UNIVERSITI TEKNIKAL MALAYSIA MELAKA
CHARACTERIZATION OF EPOXIDISED NATURAL RUBBER /
ETHYLENE PROPYLENE DIENE MONOMER (ENR/EPDM)
BLEND PREPARED VIA SOLUTION MIXING IN TOLUENE
This report submitted in accordance with requirement of the Universiti Teknikal Malaysia Melaka (UTeM) for the Bachelor Degree of Manufacturing Engineering
(Engineering Materials) (Hons.) by
MUHAMMAD ZAIM BIN AZAMAM
B050910007
901029035783
UNIVERSITI TEKNIKAL MALAYSIA MELAKA
BORANG PENGESAHAN STATUS LAPORAN PROJEK SARJANA MUDA
TAJUK: Characterization of Epoxidised Natural Rubber/Ethylene Propylene Diene Monomer (ENR/EPDM) blends prepared via Solution Mixing in Toluene.
SESI PENGAJIAN: 2012/13 Semester 2
Saya MUHAMMAD ZAIM BIN AZAMAM
mengaku membenarkan Laporan PSM ini disimpan di Perpustakaan Universiti Teknikal Malaysia Melaka (UTeM) dengan syarat-syarat kegunaan seperti berikut:
1. Laporan PSM adalah hak milik Universiti Teknikal Malaysia Melaka dan penulis. 2. Perpustakaan Universiti Teknikal Malaysia Melaka dibenarkan membuat salinan
untuk tujuan pengajian sahaja dengan izin penulis.
3. Perpustakaan dibenarkan membuat salinan laporan PSM ini sebagai bahan pertukaran antara institusi pengajian tinggi.
(Mengandungi maklumat TERHAD yang telah ditentukan oleh organisasi/badan di mana penyelidikan dijalankan)
Alamat Tetap:
No 172, Kampung Surau Lama,
Geting, 16200, Tumpat
DECLARATION
I hereby, declared this report entitled “Characterization of Epoxidised Natural Rubber/ Ethylene Propylene Diene Monomer (ENR/EPDM) blend prepared via Solution Mixing in Toluene” is the result of my own research except as cited in references.
Signature : ………..
Author’s Name : MUHAMMAD ZAIM BIN AZAMAM
APPROVAL
This report is submitted to the Faculty of Manufacturing Engineering of UTeM as a partial fulfillment of the requirements for the degree of Bachelor of Manufacturing Engineering (Engineering Materials) (Hons.). The member of the supervisory is as follow:
………
i
ABSTRAK
ii
ABSTRACT
iii
DEDICATION
To my father; Azamam Bin Hassan, my mother; Jamilah Binti Che Hamid, my siblings and friends. Your love is my driving force.
To my supervisor Dr. Noraiham Binti Mohamad, to my mentors Mazlin Aida, Nor Nadiah Hamid, Nur Sharafina, Juliana and all staffs in UTeM.
iv
ACKNOWLEDGEMENT
I would like to extend my gratitude to Allah S.W.T for His generous blessing and undying strength bestowed upon me during the course of this research.
Secondly, I would like to extend my heartiest gratitude to Dr. Noraiham Mohamad as my supervisor who had given me guidance and support during the research. Not to be forgotten to other lecturers, my friends and other person whose name is not mentioned here.
