choi NS, Yoo KH, Hahm JH, Yoon KS, Chang KT, Hyun BH, Maeng PJ, Kim SH. 2005. Purification and characterization of a new peptidase, bacillopeptidase DJ-2, having fibrinolytic activity: produced by Bacillus sp. DJ-2 from Doen-Jang. Journal of Microbiology and Biotechnology
15(1):72–79.
Choi NS, Ju SK, Lee TY, Yoon KS, Chang KT, Maeng PJ, Kim SH. 2004. Miniscale identification and characterization of subtilisins from Bacillus sp. strains. Journal of Microbiology and Biotechnology 15:537–543.
Collen D, Lijnen HR. 2004. Tissue-type plasminogen activator: a historical perspective and personal account.Journal of Thrombosis and Haemostasis
2(4):541–546.
Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S. 1993. Purification and Characterization of a strong fibrinolytic enzyme (Nattokinase) in the vegetable cheese Natto, a popular soybean fermented food in Japan.
Biochemical and Biophysical Research communications 197(3):1340-1347. Hwang KJ, Choi KH, Kim MJ, Park CS, Cha J. 2007. Purification and
characterization of a new fibrinolytic enzyme of Bacillus licheniformis KJ-31, Isolated from Korean traditional Jeot-gal. Journal of Microbiology and Biotechnology 9:1469–1476.
Jeong SJ, Kwon GH, Chun J, Kim JS, Park CS, Kwon DY, Kim JH. 2007. Cloning of fibrinolytic enzyme gene from Bacillus subtilis isolated from
Cheonggukjang and its expression in protease-deficient Bacillus subtilis
Strains. Journal of Microbiology and Biotechnology 17(6):1018–1023. Jo HD, Lee HA, Jeong SJ, Kim JH. 2011a. Purification and characterization of a
major fibrinolytic enzyme from Bacillus amyloliquefaciens MJ5-41 isolated from Meju. Journal of Microbiology and Biotechnology 21(11):1166–1173. Jo HD, Kwon GH, Park JY, Cha J, Song YS, Kim JH. 2011b. Cloning and over
expression of aprE3-17 encoding the major fibrinolytic protease of Bacillus licheniformis CH3-17. Biotechnology and Bioprocess Engineering 16:352-359.
Kim YS, Jung HJ, Park YS, Yu TS. 2003. Characteristics of flavor and functionality of Bacillus subtilis K-20 Chungkukjang. Korean Journal of Food Science and Technology 35:475-478.
Kim GM, Lee AR, Lee KW, Park JY, Chun J, Cha J, Song YS, Kim JH. 2009. Characterization of a 27 kDa fibrinolytic enzyme from Bacillus amyloliquefaciens CH51 isolated from Cheonggukjang. Journal of Microbiology and Biotechnology 19:997-1004.
20
Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikedo R, Seiki M, Maruyama M. 1991. A novel fibrinolytic enzyme extracted from the earthworm Lumbricus rubellus. The Japanese Journal of Physiology 41(3):461–472.
Olajuyigbe FM, Ajele JO. 2008. Some Properties of Extracellular Protease from
Bacillus licheniformis Lbbl-11 Isolated from “iru”, A Traditionally Fermented African Locust Bean Condiment. Global Journal of Biotechnology & Biochemistry 3(1):42-46.
Peng Y, Yang X, Zhang Y. 2005. Microbial fibrinolytic enzymes: an overview of source, production, properties, and thrombolytic activity in vivo.Applied Microbiology and Biotechnology 69:126–132.
Peng Y, Zhang YZ. 2002. Optimation of fermentation conditions of douchifibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4.
Chinese Journal of Applied and Environmental Biology 8:285-289.
Prihanto AA, Darius, Firdaus M. 2013. Proteolytic and fibrinolytic activities of halophilic lactic acid bacteria from two indonesian fermented foods. Journal of Microbiology, Biotechnology and Food Sciences 2(5):2291-2293.
Tough J. 2005. Thrombolytic therapy in acute myocardial infarction. Nursing Standard 19(37):55–64.
