STRUCTURAL CHANGES OF ARROWROOT STARCH
(
Marantha arundinacea
L.) AS THE IMPACT OF MULTIPLE
TREATMENTS BY ACID HYDROLYSIS, DEBRANCHING,
AUTOCLAVING-COOLING CYCLES, AND
HEAT MOISTURE TREATMENT (HMT)
MUTIARA PRATIWI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT LETTER OF THESIS AND
SOURCE OF INFORMATION*
I declare that this thesis entitled Structural Changes of Arrowroot Starch (Marantha arundinacea L.) as the Impact of Multiple Treatments by Acid Hydrolysis, Debranching, Autoclaving-cooling Cycles, and Heat Moisture Treatment (HMT) is based on my original work, in collaboration with the advisors and has not been submitted in any form at any college, except Bogor Agricultural University. Sources of information derived and quoted from published and unpublished works of other authors mentioned in the text are listed in the Bibliography at the end of this thesis.
Hereby I transfer the copyright of this thesis to Bogor Agricultural University. Bogor, February 2016
RINGKASAN
MUTIARA PRATIWI. Perubahan Struktur Pati Garut (Marantha arundinacea L.) sebagai Akibat Kombinasi Perlakuan Hidrolisis Asam, Debranching, Siklus Autoclaving-Cooling, dan Heat Moisture Treatment (HMT). Dibimbing oleh HANIFAH NURYANI LIOE sebagai Ketua Komisi Pembimbing dan DIDAH NUR FARIDAH sebagai Anggota Komisi Pembimbing.
Pati garut (Marantha arundinacea L.) diketahui memiliki karakteristik yang sesuai sebagai bahan baku pati resisten tipe III (RS3). Penelitian terdahulu menunjukkan bahwa pati garut yang dimodifikasi dengan menggunakan kombinasi hidrolisis asam-debranching-autoclaving-cooling menghasilkan rendemen pati resisten yang tinggi (39.39%), namun masih lebih rendah bila dibandingkan dengan pati resisten komersial (Novelose 330) (42.68%). Kombinasi perlakuan tersebut juga menyebabkan terjadinya perubahan struktur pada pati yang dapat diasosiasikan dengan sifat resistensi pati. Pada penelitian ini, kombinasi modifikasi pada penelitian terdahulu dikombinasikan kembali dengan teknik HMT pada suhu 121
oC dan kadar air 20%. Penelitian ini bertujuan untuk mempelajari perubahan
struktur pada pati garut akibat kombinasi modifikasi hidrolisis asam-debranching-autoclaving-cooling-HMT, yang belum dipelajari pada penelitian lainnya, khususnya terkait dengan sifat resistensi pati. Pada penelitian ini, perubahan struktur pati dipelajari dengan menggunakan teknik GFC, FTIR, DSC, XRD, dan SEM.
Pemisahan dengan GFC menunjukkan bahwa modifikasi yang diberikan menyebabkan perubahan profil distribusi molekul pati, yaitu terjadi penurunan fraksi amilopektin dan peningkatan fraksi amilosa. Amilosa berperan dalam retrogradasi yang menjadi salah satu mekanisme pembentukan pati resisten. Analisis dengan FTIR menunjukkan bahwa kombinasi perlakuan secara umum meningkatkan daerah amorf dan menurunkan daerah kristalin. Terbukanya struktur double helix dari molekul amilopektin serta degradasi amilopektin lebih lanjut merupakan fenomena yang dapat menyebabkan terjadinya penurunan daerah kristalin. Dugaan tersebut diperkuat dengan hasil analisis dengan GFC yang menunjukkan adanya penurunan fraksi amilopektin. Analisis dengan DSC menunjukkan terjadinya peningkatan suhu gelatinisasi (To, Tp, dan Tc), dengan
seluruh kombinasi perlakuan, kecuali pada pati yang dihidrolisis asam. Perubahan tipe kristalin dari tipe A menjadi tipe B mengindikasikan terjadinya rekristalisasi pati. Pengamatan dengan SEM menunjukkan bahwa perlakuan tunggal HMT tidak menyebabkan perubahan morfologi granula pati. Akan tetapi, ketika dikombinasikan dengan hidrolisis asam, debranching, dan autoclaving-cooling, HMT memicu terbentuknya struktur kristalit yang rapat. Hal ini mengkonfirmasi dugaan bahwa HMT berperan dalam penyempurnaan kristal pati yang merupakan salah satu mekanisme pembentukan pati resisten.
SUMMARY
MUTIARA PRATIWI. Structural Changes of Arrowroot Starch (Marantha arundinacea L.) as the Impact of Multiple Treatments by Acid Hydrolysis, Debranching, Autoclaving-cooling Cycles, and Heat Moisture Treatment (HMT). Under the supervision of HANIFAH NURYANI LIOE as the Chairman and DIDAH NUR FARIDAH as Advisory Committee Member.
Arrowroot starch (Marantha arundinacea L.) has been known to have the characteristics required for producing resistant starch type III (RS3). In the previous study, RS3 from arrowroot starch had been produced through the combination of acid hydrolysis-debranching-autoclaving-cooling, resulting high amount of resistant starch (39.39%), yet its yield was still lower than that of commercial resistant starch (Novelose 330) (42.68%). The combination of modification led to changes of starch structure which was strongly presumed to be associated with the starch resistance. In this study, the combination of modification applied in the previous study was combined with HMT at 121 oC and 20% moisture content, as
an additional treatment to the previous combination. This study was aimed to investigate structural changes of arrowroot starch as the impact of acid hydrolysis-debranching-autoclaving-cooling-HMT, which have not been revealed in other studies, particularly associated with the starch resistance after the modifications. In this study, the structural changes of arrowroot starch were studied by using GFC, FTIR, DSC, XRD, and SEM techniques.
Separation with GFC showed that the modifications led to changes of molecular distribution profile of arrowroot starch, showing the decrease of amylopectin fraction, but the increase of amylose fraction. Amylose fraction contributes to a rapid retrogradation process as a mechanism of resistant starch formation. Analysis with FTIR showed that in general the combination of treatments were found to increase the amorphous region and decrease the crystalline region. Unraveling of amylopectin double helix and/or further degradation of amylopectin were presumed to cause the decrease of crystalline region. The assumption was supported by the result of analysis with GFC, showing the decrease of amylopectin fraction. Analysis with DSC showed the increase of gelatinization temperature (To, Tp, and Tc), with the increase of temperature ranging from
indicated the occurrence of starch recrystallization. The SEM observation showed that single treatment of HMT did not change the morphology of granules in shape and size. While in combination with acid hydrolysis, debranching, and autoclaving-cooling, HMT led to the formation of smaller crystalline bodies which closely adhered to each other. This result confirmed the mechanism of HMT in inducing the perfection of crystallites as a possible mechanism of resistant starch formation. Keywords: arrowroot starch, heat moisture treatment, resistant starch, starch
© Copyright of Bogor Agricultural University, 2016
Protected by the laws
Any unauthorized quotation of all contents or any part thereof is strictly prohibited. Quotation is only for educational purpose, research, scientific writing, reports writing, critique and problem analysis; and quotation would not give any disadvantage on behalf of Bogor Agricultural University.
STRUCTURAL CHANGES OF ARROWROOT STARCH
(
Marantha arundinacea
L.) AS THE IMPACT OF MULTIPLE
TREATMENTS BY ACID HYDROLYSIS, DEBRANCHING,
AUTOCLAVING-COOLING CYCLES, AND
HEAT MOISTURE TREATMENT (HMT)
MUTIARA PRATIWI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2016 Thesis
in fulfillment of the requirement for degree of Master Science in
PREFACE
All the praise to Allah subhanahu wa ta’ala for all His grace, mercy, and blessing through all the way to complete my Master study. This study was intended as requirement to graduate in Master degree in Bogor Agricultural University. This study entitled “Structural Changes of Arrowroot Starch (Marantha arundinacea L.) as the Impact of Multiple Treatments by Acid Hydrolysis, Debranching, Autoclaving-cooling Cycles, and Heat Moisture Treatment (HMT)” was conducted in Bogor Agricultural University from January-August 2015.