v
List Abbreviations, Symbols and Nomenclatures xii
vi 2.2.2.1 Ethylene Propylene Diene Monomer (EPDM) 19
2.2.2.2 Application of EPDM 21
2.5.4.2 Scanning electron microscopy 42
2.5.5 Compositional analysis 44
CHAPTER 3: METHODOLOGY 46
3.1 Introduction 46
3.2 Raw material 48
3.3 Characterization of raw material 48
3.3.1 Epoxidised Natural Rubber (ENR) 48
3.3.2 Ethylene Propylene Diene Monomer (EPDM) 49
3.3.3 Toluene 50
3.4 Preparation of ENR/EPDM blend using solution mixing 51
3.5 Fabrication of thin film 53
vii 3.7 Utilization of NR/EPDM blend with ENR/EPDM compatibilizer 56
3.8 Testing and analysis 57
3.8.1 Density (ASTM D792) 57
3.8.2 Differential Scanning Calorimetry Analysis (DSC) 58 3.8.4 Fourier Transformation Infrared Spectroscopy (FTIR) 59
3.8.5 Scanning Electron Microscopy (SEM) 61
CHAPTER 4: RESULT AND DISCUSSION 62
4.1 Introduction 62
4.2 Raw Materials Characterization 63
4.2.1 Density 63
4.2.2 Raw Material Characterization using FTIR 64
4.3 Analysis of ENR/EPDM Compatibilizer 66
4.3.1 Compositional analysis of ENR/EPDM Compatibilizer 66 4.3.2 Thermal Analysis of ENR/EPDM Compatibilizer 75 4.4 Tensile Properties of NR/EPDM incorporated with ENR/EPDM
Compatibilizer 77
4.5 Morphological Characteristics of NR/EPDM incorporated with
ENR/EPDM Compatibilizer 82
CHAPTER 5: CONCLUSION AND RECOMMENDATION 85
5.1 Conclusion 85
5.2 Recommendations 86
REFERENCES
viii
LIST OF TABLES
2.1 Properties of natural rubber 12
2.2 Application of ENR 15
2.3 Main Types and Applications for Synthetic Rubbers 18 2.4 principle diene Monomer used in EPDM manufacture 20
2.5 Methods for production of polymeric films 30
2.6 Examples of solution mixing of polymer 32
2.7 The advantage and disadvantage of FTIR spectroscopy 45
3.1 General properties of EPDM rubber according USSA industry MSDS 49 3.2 Properties of Toluene according to ScienceLab.com MSDS 50
3.3 Sample code and ENR/EPDM blend formulation 51
3.4 NR/EPDM blend mixing component 56
4.1 Density average of ENR and EPDM 63
4.2 Density average of ENR/EPDM 64
4.3 Infrared intensity for epoxide in respect of absorption peaks 71 4.4 peak height intensity of absorbance 875 cm-1 and 2950 cm-1 73 4.5 percentage of unreacted epoxide in the numerous of ENR/EPDM blends 73
4.6 Percentage of reaction epoxide in ENR/EPDM 74
4.7 Glass Transition temperature of 70/30 ENR/EPDM blend 75
4.8 Tensile data for NR/EPDM 77
ix
LIST OF FIGURE
2.1 Polymer formation 5
2.2 Polymer chains in amorphous and crystalline regions 7
2.3 Tapping Natural latex 10
2.4 Natural rubber structure 11
2.5 A rubber polymeric chain 11
2.6 Formation of peroxy formic acid 13
2.7 Epoxidised natural rubbers formation 13
2.8 Production process for synthetic rubber 16
2.9 Some monomers used to produce synthetic rubbers 17
2.10 EPM structure 19
2.10 Chemical structure of EPDM 20
2.11 EPDM waterproofing System 22
2.13 Example Ozone resistance effect on side wall of tire 22
2.14 Interface between immiscible polymers 26
2.15 Schematic flow diagram of the melt-mixing process 27 2.16 A simple representation of the latex film formation process 28 2.17 Solvent casting method for the preparation of CNTs-polymer 31
2.18 Cross section of melt indexer furnace 36
2.19 Common sketch of MFR equipment 37
2.20 Cross-section of UTM Tensile Grip machine 38
2.21 Illustration of Charpy and Izod Impact Tests 39
2.22 Example of DSC cross-section 41
2.24 Fractured surface of NR/EPDM via SEM at X5.000 magnification 42 2.23 Principle working of Scanning Electron Microscope (SEM) 43
x
3.6 Overhead Digital Stirrer 52
3.7 Mixing using mechanical stirrer 53
3.8 Blend was aged at different aging time according to their mixing time 54
3.9 a) Rubber gum was cast on petri dish 54
b) Thin film was measured before drying process 54
3.10 Samples was dried in vacuum oven 55
3.11 Finished shape thin film of ENR/EPDM compatibilizer 55
3.12 Electronic densitometer 58
3.13 Perkin Elmer DSC-7 59
3.14 FT/IR-6100 machine 59
3.15 Zeiss EVO-50 ESEM machine 60
3.16 a) Dumbbell pressing machine 61
b) Testing shape for tensile 61
4.1 FTIR spectrum of ENR 65
4.2 FTIR spectrum of EPDM 66
4.3 FTIR spectrum of ENR/EPDM blend for 0-hour/14 day’s aging 67 4.4 FTIR spectrum of ENR/EPDM blend for 8-hour/7 days aging 68 4.5 FTIR spectrum of ENR/EPDM blend for 24-hour/1 days aging 69
4.6 Epoxide functional group 70
4.7 Calibration curve for selected blend 72
4.8 Comparison DSC thermogram for ENR blend with different mixing
time and aging time 76
