Perbandingan Efektifitas dan Efek Samping
Pemakaian Metformin XR dan Metformin IR
dalam Pengobatan PCOS yang Resisten
terhadap Clomiphene Citrate
TESIS
OLEH :
HEDY TAN
DEPARTEMEN OBSTETRI DAN GINEKOLOGI
FAKULTAS KEDOKTERAN UNIVERSITAS SUMATERA UTARA
RSUP. H. ADAM MALIK
PENELITIAN INI DI BAWAH BIMBINGAN TIM-5
Pembimbing : Dr. Binarwan Halim, Sp.OG.K
Dr. Yostoto B. Kaban, Sp.OG.K
Penyanggah : Dr. Herbert Sihite, Sp.OG
Dr. M. Fidel Ganis Siregar, Sp.OG
Prof. Dr. M.Fauzie Sahil, Sp.OG.K
Diajukan untuk melengkapi tugas-tugas dan memenuhi
salah satu syarat untuk menyelesaikan program adaptasi
dokter spesialis
i
KATA PENGANTAR
Segala Puji dan Syukur saya panjatkan kepada Tuhan Yang Maha Kuasa, karena kasih
dan karunia-Nya maka penulisan tesis ini dapat diselesaikan.
Tesis ini disusun untuk melengkapi tugas-tugas dan memenuhi salah satu syarat untuk
menyelesaikan program adaptasi dokter spesialis Obstetri dan Ginekologi lulusan luar
negeri. Sebagai manusia biasa, saya menyadari bahwa tesis ini banyak kekurangannya
dan masih jauh dari sempurna, namun demikian besar harapan saya kiranya tulisan
sederhana ini dapat bermanfaat dalam menambah perbendaharaan bacaan khususnya
tentang :
“
PERBANDINGAN EFEKTIFITAS DAN EFEK SAMPING PEMAKAIAN
METFORMIN XR DAN METFORMIN IR DALAM PENGOBATAN PCOS
YANG RESISTEN TERHADAP CLOMIPHENE CITRATE ”
Dengan selesainya laporan penelitian ini, perkenankanlah saya menyampaikan rasa
terima kasih dan penghargaan yang setinggi-tingginya kepada yang terhormat :
1.
Rektor Universitas Sumatera Utara dan Dekan Fakultas Kedokteran Universitas
Sumatera Utara, yang telah memberikan kesempatan kepada saya untuk mengikuti
Program Adaptasi Dokter Spesialis Obstetri dan Ginekologi lulusan luar negeri di
Fakultas Kedokteran USU Medan.
2.
Prof. dr. Delfi Lutan, MSc, SpOG.K, Ketua Departemen Obstetri dan Ginekologi
FK-USU Medan ; dr. M. Fidel. G. Siregar, SpOG, Sekretaris Departemen Obstetri
dan Ginekologi FK-USU Medan ; dr. Henry Salim Siregar, SpOG.K, Ketua
Program Studi Dokter Spesialis Obstetri dan Ginekologi FK-USU Medan ; dr. M.
Riza. Z. Tala, SpOG.K, Sekretaris Program Studi Dokter Spesialis Obstetri dan
Ginekologi FK-USU Medan ; Prof. dr. R. Haryono. R. Roeshadi, SpOG.K ; Prof.
ii
Prof. dr. T. M. Hanafiah, SpOG.K ; Prof. dr. Budi R. Hadibroto, SpOG.K ; dan
Prof. dr. Daulat H. Sibuea, SpOG.K ; Prof. dr. M. Fauzie Sahil, Sp.OG.K yang telah
bersama-sama berkenan menerima saya untuk mengikuti program adaptasi dokter
spesialis lulusan luar negeri di Departemen Obstetri dan Ginekologi.
3.
Prof. dr. M. Fauzie Sahil, SpOG.K, ketua tim pelaksana adaptasi dokter spesialis
obstetri dan ginekologi lulusan luar negeri; dr. Muhammad Rusda, SpOG.K,
sekretaris tim pelaksana adaptasi dokter spesialis obstetri dan ginekologi lulusan
luar negeri; Prof. dr. R. Haryono. R. Roeshadi, SpOG.K ; Prof. dr. Delfi Lutan,
MSc, SpOG.K ; dr. Deri Edianto SpOG.K, anggota tim pelaksana adaptasi dokter
spesialis obstetri dan ginekologi lulusan luar negeri selama saya menjalani masa
adaptasi, yang telah banyak mengayomi, membimbing dan memberikan
nasehat-nasehat yang bermanfaat kepada saya dalam menghadapi masa-masa sulit selama
adaptasi.