The author would like to express the deepest gratitude to beloved parents and brother in appreciation of their love and encouragements, especially during the completion of my Master study. Nothing can reply their life time support. My sincere appreciation and deepest gratitude is also expressed to the advisors, Dr. Ir. Hanifah Nuryani Lioe, MSi and Dr. Ir. Didah Nur Faridah, MSi for their intense supervision and constant support. The valuable help of constructive comments and suggestions throughout the experimental and thesis work have contributed to the success of this research. The author also would like to express a sincere gratitude to Dr. Ir. Sukarno, MSc as the examiner in thesis examination, for his time, suggestion, and correction for the improvement of this thesis. Sincere thanks is also expressed to Indonesian Directorate General of Higher Education (DIKTI) for supporting the study through fresh graduate scholarship program and for supporting the research through funding incentive of Penelitian Unggulan Dasar (PUD) program 2014.
A sincere gratitude is also expressed to Dr. Ir. Dahrul Syah, MSc as the Dean of Postgraduate School and Prof. Dr. Ir. Ratih Dewanti, MSc as the Head of Food Science Program for the support and help towards my postgraduate affairs. The author also would like to express her thanks to all the laboratory technicians of Department of Food Science and Technology: Pak Taufik, Pak Sobirin, Mbak Ari, Pak Rojak, Mas Edi, Mbak Nurul, Mbak Irin, and Pak Yahya for the technical help during my research. The appreciation also goes to Mbak Ririn as the laboratory technician of the department for the guidance and suggestion for the chromatography column preparation. Special thanks to Fathma, Fitria, Mbak Ratna, and Anand for being a cooperative partner in conducting the research. The author also thanks her colleagues, especially Nesya, Silvie, and Florentina for the support, advice, and help during the study and research completion. Last but not least, sincere gratitude also goes to Rio for his constant supports and advices from the beginning until this stage.
Hopefully this thesis could be useful for the readers and contribute in a wider scope of food science realm.
Bogor, February 2016
TABLE OF CONTENT
LIST OF TABLES xviii
LIST OF FIGURES xviii
LIST OF APPENDIXES xviii
1 INTRODUCTION 1
Background 1
Potential 3
Objectives 3
Significance 4
Hypothesis 4
2 LITERATURE STUDY 4 Arrowroot (Marantha arundinacea L.) 4
Starch 5
Resistant starch 8
Starch modification 9
Acid hydrolysis 10
Debranching 11
Autoclaving-cooling 12
Heat Moisture Treatment (HMT) 13
Combination of modification to produce resistant starch 13
Changes of molecular structure in starch modification 14
Gel Filration Chromatography (GFC) profile 14
Fourier Transform Infra-Red (FTIR) spectra profile 16
Differential Scanning Calorimetry (DSC) profile 18
X-ray Diffraction (XRD) pattern 21
Scanning Electron Microscopy (SEM) microstructure profile 22
3 RESEARCH METHODOLOGY 23 Time and place 23 Material and instruments 24 Methods 24 4 RESULT AND DISCUSSION 28 Profiles of starch molecular distribution analyzed by GFC 28 Changes of crystalline and amorphous regions analyzed by FTIR 33 Gelatinization properties analyzed by DSC 36 Crystallinity of starch analyzed by XRD 39
Morphology of starch granules analyzed by SEM 43
5 CONCLUSION AND RECOMMENDATION 46 Conclusion 46 Recommendation 47 Acknowledgement 47
BIBLIOGRAPHY 48
APPENDIXES 59
LIST OF TABLES
1 Studies of starch molecular distribution by using Gel Filtration 15 Chromatography (GFC) technique
2 Studies of starch structure by using Fourier Transform Infra-Red 17 (FTIR) spectroscopy
3 Thermal studies of starches by using Differential Scanning 19 Calorimetry (DSC) technique
4 Studies of crystallinity in various starches by using X-ray 21 Diffraction (XRD)
5 Studies of microstructure in various starches by using Scanning 23 Electron Microscopy (SEM)
6 Condition for analysis of molecular distribution profile of arrowroot starch by using Gel Filtration Chromatography (GFC) 26 7 Gelatinization properties of native and modified arrowroot starch 36 8 X-ray diffraction pattern of native and modified arrowroot starch 40
LIST OF FIGURES
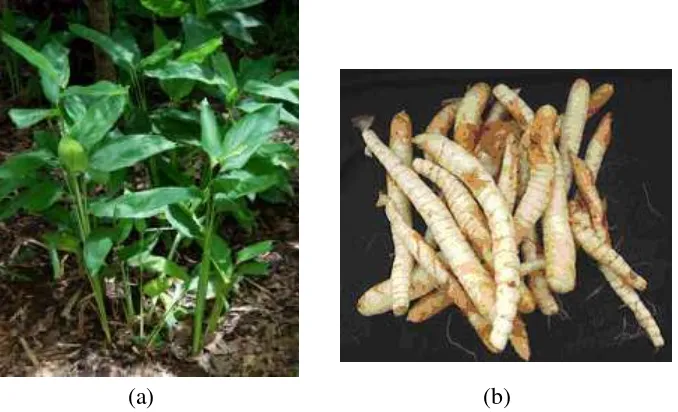
1 Arrowroot plant Marantha arundinacea L. (a) and arrowroot tuber 5 Marantha arundinacea L. (b)
2 Schematic diagram of starch granule architecture 7
3 Cluster model of amylopectin 7
4 Typical DSC thermogram with the To, Tp, Tc, and ∆H values 20
5 GFC profiles of arrowroot starch using Sephacryl S-HR 400 column 29 (2.6 cm i.d. x 90 cm)
6 GFC profiles of arrowroot starch using Sephadex G-50 column 31 (2.6 cm i.d. x 135 cm)
7 Changes of crystalline and amorphous regions of native and 34 modified arrowroot starches analyzed by Fourier Transform Infra-Red (FTIR) spectroscopy
8 DSC gelatinization curve of native and modified arrowroot starch 37 9 X-ray diffraction patterns of native and modified arrowroot starch 41 10 Scanning Electron Microscopy (SEM) micrographs of native and 45
modified arrowroot starches: 1. native; 2. AH; 3. AC; 4. HMT. (a) magnification = 500x; (b) magnification = 1500x
11 Scanning Electron Microscopy (SEM) micrographs of native and 46 modified arrowroot starches: 1. DE-AC; 2. AC; 3. AH-DE-AC-HMT (a) magnification = 500x; (b) magnification = 1500x
LIST OF APPENDIXES
1 Reagents for making acetate buffer pH 5.2 solution 59
2 Reagents for making mobile phase solution 60
3 Reagents for total carbohydrate analysis 61
4 Reagents for blue value analysis 62
5 Sephadex G-50 column preparation 63
1
INTRODUCTION
Background
Resistant starch (RS) has been known for its potential health benefits and functional properties (Sajilata et al. 2006). Resistant starch is defined as the fraction of starch which is not hydrolyzed to D-glucose in the small intestine within 120 min of being consumed, but which is fermented by bacteria in the colon to short chain fatty acids and gases (Fuentes-Zaragoza et al. 2010, Topping and Clifton 2001). Resistant starch is further divided into five distinct classes: RS1, RS2, RS3, RS4 and RS5. RS1 is physically inaccessible starch, which is entrapped within whole or partly milled grains or seeds. RS2 belongs to some types of raw starch granules. RS3 is retrograded starch (either processed from unmodified starch or resulting from food processing applications). RS4 belongs to starches which are chemically modified to obtain resistance to enzymatic digestion (Ratnayake dan Jackson 2008, Sanz et al. 2009). RS5 belongs to resistant starch formed as a single-helix complex of lipid-amylose (Ratnayake and Jackson 2008, Sanz et al. 2009, Fuentes-Zaragoza et al. 2010, Ai et al. 2013).