Wei X, Luo M, Xu L, Zhang Y, Lin X, Kong P, Liu H. 2011. Production of Fibrinolytic enzyme from Bacillus amyloliquefaciens by fermentation of chickpeas, with the evaluation of the anticoagulant and antioxidant properties of chickpeas. Journal of Agricultural and Food Chemistry
59:3957–3963.
2
Telah dipresentasikan secara oral pada International Symposium on Food and Agro-Biodiversity di Semarang pada 16 – 17 Sepetember 2014 dan artikel lengkapnya sedang dalam proses telaah untuk diterbitkan pada Proceedia Food Science Elsevier
4 THE USE OF RED ONCOM POWDER
AS POTENTIAL PRODUCTION MEDIA FOR
FIBRINOGENOLYTIC PROTEASE DERIVED FROM
Bacillus licheniformis RO3
2Abstract
The high cost of enzyme production is one of the barriers to successful application of enzyme in the industry. Selection of media is a critical factor for the enzyme production. Main factors for optimization of enzyme production include nutritional components and environmental conditions for growth and production of fibrinogenolytic protease. In this study, Bacillus licheniformis RO3 isolated from red oncom, an Indonesian fermented food was tested for its fibrinogenolytic protease production by using several media. Three types of media were analyzed, i.e. Luria-Bertani broth (LB), ½ LB + 1% skim milk (LBS), and ½ LB + 1% red oncom powder (LBO). Protease activity was tested by using spectrophotometric method with casein as a substrate and fibrinogenolytic activity was confirmed based on zymography assay using fibrinogen substrate. In LB media, B. licheniformis RO3 was able to produce protease with activity of 0.024 U/ml or 0.157 U/mg at 36 h fermentation. In LBS media, the highest protease activity was 0.022 U/ml or 0.152 U/mg at 48 h fermentation. The best result was shown by B. licheniformis RO3 grown in LBO media with the highest protease activity of 0.051 U/ml or 0.283 U/mg at 48 h. Zymographic profiles showed that crude enzyme from B. licheniformis RO3 consisted of six fibrinogenolytic bands with molecular weight of 20, 27, 32, 40, 70, and >140 kDa. These results indicate that
red oncom powder can be used as a potential media for fibrinogenolytic protease production.
Keywords: red oncom powder, fibrinogenolytic protease, production media, B. licheniformis
Introduction
22
The Bacillus genus from fermented foods is one of microbes that is found to be able to produce a strong fibrinolytic enzyme, such as Bacillus natto from natto, a traditional Japanese soy fermented food that produce nattokinase (NK). The supply of natto or its enzyme orally is not only able to degrade the thrombus directly, but also able to increase the release of endogenous plasminogen activator in experimental animals and human subjects (Sumi et al. 1990). Other Bacillus
genus of various fermented foods have also been found to be able for producing strong fibrinolytic enzymes, including B. amyloliquefaciens DC-4 from douchi, a fermented soy food from China (Peng et al. 2002), Bacillus sp. CK from
chungkookjang, a fermented soy sauce from Korea (Kim et al. 1996), Bacillus sp. DJ-2 strains and DJ-4 from doenjang, a Korean fermented soybean paste (Choi et al. 2005; Kim et al. 2000), and Bacillus sp. KA38 from jeotgal, a fermented salted fish from Korea (Kim et al. 1997).
Oncom is one of traditional Indonesian fermented food in West Java. This food is made from a fermentation process generated by several fungi. There are two types of oncom, i.e. red oncom and black oncom. The red oncom is degraded by the fungus of Neurospora sitophila or N. intermedia while the black oncom is degraded by the fungus of Rhizopus oligosporus and other types of Mucor
(Sastraatmadja et al. 2002; Wood 1998). Generally, red oncom is made from tofu waste, i.e. the soy which its protein has been taken in its make, while the black oncom is generally made from the peanuts dregs mixed with cassava dregs or cassava powder, i.e. tapioca, in order to make a better texture and more tender. Although both the substrate material is a kind of waste, its nutrient is still high enough to be exploited by human.