4.
dr. Ichwanul Adenin, SpOG.K selaku Kepala Sub Divisi Fertilitas Endrokrinologi
dan Reproduksi atas kesempatan yang diberikan kepada saya untuk melakukan
penelitian ini.
5.
dr. Binarwan Halim, SpOG.K dan dr. Yostoto B Kaban, SpOG.K selaku
pembimbing utama penelitian ini yang dengan rela dan dengan penuh kesabaran,
yang telah meluangkan waktunya yang sangat berharga untuk membimbing,
memeriksa dan melengkapi penulisan tesis saya ini dari awal hingga selesai.
6.
dr. Herbert Sihite, SpOG ; dr. M. Fidel. G. Siregar, SpOG ; dan Prof. dr. M. Fauzie
Sahil, SpOG.K, selaku penyanggah dan nara sumber dalam penulisan tesis ini, yang
telah banyak memberikan bimbingan dan masukan dalam perbaikan tesis ini.
7.
Prof. dr. Daulat H. Sibuea, SpOG.K, selaku pembimbing referat mini fetomaternal
iii
Sarma N Lumbanraja, SpOG.K, selaku pembimbing referat mini fetomaternal saya
yang berjudul
”Pemakaian Low-molecular-weight Heparin selama Kehamilan
dan Nifas“
; dr. Aswar Aboet, SpOG.K selaku pembimbing referat mini Fertilitas
Endokrinologi dan Reproduksi saya yang berjudul
”Keguguran Kahamilan
Berulang”
dan dr. Hj. Sarah Dina, SpOG.K selaku pembimbing referat mini
Onkologi saya yang berjudul
”Human Papillomavirus (HPV) dan Vaksinasi”
8.
Kepada dr. Surya Dharma, M.Kes, yang telah meluangkan waktu dan pikiran untuk
membimbing saya dalam penyelesaian uji statistik tesis ini.
9.
Seluruh Staf Pengajar di Departemen Obstetri dan Ginekologi FK-USU Medan,
yang secara langsung maupun tidak langsung telah banyak membimbing dan
mendidik saya sejak awal hingga akhir program adaptasi. Semoga Yang Maha
Pengasih membalas budi baik guru-guru saya tersebut.
10.
Direktur RSUP. H. Adam Malik Medan beserta staf yang telah memberikan
kesempatan dan sarana kepada saya untuk bekerja selama mengikuti program
adaptasi di Departemen Obstetri dan Ginekologi.
11.
Direktur RSUD. Dr. Pirngadi Medan ; Kepala SMF Obstetri dan Ginekologi
RSUD. Dr. Pirngadi Medan beserta staf yang telah memberikan kesempatan dan
sarana kepada saya untuk bekerja selama mengikuti program adaptasi di
Departemen Obstetri dan Ginekologi.
12.
Direktur RS. PTPN 2 Tembakau Deli ; dr. Sofian Abdul Ilah, SpOG dan dr.
Nazaruddin Jaffar, SpOG.K beserta staf yang telah memberikan kesempatan dan
sarana kepada saya untuk bekerja selama bertugas di Rumah Sakit tersebut.
13.
Direktur RS Haji Mina Medan, beserta staf yang telah memberikan kesempatan
iv
14.
Ka. Rumkit Tk II Putri Hijau KESDAM I/BB & Ka. SMF OBSGYN Mayor. CKM.
dr. Gunawan Rusuldi, SpOG beserta staf yang telah memberikan kesempatan dan
sarana kepada saya untuk bekerja selama bertugas di Rumah Sakit tersebut.
15.
Direktur RSU Sundari Medan, beserta staf yang telah memberi kesempatan dan
sarana kepada saya untuk bekerja selama bertugas di Rumah Sakit tersebut.
16.
Direktur RSU HKBP Balige, beserta staf yang telah memberikan kesempatan kerja
dan bantuan moril selama saya bertugas di rumah sakit tersebut.
17.
Kepada teman-teman sejawat saya yang telah menyelesaikan Program Pendidikan
Dokter Spesialis Obstetri dan Ginekologi : dr. Ronny. P. Bangun, SpOG, dr. Siti
Sylvia. S, SpOG, dr. Gorga. I. V. W. Udjung, SpOG, dr. Ilham S Lubis, SpOG, dr.