Arrowroot starch (Marantha arundinacea L.) has the characteristics which are suitable for producing RS3. Arrowroot has an A-type crystalline and it has amylopectin, containing branching chains with degree of polymerization (DP) of about 9-30 found in a high amount (up to 96%) (Srichuwong 2006). Arrowroot starch also has a higher density in the helical structure region (Wang et al. 1998), higher proportion of short branch chain of amylopectin (Hizukuri et al. 1983), and greater amount of chains found in each cluster, compared to B-type crystalline (Takeda et al. 2003). Arrowroot starch has been known to contain observable amount of amylose, reaching 29.39% (Aprianita 2010), which contributes to a rapid retrogradation. Besides, arrowroot starch also has low content of fat (0.68%) which can minimize the possibility of fat-amylose formation that may reduce retrogradation to some extent. This will enable higher amount of amylose which can bind with another amylose or amylopectin during retrogradation (Faridah 2011). RS3 can be obtained through heating and cooling cycles of starch which respectively involves a gelatinization and retrogradation of starch. Acid modification and enzymatic debranching of starch are two methods known to increase short chain amylose which contributes in more rapid retrogradation (Sandoval et al. 2008, Milasinovic et al. 2010).
2
temperature above the gelatinization temperature (84-120 oC) for a certain period
(15 min-16 h) (Arns et al. 2014). In this study, HMT was carried out at 20% moisture content at 121 oC for 15 min.
The modifications of starch could change molecular composition, organization, and structures of the starch granule. Amylose, amylopectin, and intermediate molecules have different characteristics of molecular weight, molecular structure, and physical and chemical properties. Thus, the composition, molecular structures, and organization of these molecules on the starch granule have significant effects on structure and properties of the starch (Yangcheng 2012). This research was aimed to investigate the structural changes of arrowroot starch after the modification using Gel Filtration Chromatography (GFC), Fourier Transform Infra-Red spectroscopy (FTIR), and Differential Scanning Calorimetry (DSC) techniques.
GFC, also referred to as Size Exclusion Chromatography (SEC) is a mode of liquid chromatography in which components of mixture are separated on the basis of size. In GFC, large molecules elute from the column first, followed by smaller molecules. The molecular distribution of various native and modified or treated starches have been determined by using GFC technique (Bradbury and Bello 1993, Lu et al. 1996, John et al. 2002, Han et al. 2003, Ferrini et al. 2008, Ozturk et al.2011). GFC can quantitatively separate starch using molecular weight distribution and mean degree polymerization (Lu et al. 1996). In most studies, there are commonly two distribution areas which appear in chromatographic profile of starch separated by using GFC technique. The first distribution area belongs to amylopectin with higher molecular weight, while the second distribution area belongs to amylose with smaller molecular weight (Bradbury and Bello 1993, Wang et al. 1993, Lu et al. 1996, Ferrini et al. 2008). Modifications of starch applied in this study were hypothesized to increase the amylose fraction while decrease the amylopectin fraction due to the degradation caused by thermal, enzymatic, and/or chemical treatment applied. Thus, this technique could differentiate the native and modified starches by observing the changes of molecular distribution profile of the starches.
Structural changes of starch as the impact of modification could be observed as well with FTIR. FTIR technique could detect the absorption of different bond vibrations in starch molecules during gelatinization (Goodfellow and Wilson 1990). FTIR could inform the changes of crystalline and amorphous region by observing the IR absorbance at 1045, 1022, and 995 cm-1.The bands at 1045 and 1022 cm-1
have been linked with order/crystallinity and amorphous regions in starch, respectively. Intensity ratio for 1045/1022 cm-1 is associated with crystalline, while
ratio for 1022/995 cm-1 is associated with amorphous region (Sevenou et al. 2002,
Chung et al. 2009).
DSC has commonly been used for investigating thermal behavior of starches (Lu et al. 1996). DSC data can provide a quantitative measure of gelatinization (Stevens and Elton 1971, Biliaderis et al. 1980, Nakazawa et al. 1984, Krueger et al. 1987), retrogradation (Atwell et al. 1980), and the phase transitions (Donovan 1979, Biliaderis et al. 1986) in variety of starches. In this research, DSC was used to give information about gelatinization transition temperature (To, Tp, and Tc) and
3 describe the mechanism of double helix dissociation during gelatinization and also the melting of crystallites.
XRD is a widely used technique to evaluate the crystalline fraction of variety of starches (Keren et al. 2003, Frost et al. 2009, Mutungi et al. 2009, Miao et al. 2009, Zeng et al. 2015a). Native arrowroot starch was known to have an A-type crystalline pattern (Srichuwong et al. 2005, Faridah 2011). In this study, XRD was used to evaluate the change of crystalline type as the impact of the modification, by observing the peak at certain diffraction angle (2θ). The diffraction peaks are used to identify the particular crystalline form(s) in the material (Singh et al. 2005). The intensity profile of the X-ray reflection from a partially crystalline sample like starch granules is a function of distribution of crystal size and of lattice disorder (Singh et al. 1995). The relative crystallinity could actually be observed quantitatively by using Lorentz, Gaussian, and log-normal distribution (Frost et al. 2009). However, in this study, the observation was conducted by using only descriptive and qualitative method, based on the intensity of peak in certain diffraction angle (2θ).
Scanning electron microscopy (SEM) has been known as a very useful tool to visualize food structure, because it combines in many ways the best features of light microscopy (LM) and transmission electron microscopy (TEM) (Fazaeli et al. 2012). It has been applied in many studies of starch to evaluate the granule structure of native and modified starch (Ferrini et al. 2008, Mutungi et al. 2009, Miao et al. 2009, Cai et al. 2010, Li and Gao 2010). In this study, analysis with SEM was conducted to observe the change of granular structure of arrowroot starch after modifications, which might relate with the resistant behavior of the starch.
Potential
Arrowroot starch has suitable characteristics required for RS3 production. Retrogradation of starch through heating and cooling cycles was known to increase the amount of resistant starch. Starch tends to retrograde more easily when it contains higher short chain amylose. This could be achieved through partial hydrolysis of glycosidic bonds which could be done with acid hydrolysis and enzymatic debranching. Hydrolysis with acid could attack amorphous region of starch and generate the short chain amylose. While enzymatic debranching by pullulanase could cleave the α-1,6 branching point of amylopectin molecules, so that the short chain amylose would be produced. Faridah (2011) modified arrowroot starch by applying combination of acid-hydrolysis-debranching-autoclaving-cooling method which resulted in high amount of resistant starch, up to 39.39%, which is yet still lower than commercial starch (Novelose 330), reaching 42.68%. In this research, HMT was applied as additional treatment to the existing combination in order to obtain higher yield of resistant starch. Combination of the modifications given could lead to structural changes of starch which were analyzed by using GFC, FTIR, DSC, XRD, and SEM.
Objectives
4
analyzed by GFC, changes of amorphous and crystalline regions analyzed by FTIR, gelatinization properties including gelatinization temperature and gelatinization enthalpy analyzed by DSC, crystallinity of starch analyzed by XRD, and morphology of starch granules analyzed by SEM.
Significance
This research was expected to give scientific knowledges about how the subsequent modifications: acid hydrolysis-debranching-autoclaving-cooling-HMT affects the structural changes of arrowroot starch, particularly related to resistant characteristics of the starch and its application in functional food. The utilization of arrowroot starch as food ingredient could give an added-value of arrowroot tuber, which is not yet optimally exploited in Indonesia.