xi 4.10 Elongation at break of NR/EPDM with and without compatibilizer 80 4.11 100 Modulus of NR/EPDM with and without compatibilizer 81 4.12 SEM image of NR/EPDM at 1000X magnification 82 4.13 NR/EPDM with 0-hour/14 day’s compatibilizer at 1000X
magnification 83
4.14 NR/EPDM with 8-hour/7 days compatibilizer at 1000 X
magnification 84
4.15 NR/EPDM with 24-hour/1 day compatibilizer at 1000 X
xii EPDM - Ethylene Propylene Diene Monomer EV - Efficient vulcanisation
IISR - International institute of synthetic rubber
IR - Infrared
PTFE - Polytetrafluoroethylene PVC - Polyvinyl chloride SBR - Styrene butadiene rubber Semi-EV - Semi-efficient vulcanisation SEI - Secondary Electron Image
TEM - Transmission Electron Microscopy TOR - Trans-polyoctylene
TPE - Thermoplastic elastomer TSE - Thermoset elastomer TCE - Trichloroethylene
1
CHAPTER 1
INTRODUCTION
1.1Background
Polymer blends have received much attention since blending is a simple, effective approach to develop new materials exhibiting combinations of properties that cannot be obtained by individual polymers. Blending two or more elastomers is carried out for particular objectives such as enhancement of technical properties, improvement of ageing resistance and also processing characteristics (Alex et al., 2003). It is most common for compatibilization to be succeeded by addition of a third component (compatibilizer) or by inducing in situ chemical reaction between blend components (reactive blending) leading to modification of the polymer interfaces in two-phase blends, and thereby for tailoring of the phase structure and properties (Hussain et al., 2010).
Natural rubber (NR) is normally blended with ethylene propylene diene rubber (EPDM) to improve ageing resistance of the former without losing its good mechanical properties. Due to the difference in unsaturation level between these components, a mutual incompatibility can exist, which contributes for a decreasing of mechanical performance.
2 mechanical and acoustic damping devices and in specialty shoe soles to give high wet grip. ENR also are used in the construction of composites conveyor belts because of their adhesives properties. The combination of high strength and low resilience of ENR based conveyor belts are advantageous under severe service conditions (Gelling, 1991)
1.2 Problem statement
Nowadays, rubber-rubber mixture has attracted number of researcher around the world due to its potential to combine the attractive properties of both the constituents in the blend. One of the famous types of rubber-rubber mixtures is NR/EPDM .The NR/EPDM blend is one most used blend in the market nowadays. The application of NR/EPDM is used especially to make the automotive weather-strips, engine mounts, washing machine gaskets and grommets.
In the application of NR/EPDM blend, it has been shown that most of the blend are immiscible and incompatible thus resulting in poor mechanical properties. They have coarse phase morphology with sharp interface. Furthermore, the adhesion between both blend phases is poor, so that these blends are useless without being compatibilized. Although there are many compatibilizer agents such as maleic anhydride (MA) and trans-polyoctylene (TOR) can be incorporated into this blend to increase the compatibility, it has their own drawback due to expensive price.
3 application. Epoxidised natural rubber (ENR) is a best material for the present work since the introduction of epoxide groups not only reduces the number of double bond but also increase the hydrophilicity of NR (Yoksan, 2008); which will be very critical in the production of any NR/EPDM based composites.
Thus far, based on ENR capability, the introduction of ENR/EPDM blend as a compatibilizer agent to the NR/EPDM will improve the compatibility and increase the interaction between immiscible phases.
1.3Objective
The main objectives on this research are:
a) To synthesis ENR/EPDM blend through solution mixing
b) To study the effect of formulation, mixing and aging time of ENR/EPDM blend. c) To characterize the physical and thermal properties of ENR/EPDM as a potential
compatibilizer agent to the NR/EPDM blend
1.4Scope
4
1.5 Chapter review
There are three chapters in this report.
a) Chapter 1 is the introduction of the research. This chapter consists of background, problem statement, objectives of the report, scope and chapter review of the report.
b) Chapter 2 is the literature review. This chapter presents the published literatures that are relevant to particular topic of this research, demonstrating the information of any previous work and awareness of related theories and discussions.