Tofu waste still contains high nutrient values, however, most of its organoleptic properties are less preferred. Tofu waste with a fermentation process, i.e. red oncom, is preferred as food product than its original waste without fermentation. Tofu waste is a processed product from tofu in which its protein nature is probably similar to tofu and soy, although it has undergone many changes because of certain treatments during the manufacturing process of tofu, such as heating. The high nutrient content of tofu and its large amounts provide a significant opportunity to be used as a growth media for enzymes-producing microbes for health. Therefore, this study was focused for selecting the optimal production media for production of fibrinogenolytic protease by fibrinogenolytic protease-producing microbe, i.e. Bacillus licheniformis RO3 isolated from red oncom, an Indonesian fermented food. Instead of commercial media of LB and skim milk, the use of red oncom powder as the alternative media for enzyme production was also tested since the microbe tested was originally isolated from fresh red oncom. Fibrinogenolytic protease with the highest activity was further characterized for its optimum pH and temperature.
Materials and Methods
Microorganism
Production of fibrinogenolytic protease
A total of 2 ose microbes was grown in 25 mL sterile Luria-Bertani broth media (LB) to obtain the optical density value (OD) of 0.8 at 620nm. LB media
contained isolates that had reached a value of OD = 0.8 and then it was taken 10% to be added in three different media, LB, ½ LB + skim milk 1% (w/v), herein after referred to as LBS, and ½ LB + red oncom powder 1% (w/v), herein after referred to as LBO. Microbial incubation was performed for 72 hours at 37°C in a waterbath at 120 rpm. OD620 value was measured every 12 hours to determine the
growth curve of microbes. The production media that has contained with fibrinolytic protease was taken once every 12 hours and centrifuged at 6000 g for 15 minutes at 4°C. The supernatant was taken to be calculated for its fibrinogenolytic protease activity.
Analysis of protease activity
Protease activity was quantitatively measured by a modification of Bergmeyer and Grassl method (Bergmeyer & Grassl 1983) using Hammarsten casein substrate (1% w/v). Three steps of analysis treatment were conducted, including the blank, standard, and samples. Enzyme solution that was heated to a certain temperature and incubation time (which produces maximum activities) was added to microtube containing 250 µL of 50 mM phosphate buffer pH 8. For treatment of blank and standard, the enzyme was replaced with distilled water and 5 mM tyrosine.
The solution was incubated at 37°C for 10 minutes. The hydrolysis reaction was stopped by the addition of 500 µL of trichloroacetic acid (TCA) 0.1 M. Blank and standard were added with 50 µL of enzyme solution, while sample was added with 50 µL of distilled water, and then the solution was reincubated at 37°C for 10 minutes, followed by centrifugation at 4000 g and temperature of 4°C for 10 minutes. Briefly, a 375 µL of supernatant was added to a microtube containing 1.25 mL Na2CO3 0.4 M and the Folin Ciocalteau reagent, and then incubated
again at 37°C for 20 minutes. The absorbance of the solution was measured at 578 nm.
Analysis of protein concentration
The protein concentration was determined by Bradford method (Bradford 1976) using bovine serum albumin (BSA) as protein standard. A total of 100 µL enzyme was added to the tube containing 1 mL distilled water and 1 mL Bradford reagent. For blank treatment, the enzyme solution was replaced with distilled water. Then, the solution was mixed using vortex and allowed to stand for 20 minutes at room temperature. Absorbance of the solution was measured at a wavelength of 595 nm. For standard, enzyme solution was replaced with BSA Fraktion V with concentration ranges of 0-250 µg/mL. The protein concentration of enzyme solution was determined by linear equation of relationship between standard concentration of protein and absorbance.
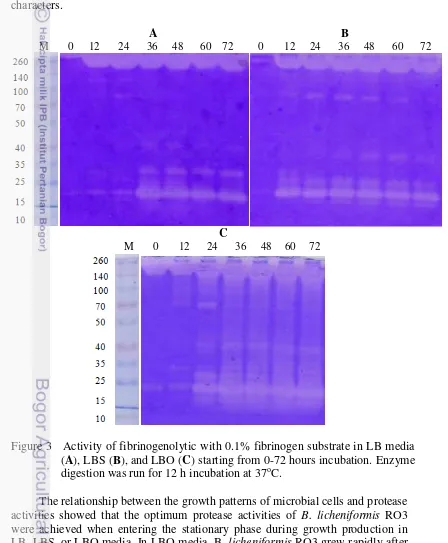
Activities of fibrinogenolytic with zymography