Hypothesis
The combination of modifications applied was hypothesized to bring about the structural changes of arrowroot starch, namely: the increase of amylose fraction and the decrease of amylopectin fraction which reflect the degradation of amylopectin and production of more short chain amylose which involved in starch retrogradation, as a mechanism of resistant starch formation; change of amorphous and crystalline region proportion in the modified starches; change of gelatinization temperature and enthalpy; change of crystalline type of starch after modification in which it has been known that recrystallized/retrograded starch generally owns a B-type crystalline pattern; change of starch granules morphology, except for single treatment of HMT which has been known as a treatment that will retain the morphology of native starch.
2
LITERATURE STUDY
Arrowroot (Marantha arundinacea L.)
Arrowroot is one of the alternative carbohydrate sources which is potential to develop. Arrowroot is cultivated to get the tuber, particularly the starch extracted from its tuber (Sukamto et al. 2010). Arrowroot is not originally coming from Indonesia. It firstly came from tropical region in United States which later spread to another tropical region, including Indonesia. It was distributed evenly along India, Indonesia, Sri Lanka, Hawaii, Philippines, and St. Vincent. In Indonesia, it could be found in several areas such as Java, Sulawesi, and Maluku. There are about 23 species of arrowroots from Marantha genus. Arrowroot which is hugely planted in Indonesia is Marantha arundinacea L. (Figure 1a). This type of arrowroot owns large tuber and doesn’t penetrate too much into the ground, also known well as Sumbawa type. The tuber of arrowroot starch is depicted in Figure 1b. Another species of arrowroot with little and long tuber, penetrating much into the ground, is also known as Chili type. This type of arrowroot owns higher content of starch, compared to Sumbawa type (Djaafar et al. 2010).
5 predominantly carbohydrate, especially starch. The freshly harvested arrowroot contains starch ranging from 16-18%, with high content of water (Villamayor and Jukema 1996).
Other than its use as carbohydrate source, arrowroot tuber also has health benefit especially for diabetic people. Arrowroot tuber has low glycemic index (14) compared to another tubers such as lesser yam (90), blue taro (95), achira (105), and sweet potato (79) (Marsono 2002). The GI is a ranking system that indicates how quickly a carbohydrate food raises blood glucose. This is determined by measuring the area under the curve in the 2 hours after the consumption of a test food. These values are then compared to the area under the curve 2 hours after the consumption of a similar weight of glucose or bread. Foods ranked <55 are considered to have a low GI. Low-GI foods include many fruits and vegetables, legumes, whole grains, and dairy products. Foods with a ranking between 56 and 75 are considered to have a moderate GI. High-GI foods, those with a ranking between 76 and 100, often include highly processed and refined carbohydrates such as instant oatmeal, white bread, and cornflakes (Kirpitch et al. 2011).
Figure 1 Arrowroot plant Marantha arundinacea L. (a) (Anonymous 2015)
and arrowroot tuber Marantha arundinacea L. (b) (Budi 2010)
Starch
Starch is the second most abundant carbohydrate in nature next to cellulose. Native starch is in form of white and semi-crystalline granules, which are synthesized in chloroplasts or amyloplasts of plant organs including seeds, stems, tubers, roots, leaves, and fruits (Robyt 1998). Starch has characteristic features varying in molecular structure, molecular organization of granule, morphological properties, gelatinization and pasting properties, starch polymorphs and percentage crystallinity, and enzyme digestibility (Jane 2004). Starch is a large-producible, economical, and environmentally friendly ingredient for food and non-food applications, which serves as the major energy source in food and feed but also as
6
a thickener, a binding agent, a texturizer, a filler, a film forming agent, and feedstock for fermentation to produce biomaterial and fuel (Jiang 2010).
Starch is structurally a carbohydrate made up of glucose units linked together by glycosidic bond (Whistler and BeMiller 1997). The molecules of the main components in starch, the polysaccharides amylose and amylopectin, are built up of a number of glucose monomers, but their structure differs tremendously. Amylose is mainly found as a linear chain of D-glucose units linked by α-(1,4) linkages. Amylopectin is a highly branched polymer, built up of glucose unit, linked with α-D-(1,4) glycosidic bond with the branching of α-D-(1,6) bond found every 24 to 30 glucose units (Wurzburg 1986, Bule´on et al. 1998). Molecular weight of amylose is ranging from 105-106 Da, while amylopectin is a much larger, having
molecular weight of 107-109 Da, in which thousands of chains of glucose are
connected to each other through their reducing end (Bule’on et al. 1998). High-amylose starch also consists of another polysaccharide known as the intermediate component (IC), which has a similar molecular weight to that of amylose but contains relatively more branched structure (Baba and Arai 1984).
Starch granules are built up of blocklets forming alternating amorphous and semi-crystalline growth ring (Gallant et al. 1997, Tang et al. 2006). These semi-crystalline growth rings consists of alternating semi-crystalline and amorphous lamellae (Gallant et al. 1997, Yamaguchi et al. 1979) with a 9 nm repeat distance (Jenkins et al. 1993). The external chain segments of amylopectin form double helix that build up the crystalline lamellae, whereas the internal part of the molecule, containing the branching points of the chains, exist in the amorphous lamellae (Waigh et al. 2000). The branch chains of amylopectin molecules form double helix and contribute to the starch crystallinity. Amylose molecules in normal starch are in an amorphous form, which are interspersed and intertwined with amylopectin molecules because of the low concentration of amylose (Jane et al. 1992, Kasemsuwan and Jane 1994). The exact location of amylose is poorly defined, although it is suggested to be associated with the amylopectin chains (Jenkins and Donald 1995). However, a study by Jenkins and Donald (1995) proposed that amylose is predominantly located in the amorphous growth ring. It had been also suggested that amylose was more concentrated at the periphery of the granules (Jane and Shen 1993) as well as at the center (Blennow et al. 2003). Schematic diagram of starch granule architecture was presented in Figure 2.
There are basically three types of X-ray diffraction patterns, depending on the organization of the double helix in the granules. Based on this, starch can be classified into A-, B-, or C- type (Bule’on 1998, Imberty et al. 1991). A-type pattern is usually owned by starch from cereal group, while B-type pattern is commonly found in root and tuber starch (Bule’on et al. 1998). The C- type pattern is found in legume starch and is a mixture of A- and B- type crystallites. The crystals in B-type are packed less densely compared to those in A-type starch, and B-type starch contains more water (Imberty et al. 1991). There is actually a V-type pattern which comprises crystalline amylose inclusion complexes (Bule’on et al. 1998).
7 and Shen 1993, Morrison and Gadan 1987), as well as at the center (Blennow et al. 2003).
Figure 2 Schematic diagram of starch granule architecture (Kallman 2013) Amylose has a degree of polymerization up to DP 6000, and has a molecular mass of 105 to 106 g/mol. The chains can easily form single or double helix (Takeda
et al. 1989). Amylopectin (107 to 109 g/mol) has an average DP of 2 million, making it one of the largest molecules in nature. Chain lengths of 20 to 25 glucose units between branch points are typical. Branch chains of amylopectin are designated as A, B, and C chains, which are arranged in clusters (Hizukuri 1986). Cluster model of amylopectin was presented in Figure 3.
8
The A chains are chains whose reducing ends attach to B or C chains but do not carry any other chains. The B chains have reducing ends attached to B or C chains and also carry A or other B chains. The C chain possesses a reducing end and carries A or B chains. The A chains are generally short and extend within one cluster. The B chains are further classified into B1, B2, and B3 chains, extending through one, two, and three clusters, respectively (Hizukuri 1986). Short chains (A) of DP 12-16 that can form double helix are arranged in clusters. The clusters comprise 80% to 90% of the chains and are linked by longer chains (B) that form the other 10% to 20% of the chains. Most B-chains extend into 2 (DP about 40) or 3 clusters (DP about 70), but some extend into more clusters (DP about 110) (Sajilata et al. 2006).