5
CHAPTER 2
LITERATURE REVIEW
2.1 Polymer
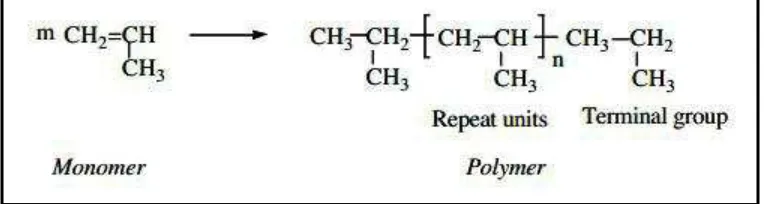
According to Hatsuo (2006) the word "polymer" consists of "poly; many", and “mer; unit", and indicates a large molecule with many repeating units. Other words, “high polymer” and “macromolecule” are often used synonymously. However, macromolecule strictly means a large molecule and does not necessarily indicate a repetitive structure. Thus, macromolecule is more general than polymer. The repeating unit of the polymer is called, “chemical repeat unit,” and the original small molecule that leads to the repeat unit is called, “monomer," meaning a single unit. Typically the molecular weight of a monomer is from several tens to several hundred whereby that of a polymer is tens of thousands to several millions. An example of the relationship between the monomer and polymer is shown in Figure 2.1.
6 In many polymers, only one monomer is used. In others, two or three different monomers may be combined. Polymers are classified by the characteristics of the reactions by which they are formed. If all atoms in the monomers are incorporated into the polymer, the polymer is called an addition polymer. If some of the atoms of the monomers are released into small molecules, such as water, the polymer is called a condensation polymer. Most addition polymers are made from monomers containing a double bond between carbon atoms. Such monomers are called olefins, and most commercial addition polymers are polyolefins. Condensation polymers are made from monomers that have two different groups of atoms which can join together to form, for example, ester or amide links. The most common ways of classifying the polymer is to separate it into three large group which is thermoplastic, thermoset and elastomer.
2.1.1 Thermoplastic
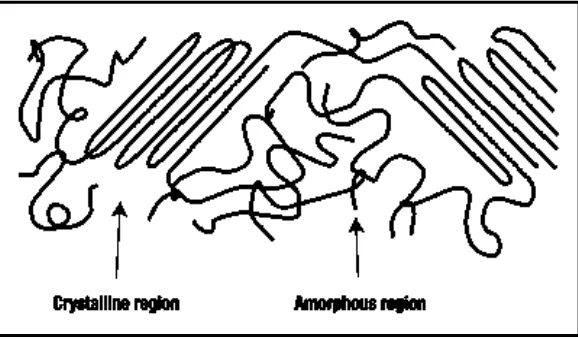
Thermoplastics are polymers that solidify as they are cooled, no longer allowing the long molecules to move freely .They also becomes soften when heated (Callister, 2008). In overall, thermoplastic polymer can be divided into two sub classes which are amorphous and semi-crystalline as shown in Figure 2.2.
Amorphous thermoplastic are those with molecules that remain in disorder as they cool, leading to material with a fairly random molecular structure (Oswald et al., 2003). An amorphous polymer also solidifies, or vitrifies, as it cooled below its glass transition material. The examples of amorphous polymer are polystyrene, polycarbonate, plasticized polyvinyl chloride.
7 lose their flow ability below their glass transition temperature. Most semi-crystalline polymer has a glass transition temperature at subzero so it behaving at room temperature as rubbery or leathery material (Callister, 2008). The example of semi-crystalline material is high density polyethylene, polypropylene, poly amide and polytetrafluoroethylene.
Figure 2.2: Polymer chains in amorphous and crystalline regions (Rosner, 2001).
2.1.2 Thermoset
According to Prime (1997), thermosets are network-forming polymers. Thermoset material solidifies when it is chemically cured. The long macromolecules cross link with each other resulting in a network of molecules that cannot slide past each other (Oswald et al., 2003). The formation of these networks causes the material to lose the ability flow after reheating. The high densities of cross linking between the molecules make thermosetting material stiff and brittle.
8 2.1.3 Elastomer
Elastomers are based on polymers which have the property of elasticity. They are made up of long chains of atoms, mainly carbon, hydrogen and oxygen, which have a degree of cross-linking with their neighboring chains. It is these crosslinking bonds that pull the elastomer back into shape when the deforming force is removed. However, there are two type of elastomer used today which is thermoplastic elastomer (TPE) and thermoset elastomer (TSE).
2.1.3.1 Classification of elastomer
Elastomer can be divided into thermoplastic elastomer, thermoset elastomer, and rubber. Thermoplastic elastomers are materials which combine the easy process ability of thermoplastics and elastic behavior of rubbers. Amin et al., (2011) stated that the unique properties of both materials exist because TPE material is created only by physical mixing of a thermoplastic and elastomer and no chemical or covalent bonding exist between the two. This behavior has opened a new field of polymer science.