Depending on the plant, starch contains 20 to 25% amylose and 75 to 80% amylopectin (Whistler and BeMiller 1997, Bule’on et al. 1998). Waxy starch contains only amylopectin (Yoo and Jane 2002), whereas high-amylose starch usually consists of more than 50% amylose (Campbell et al. 2007, Li et al. 2008, Regina et al. 2006, Shi et al. 1998). Amylose molecules can be separated from amylopectin using either gel permeation/filtration chromatography (Jane and Chen 1992, Li et al. 2008) or n-amyl alcohol fractionation (Kasemsuwan et al. 1995, Li et al. 2008, Schoch 1942, Takeda et al. 1989). The ratio of amylose and amylopectin influences physical properties such as gelatinization temperature (Fredriksson et al. 1998) and retrogradation (Fredriksson et al. 1998, Jane et al. 1999, Zhu et al. 2010). Amylopectin doesn’t undergo retrogradation and also doesn’t form gel unless in a high concentration (Belitz and Grosch 1999). Resistant starch, which could be formed through retrogradation, is therefore preferred to be produced from high-amylose starch (Herawati 2010).
Resistant Starch
Resistant starch (RS) refers to the portion of starch and starch products that resist digestion as they pass through the gastrointestinal tract (Fuentes-Zaragoza et al. 2010). RS which isolated from ragi starch (Eleusine coracana) was revealed as a linear molecule of α-1,4-D-glucan, essentially derived from the retrograded amylose fraction, and has a relatively low molecular weight (1.4 x 106 Da) (Mangala
9 RS is an extremely broad and diverse range of materials and a number of different types exist (RS1–5). Those different type of resistant starches are mostly defined based on physical and chemical characteristics (Nugent 2005). The distinct five classes of RS in foods are: (1) RS1- physically protected form of starch found in whole or partly milled grains and seeds; (2) RS2-ungelatinized resistant granules with type B crystallinity which slowly hydrolyzed by α-amylase such as raw potatoes, green bananas, high amylose corn; (3) RS3-retrograded starch such as cooked and cooled potatoes, bread, and food products with repeated moist heat treatment; (4) RS4-chemically modified starches due to cross-linking with chemical reagents; (5) RS5-resistant starch formed as a complex of lipid-amylose (Nugent 2005, Ratnayake and Jackson 2008, Sanz et al. 2009, Fuentes-Zaragoza et al. 2010, Ai et al. 2013). RS1 and RS2, which are native starches, were known to lose the potential of RS if gelatinized during the processing of food. While RS3 is stable when heated above 100 oC because retrograded starch (amylose) melts at ~155 oC
(Keren et al. 2003 at Mun and Shin). On the other hand, RS4 formed by cross-linking, is also known to be stable when treated by enzyme or acid. RS5 requires higher temperatures for gelatinization and are more susceptible to retrograde, thus it is also known to be heat stable (Fuentes-Zaragoza et al. 2011).
Generally, resistant starch behaves physiologically as a fiber (Haralampu 2000). Resistant starch has been known to increase fecal bulk and lower colonic pH (Slavin et al. 2009). Like soluble fiber, RS is a substrate for the colonic microflora, forming metabolites including short-chain fatty acids (SCFA), i.e. mainly acetic, propionic, and butyric acid (Elmstahl 2002). As butyrate is one of the main energy substrates for large intestinal epithelial cells and inhibits the malignant transformation of such cells in vitro; this makes easily fermentable RS fractions especially interesting in preventing colonic cancer (Asp and Bjorck 1992). The consumption of RS has also been related to reduce postprandial glycemic and insulinemic responses, which contributes positively to diabetes management (Tharanathan and Mahadevamma 2003). In addition to colonic cancer prevention and diabetes management, hypocholesterolemic effects of RS have been also widely demonstrated. According to several studies, RS ingestion may decrease the serum cholesterol level in rats fed a cholesterol-free diet (De-Deckere et al. 1993, Hashimoto et al. 2006).
Starch Modification
Native starch exhibits limited application due to low shear stress resistance and thermal decomposition, high retrogradation and syneresis, in addition to poor processability and solubility in common organic solvents (Neelam et al. 2012). It also has low resistance to acid, inconsistent viscosity, low solubility, and is also unstable (Maulani et al. 2013). Thus, to meet the demanding technological needs of today, the properties of starch are modified by a variety of modification methods. Starch modification is aimed to correcting one or some of the abovementioned shortcomings, which will enhance its versatility and satisfy consumer demand. The techniques for starch modification are generally classified into four categories; physical, chemical, enzymatic, and genetical modification (Neelam et al. 2012).
10
and convert native starch into cold water-soluble starch or small-crystallite starch (Neelam et al. 2012). Heat-treatment processes include heat-moisture and annealing treatments, both of which cause a physical modification of starch without any gelatinization, damage to granular integrity, or loss of birefringence (Abbas 2010). Chemical modification involves the introduction of functional groups into the starch molecules, resulting in markedly altered 10 hysic-chemical properties. Such modification of native granular starches profoundly alters their gelatinization, pasting, and retrogradation behavior (Neelam et al. 2012). This technique is the mainstream of the modified starch in the last century (Abbas 2010). Enzymatic modification of starch is hydrolysis of some part of starch into a low molecular weight of starch called maltodextrin, or dextrin using amylolytic enzymes (Miyazaki et al. 2006). Genetic modification involves transgenic technology that targets the enzymes involved in starch biosynthesis thus avails the advantage over environmentally hazardous post-harvest chemical or enzymatic modifications. This kind of technique can be applied either by the traditional plant-breeding techniques or through biotechnology (Johnson et al. 1999).
In addition to functional properties improvement of native starch, modification of starch is also hugely carried out to meet the needs for high nutritional claim. Resistant starch can be produced through modification of starch and claimed as a functional fiber as it allows high fiber nutritional claim and is well suited for food applications. Commercial resistant starch is a special high-amylose starch that has been modified by biochemical or physical processing to maximize its total dietary fiber content (Abbas 2010).
In this research, starch modification was conducted to optimize the amount of resistant starch for application in a variety of functional food. In many researches dealing with resistant starch, it has been shown that the formation of resistant starch involves the change of granular structure. Therefore, the focus of this research was to investigate the structural change of starch granule which might be associated with its resistant characteristics. Each treatment applied might contribute differently to the change of starch granular structure, which further affect the resistant characteristics of starch.
Acid Hydrolysis
Resistant starch can be produced from a highly retrograded amylose fraction, the amount of RS formed being directly proportional to the amylose content of the starch (Annison and Topping 1994). Acid hydrolysis (lintnerization) has been a common method conducted to increase the amount of short linear chains, like amylose, that favor the phenomenon of retrogradation (Dundar and Gocmen 2013, Aparicio-Saguilan 2014). Lintnerization which applied in this research belongs to chemical modification. It has been the most widely used chemical method to modify the starch structure and generate crystalline structures that resist enzymatic hydrolysis (Aparicio-Saguilan 2014). The hydrolysis of starch by acid takes place in two stages (Jayakody and Hoover 2002, Robin et al. 1975, Singh and Ali 2000, Wang et al. 2003): first, the hydrolysis of an amorphous region that rapidly progresses and second, the hydrolysis of a crystalline region that progresses slowly.
11 for RS formation (Lehmann et al. 2003, Ferrini et al. 2008, Faridah et al. 2010, Ozturk et al. 2011, Aparacio-Saguilan 2014). According to Mun and Shin (2006), lintnerized starch is generally manufactured by reacting starch with 2.2 N HCl at 30–40 oC. In this research, 2.2 N HCl is also used at temperature 35 oC. Chung et
al. (2003) conducted mild acid hydrolysis of high amylose corn starch by using 2.2 M HCl at 35 oC for 0.5, 1, or 2 h to increase the degree of retrogradation. Chung et
al. (2003) described that mild acid hydrolysis of starch prior to the freeze-thawing treatment allowed more amylose to associate because it generated short amylose chains, suitable for the formation of double helix, and thus increased the degree of retrogradation.
In acid modification, the hydroxonium ion attacks the glycosidic oxygen atom and hydrolyses the glycosidic linkage. An acid acts on the surface of the starch granule first before it gradually enters the inner region. Acid modification changes the physicochemical properties of starch without destroying its granule structure (Bentacur et al. 1997). According to John et al. (2002), the product obtained has the same appearance as the native granule though it presents higher fragmentation, lower swelling during gelatinization, and lower viscosity. The gelatinization temperature and the width of the gelatinization endhoterm have also been shown to increase on acid hydrolysis. The method for the manufacture of acid modified starch entails treating concentrated starch slurry with mineral acid like HCl, HNO3, H2SO4,
and H3PO4 at temperatures below gelatinization temperature for specific period
depending on the desired degree of hydrolysis (Singh and Ali 2000).
Debranching
Debranching term generally refers to a hydrolysis of amylopectin to form linear starch molecules. In this study, debranching term refers to the kind of hydrolysis by using debranching enzymes. Debranching by using enzyme has been widely used to produce linear chains that contributes to a high RS content (Milasinovic et al. 2010, Sun et al. 2014). Pullulanase (Pullulan 6-glucanohydrolase, EC 3.2.1.41), an important debranching enzyme in starch processing, can cleave α -1,6 linkages in pullulan, amylopectin, and other related polysaccharides. Debranching of amylopectin will provide an increased opportunity to molecule alignment or aggregation, to form crystalline structures, and is, thus favorable in RS formation.
In this study, debranching using pullulanase was applied to produce linear, low molecular weight, and recrystallizable polymer chains. The effect of debranching using pullulanase in increasing the yield of RS3 has been reported in several studies (Gonzales-Soto et al. 2007, Leong et al. 2007, Zhao and Lin 2009, Faridah et al. 2010). Zhao and Lin (2009) reported that debranching of gelatinized or retrograded maize starch at 60 oC with pullulanase at addition level of 3 PUN g -1 starch showed a more favorable effect on RS formation. In the study it was
12
effective, indicating this treatment is applicable in RS preparation to increase the RS yield (Zhao and Lin 2009). In this study, arrowroot starch was also allowed to be retrograded first through one cycle of autoclaving-cooling process before being debranched by using pullulanase enzyme. Gonzales-Soto et al. (2004) conducted debranching of banana starch by using pullulanase enzyme at different concentration, followed by autoclaving treatment, to produce resistant starch. It was described in the study that debranching by using pullulanase at 10.6 U/g for 5 h, exhibited the optimal yield of resistant starch, reaching 18.0%, which was significantly different with the native starch which only 9.1%.
Autoclaving-cooling
It is well-documented that when starch dispersion is heated to fixed temperature, it forms a starch-based gel. Upon cooling, the gel will undergo transformations, leading to a partially crystalline structure. The process has been known as autoclaving-cooling process which is a physical modification to convert native starch into resistant starch type III (RS3). Autoclaving process involves gelatinization which includes disruption of the granular structure by heating with excess water. It is well known that gelatinization temperature has an important influence on RS yields (Escarpa et al. 1997). While upon cooling of the starch solution, there is retrogradation phenomenon which involves slow recrystallization of the amylose molecules (Zabar et al. 2008). During retrogradation, starch molecules are re-associated and can form tightly packed structures stabilized by hydrogen bonding (Haralampu 2000). The resulting RS3 consists primarily of amylose with relatively high degree of crystallization (Sievert et al. 1991, Eerlingen et al. 1993). The autoclaving-mediated formation of RS can be affected by amylose content (Escarpa et al. 1997), treatment time, temperature (Onyango et al. 2006), and lintnerization (Aparicio-Saguilan et al. 2005). According to Haralampu (2000), starch retrogradation actually includes not only short term retrogradation by amylose, but also long term retrogradation by amylopectin.
Isothermal formation of RS is favored at 100 oC (Eerlingen et al. 1993). High
temperature is optimal, but not accessible to 1 atm operations. Thermal cycling to 134 oC is advantageous for the formation of extremely stable RS (Haralampu 2000).
Dundar and Gocmen (2013) conducted three cycles of autoclaving-cooling of amylotype corn starch using autoclaving temperature at 140 and 145 oC for 30 min
and cooling time 24, 48, and 72 h at 4 oC. It was found out in the study that the
higher autoclaving temperature and longer storing time showed a beneficial impact on RS formation. Faridah et al. (2010) also conducted three cycles of autoclaving-cooling of arrowroot starch, by heating at 121 oC for 15 min and cooling at 4 oC for
13
Heat Moisture Treatment
Heat Moisture Treatment (HMT) is a form of physical modification of starch, without gelatinization or damage to granular integrity with respect to size, shape or birefringence, through controlled application of heat and moisture (Jacobs and Delcour 1998). This method involves treatment of starch granules at low moisture levels (<35% moisture w/w) over a period of time (15 min-16 h) at temperature above the gelatinization temperature (84-120 oC) (Arns et al. 2014). HMT technique
can increase the tendency of retrogradation which responsible for RS3 formation (Singh et al. 2005). This technique can promote the interaction of polymer chains by initially disrupting the crystalline structure and dissociating the double helical structure in the amorphous region followed by rearrangement of the disrupted crystals (Gunaratne and Hoover 2002). HMT is also known to trigger perfection of starch crystallites. This is possibly initiated by incipient swelling and the resulting mobility of amorphous α-glucans which facilitate ordering of double helix (that is, increased inter- and intramolecular hydrogen bonding) (Lawal and Adebowale 2005).
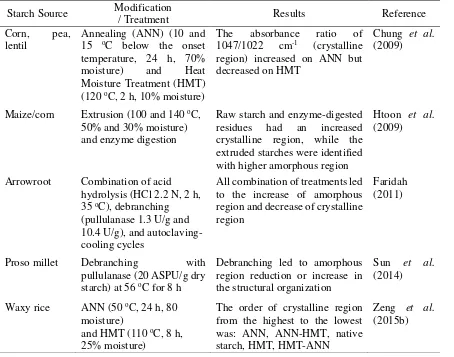
Chung et al. (2009) applied HMT at 120 oC for 2 h with 30% moisture content in corn, pea, and lentil starch. It was described in the study that HMT led to the increase of resistant starch in corn, pea, and lentil up to 7.7, 11.2, and 10.4% respectively. In this study, HMT was conducted at 121 oC for 15 min with 20%
moisture content. The changes occurred in HMT-modified starch were attributed to an interplay of factors such as: (1) amylose content, (2) interaction between starch chains, (3) arrangements of amylose chains within the amorphous domains, and (4) lipid-amylose complex (Neelam et al. 2012).
Combination of Modification to Produce Resistant Starch
14
In other studies, HMT was revealed to contribute in promoting the yield of resistant starch, in combination with other treatments. Satmalee and Matsuki (2011) reported that combination of debranching and heat moisture treatment applied in low amylose Thai rice flour resulted in the increase of resistant starch from 11.59% (in native flour) to 18.31%. The effect of HMT to increase the resistant starch yield when combined with other treatments was also revealed in Mutungi et al. (2009), showing that the resistant starch yield increased from 21.4% in debranched starch to 47.8% in debranched starch subjected to temperature cycling, and further to 88.4% in debranched starch subjected to temperature cycling and HMT. In other study conducted by Shin et al. (2004), combination of acid hydrolysis, autoclaving-cooling, and HMT at 30% moisture content also led to the increase of resistant starch yield from 5.4% in native starch to 22.7% in modified starch. The study also showed that compared to the sample subjected only with acid hydrolysis and autoclaving-cooling, the sample treated with HMT at 30% moisture content as additional treatment to the previous combination showed higher yield of resistant starch. The effect was somehow different when HMT was applied at 20% moisture content, which showed lower resistant starch yield, compared to sample subjected only with acid hydrolysis and autoclaving-cooling.
Changes of Molecular Structure in Starch Modification
Gel Filtration Chromatography (GFC) Profile
15 Table 1 Studies of starch molecular distribution by using Gel Filtration
Chromatography (GFC) technique
Starch
Source Modification / Treatment Material GFC Mobile Phase Results Reference
High-available Samples were separated into 27 fractions. GFC indicated a greater proportion of
and corn Acid-methanol treatment using 0.36% concentrated treatment for both starches, amylose content of maize is
NaCl 50 mM Samples were separated into 45 fractions. There was a led to the reduction of Mw
16
Many oligo- and polysaccharides, especially starch, have been separated, prepared, and analyzed by using GFC technique (Praznik and Beck 1985, Suortti and Pessa 1991, Tester and Karkalas 1996, Han et al. 2003, Ozturk et al. 2011). Starches are used in foodstuffs and in various technological applications. For technological purposes, starches are often modified by various chemical, enzymatic, or physical treatments, which change their molecular weight distribution and the ratio between the two major components, namely amylose and amylopectin (Suortti and Pessa 1991). The change of molecular weight distribution can be very well described by using the GFC technique.
As previously described, GFC profile of starch could give information about the structural changes occur in the starch which shown by the change of molecular weight distribution and the ratio between amylose and amylopectin as the main components of starch. As seen in Table 1, various modification applied in starch reflected alteration of molecular weight distribution and proportion of amylose and amylopectin. Rodis et al. (1993) illustrated that extrusion which subjected to high-amylose and amylopectin enriched corn starch led to fragmentation of starch confirmed by appearance of lower molecular weight material. The GFC profile exhibited an alteration of molecular weight distribution of corn starh after subjected to extrusion. In a study by Lu et al. (1996), GFC profile could confirm the altered ratio of amylose and amylopectin in rice starch caused by heat moisture treatment (HMT). It was described in the study that HMT led to the decrease of amylopectin but increase of amylose, suggesting the thermal degradation of amylopectin due to HMT. The altered ratio of amylose and amylopectin was also shown in GFC profile of arrowroot, cassava, and corn starch which subjected to single treatment of acid hydrolysis and/or combined treatment of acid hydrolysis, debranching, and autoclaving-cooling (John et al. 2002, Ferrini et al. 2008, Faridah et al. 2010). The GFC profile could confirm the decrease of amylopectin and the increase of amylose fraction in the modified starches, as the impact of the modification applied. While in Othman et al. (2010), a study with GFC reflected that debranching of sago starch led to a decrease of the starch molecular weight (Mw).
Fourier Transform Infra-Red (FTIR) Spectra Profile
17 Table 2 Studies of starch structure by using Fourier Transform Infra-Red (FTIR)
spectroscopy
Starch Source Modification / Treatment Results Reference Corn, pea, crystalline region, while the extruded starches were identified
Proso millet Debranching with pullulanase (20 ASPU/g dry starch) at 56 oC for 8 h
Debranching led to amorphous region reduction or increase in
The region of CO bending vibrations (800-1300 cm-1) has shown a relation
with the water content and the crystalline (types B and V) and amorphous phases (Bernazzani et al. 2008). According to Htoon et al. (2009), the bands in the spectral region of 800-1200 cm-1 reflect changes in polymer conformation and hydration
processed starches. FTIR have been used to study the gelation and retrogradation of amylose and amylopectin (Goodfellow and Wilson 1990). FTIR have been also applied to study various starch in rubbery and glass states (van Soest et al. 1995, Capron et al. 2007, Chung et al. 2009, Zeng et al. 2015b). In the studies, they had identified amorphous starch by a FTIR band around 1022 cm-1 and the crystalline
state by a band at 1047 (or 1045) cm-1 . van Soest et al. (1995) described that the IR
absorbance band at 1047 cm-1 is sensitive to the amount of ordered or crystalline
starch, and the band at 1022 cm-1 is characteristic of amorphous starch, hence the
ratio of 1045/1022 cm-1 could express the amount of crystalline region in starches
(Zeng et al. 2015b). While the ratio of 1022/995 cm-1 have been revealed to express
18
As presented in Table 2, various modification applied upon different starches led to different effect of the ratio of crystalline and amorphous regions in starch. The same treatment when applied as a single treatment and as a combined treatment might give the different effect to the ratio of crystalline and amorphous regions. Crystalline region has been known to be region with a more ordered structure, while the amorphous region with the less ordered (random) structure. Chung et al. (2009) described that annealing and HMT subjected to corn, pea, and lentil starch have shown different effect to the ratio of amorphous and crystalline region in starch. The former led to the increase of crystalline region (decrease of amorphous region), while the later led to the decrease of crystalline region (increase of amorphous region). However, when HMT and annealing was applied as a combined treatment, they could show different effect when applied in different order. When annealing was applied prior to HMT, the crystalline region is higher than if HMT was applied before annealing (Zeng et al. 2015b). Extrusion treatment of corn starch applied at certain moisture content (50% and 30%) was also found to increase the amorphous region (Htoon et al. 2009). Single treatment of debranching has been known to reduce the amorphous region (Sun et al. 2013), while if it was combined with acid hydrolysis and autoclaving-cooling, the amorphous region was found to increase (Faridah et al. 2010). Hence, FTIR could detect the change of structural organization occurred in the starch due to the treatments applied.
Differential Scanning Calorimetry (DSC) Profile
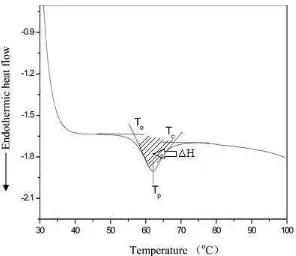
Starch gelatinization is an important phenomenon occurring in various food processing operations (Biliaderis et al. 1980). The term refers to an irreversible change from a semi-crystalline structure to an amorphous structure with the presence of sufficient water at high temperature, which is associated with the dissociation of double helices, loss of birefringence, and disruption of granular structure (Jane 2004). The most common technique used in thermal studies of starches is Differential Scanning Calorimetry (DSC) (Biliaderis et al. 1986, Lu et al. 1996), which can give the information of transition temperature during gelatinization and enthalphy change of starch (∆H). Transition temperature includes To (onset temperature), Tp (peak temperature), and Tc (conclusion temperature).
Figure 4 illustrated To, Tp, Tc, and ∆H values in a typical DSC thermogram.
DSC is particularly well suited to investigate phase transitions of starch/water systems because it allows: (1) study of starch gelatinization over a wide range of starch/water ratio; (2) determination of gelatinization temperatures above 100 oC; and (3) estimation of transition enthalpies. The technique, which detects the
19 Table 3 Thermal studies of starches by using Differential Scanning Calorimetry
(DSC) technique
Starch
Source Modification / Treatment Results Reference Waxy rice Annealing (ANN) (50 oC,
gelatinization enthalpy (∆H), except in single annealing treatment
Arrowroot Combination of acid hydrolysis (HCl 2.2 N, 2 h,
enthalpy (∆H) in all combination of treatments gelatinization increased when the acid-treatment time increased
Aparicio-Saguilan et al. (2014)
Cassava
20
Figure 4 Typical DSC thermogram with the To, Tp, Tc, and ∆H values (Wang and
Copeland 2013)
Analysis with DSC could explain the change of gelatinization temperature and enthalpy of modified starch as the impact of structural changes in molecular level. The applied treatments, for instance, could affect the hydrogen bond in the starch structure, influence the crystallite perfection of starch, and also molecular interaction within the crystalline and amorphous region, which further bring about the alteration of the starch gelatinization properties. As described in Table 3, different treatment led to a different gelatinization properties of starch. HMT was generally shown to increase To, Tp, and Tc, while decrease ∆H in waxy rice, potato,
corn, pea, and lentil starches (Zeng et al. 2015b, Vermeylen et al. 2006, Chung et al. 2009). When combined with annealing, HMT still showed the similar effect upon the gelatinization properties of starch (Zeng et al. 2015b). Acid hydrolysis subjected to banana, cassava, and corn starch generally led to the increase of To, Tp,
Tc, and ∆H, except ∆H of cassava starch which remained unchanged
(Aparicio-Saguilan et al. 2014, Ferrini et al. 2008). Autoclaving-cooling cycles was known to decrease To, Tp, Tc, and ∆H in high-amylose barley starch (Szczodrak and Pomeranz
1991). While debranching of proso millet starch was found to increase Tp and Tc,
but decrease ∆H. On the other hand, Faridah (2011) described that combination of acid hydrolysis, debrancing, and autoclaving subjected to arrowroot starch would lead to the increase of To, Tp, Tc, and ∆H. The increase of gelatinization temperature
21
X-ray Diffraction (XRD) Pattern
XRD have been used to evaluate the crystalline fraction of variety of starches (Frost et al. 2009). The crystal structures in native starches are formed by packing of hexagonal arrays of amylopectin in helical coils (Zobel et al. 1988). The diffraction peaks are used to identify the particular crystalline form(s) in the material (Singh et al. 2005). The intensity profile of the X-ray reflection from a partially crystalline sample like starch granules is a function of distribution of crystal size and of lattice disorder (Singh et al. 1995). Table 4 presented list of studies which analyzed crystallinity of various starches by using XRD technique. Table 4 Studies of crystallinity in various starches by using X-ray Diffraction
(XRD)
Starch
Source Modification / Treatment Results Reference Corn Acid hydrolysis with acid hydrolysis. The crystallinity of starches increased gradually following the acid thinning
Crystallinity of the starch was unaffected by ANN, but with HMT there was a
formation of a B-type structure, while at 95 oC yielded a mixture which contains
mainly A-type crystal. Residues of V or B polymorph were identified in all samples treated with retrogradation at 95 oC
Zabar et al.
Native proso millet starch showed A-type crystal, while the debranched-recrystallized starch showed B-type crystal. The characteristic of B-type at
22
opposita starch led to different change of crystallinity type. Acid hydrolyzed corn starch was found to lose its A-type crystallinity due to the acid treatment (Beninca et al. 2008), while rhizome Dioscorea opposita underwent a change from C-type to A-type after acid hydrolysis (Shujun et al. 2007). Annealing was found to cause no change of crystallinity type in potato starch, while HMT led to alteration from B- to A-type crystallinity (Vermeylen et al. 2006). In other study, corn and wheat starch subjected to autoclaving-cooling cycles led to formation of B-type structure when retrogradation was carried out at 40 oC, while at 95 oC the crystallinity type
was found to be mainly A-type crystal (Zabar et al. 2008). On the other hand, recrystallized debranched proso millet starch exhibited a B-type crystalline pattern after the treatment (Sun et al. 2014). Generally, the formation of B-type crystalline is commonly found in recrystallized starch as a result of reorganization of starch chain during retrogradation (Sun et al. 2014). However, the impact of modification towards the type of starch crystallinity is very typical among different starches and depending on the specific condition of treatments applied.
Scanning Electron Microscopy (SEM) Microstructure Profile
Electron microscopy has been widely employed for the evaluation of the microstructure of food and biological products, including starch (Fazaeli et al. 2012). Scanning electron microscopy (SEM) is a very useful tool to visualize food structure, because it combines in many ways the best features of light microscopy (LM) and transmission electron microscopy (TEM). Compared to light microscopy, SEM has been more convenient because both surface and internal features can be studied, a wide range of magnification is possible and the SEM can achieve a depth of field roughly 500 times greater than that of light microscopy (Fazaeli et al. 2012). Table 5 presented a list of studies of starch microstructure by using SEM.
Microstructure investigation can help quantifying product changes during processing and may also improve the understanding of mechanisms and changes in quality factors, especially the changes in food texture (Aguilera and Stanley 1999). For example, the pore sizes and the number of pores can significantly influence the texture of food. Smaller number of pores and small sizes led to the dense structure. While, larger number of pores and large pore size can cause a decrease of the hardness of the product (Xiao and Gao 2012).
23 profile of various starches are different each other by size, shape, or birefringence, thus the effects observed after the treatment might differ in different starches. Condition of treatment is also influential to the extent of change observed in modified starch. For instance, acid modification which carried out with different kind or concentration of acid, and also with different period of time, would result in different effect towards the starch microstructure.
Table 5 Studies of microstructure in various starches by using Scanning Electron Microscopy (SEM)
Starch Source Modification / Treatment Results Reference Cassava and
maize Acid-methanol treatment using 0.36% concentrated HCl at 54 oC for 1-8
h
The surface of maize and cassava starch granular were found rougher, compared to their native. Part of the granules was eroded. The cassava granule size was found smaller granules. There was an increase of surface area per unit weight in
The granules were smaller than that of native starches. Some granules
barley Autoclaving-cooling cycles with 1-20 cycles (121 oC,
1 h - 4 oC,
overnight)
The granular structure disappeared, irregularly shaped particles were visible, very compact and dense to bread-like or cake-like in shape. Longer time of hydrolysis led to
24
Pusat Studi Biofarmaka (Biopharmaca Research Center), Bogor Agricultural University, Bogor.
Materials and Instruments
Arrowroot starch was obtained from Kelompok Wanita Tani or Woman Farmer Association (Yogyakarta, Indonesia). Chemicals used were HCl 2.2 N, Pullulanase 10.4 U/g (EC 3.2.1.4.1, Sigma), Sephadex G-50 superfine (Sigma, Sigma-Aldrich, USA), Sephacryl S-400 HR (Sigma, Sigma-Aldrich, USA), and other chemicals for analysis. All chemicals used were analytical grade.
Instruments used for this research were autoclave, waterbath with temperature control, refrigerator, chromatography glass columns, fraction collector model SF-100 (Toyo, Toyo Kagaku Sangyo Co., Japan), Fourier Transform Infra-Red (FTIR) spectrometer type tensor 37 (Bruker Inc., Germany), equipped with deuterated L-alanine doped triglycene sulphate (DLATGS) detector at a resolution of 4 cm-1 by
32 scans, Differential Scanning Calorimeter (DSC-60A, Shimadzu, Japan), X-Ray Diffraction (XRD) instrument GBC type EMMA (GBC Scientific Equipment Pty. Ltd., Australia), and Scanning Electron Microscope (SEM) JEOL JSM-5310LV (JEOL Ltd., USA).
Methods
This research generally consisted of two parts: 1) Modification of starch, 2) Characterization of starch. Modification of starch consisted of acid hydrolysis (AH), debranching using pulullanase enzyme (DE), autoclaving-cooling (AC), heat moisture treatment (HMT), and combination of those treatments. The analysis conducted in this research including 1) Profile of starch molecular distribution by using Gel Filtration Chromatography (GFC) technique, 2) Changes of the crystalline and amorphous regions by using Fourier Transform Infra-Red spectroscopy (FTIR), 3) Gelatinization properties (gelatinization temperature and gelatinization enthalpy) by using Differential Scanning Calorimetry (DSC) technique, 4) Crystallinity by using X-ray Diffraction (XRD), 5) Morphology of starch granules by using Scanning Electron Microscopy (SEM).
Modification of Starch
Acid Hydrolysis (AH) (Faridah et al. 2010)
As much as 250 g of arrowroot starch was hydrolyzed with HCl 2.2 N (1:1) for 2 h in waterbath at 35 oC. Sample was neutralized using NaOH 1 N and allowed
to separate. HCl-NaOH solution which has been separated was removed from the sample. Distilled water was added approximately two times of the HCl volume, then it was stirred and let to separate again. The liquid part above the precipitate was removed and separated from the starch. The starch was oven-dried at 50 oC for