Anggia Lubis, SpOG, dr. Maya Hasmita, SpOG, dr. M. Ikhwan, SpOG, dr. Edward
Muldjadi, SpOG, dr. Zilly Adein, SpOG, dr. Lili kuswani, SpOG, dr. Ari. A. Lbs
SpOG, dr. T. Jeffrey. A, SpOG, dr. M. Rizky Yaznil, SpOG, dr. Made Surya.K,
SpOG, dr. M. Jusuf. R SpOG, dr. Sri Jauhara Laily, SpOG, dr. G. Joshimin F,
SpOG, dr. Alfian. Z. Srg, SpOG, dr. Firman Alamsyah, SpOG, dr. Aidil Akbar,
Sp.OG, dr. Andri. P. Aswar, SpOG, dr Errol Hamzah, SpOG terima kasih banyak
atas segala bantuan dan dukungannya yang telah diberikan selama ini.
18.
Kepada teman-teman sejawat saya PPDS Obstetri dan Ginekologi : dr. Riza. H. Nst,
dr. Boy. R. P. Srg, dr. Hatsari. M. P. S.S, dr. Rizka Heriansyah, dr. Reynanta dr. T.
Johan. A, dr. Elvira. M. S, dr. Yuri Andriansyah, dr. Tigor Hasugian, dr. Riske Eka
Putri, dr. Ulfa WK, dr. Hendryadi, dr. Heika. N. Silitonga, dr. Irwansyah. P, dr. Ali
Akbar. Hsb, dr. Ismail Usman, dr. Arjuna. S, dr. Janwar. S, dr. M. Yusuf, dr. Meity
Elvira, dr. Hendri Ginting, dr. Dany Ariyani, dr. Fatin Atifa, dr. Eka Handayani, dr.
Hendri Gunawan, dr. Ferdiansyah Putra, dr. Kiko Marpaung, dr. M. Wahyu
v
dr. M. Arif Siregar, dr. Pantas Saroha Siburian, dr. Abdur Rohim Lubis, dr. Hotbin
Purba, dr. Hiro HD Nasution, dr. Anindita Novina, dr. Nureliani Amni, dr. Liza
Marosa, dr. Julita A Nasution, dr. Aries Misrawani, dr. Ivo F Canitri, dr. M Rizky
Pranata, dr. Ray Barus, dr. Robby. P, dr Edward S Manurung, Edy Rizaldy, Erwin
E Saputra, dr. Rizal Sangadji, dr. Ricca P Rahim, dr. Ika Sulaika, dr. Fifianti Putri
Adela, dr. Chandran F Saragih, dr. Dona Wirniaty, dr. Apriza Prahatama, dr Hilma
Putri Lubis, dr. Rahmanita Sinaga, dr. Muhammad Dezarino, dr. Alfred Hara
Sinuhaji, dr. Ninong Ade Putri, dr. M Faisal Fahmi, dr. Renny Anggraini, dr.
Yasmien Hasby, dr. Johan Ricardo, dr. Arvitamurianty T Lubis, dr. Juhriyani M
Lubis, dr. Hermima Nurul A, dr. Meifi Elfira, , dr. Bandini, dr. Hendrik A Tarigan
Tua, dr. Dina K Wiratma, dr. Dewi Andriyati, dr. Aliya Hanifa, dr. Daniel Hendra
Simbolon, dr. Servin Pandu Djagadinata, dr Yufi Permana, dr. Trishna, dr. Renny
Junitasari, dr. Tri Sugeng Hariadi, dr. Masithah Thaharuddin, dr. Jesurun. B. D.
Hutabarat, dr. Adrian Octara Sinuhaji, dr. Eva Maya Puspita, dr. M Gamal Darus,
dr. Nafon Zaitun, dr. Rizal K Aritonang, dr. Obed P A Simatupang, dr. Aurora M
Farrah, dr. Indra Setiawan, dr. M Wahyu Utomo, dr. Eunike W Zega dan seluruh
PPDS obstetri & Ginekologi FK-USU yang tidak dapat saya ucapkan satu per satu,
saya menyampaikan terima kasih atas dukungan dan bantuan yang diberikan selama
penelitian dan pembuatan tesis saya ini.
19.
Dokter Muda, Bidan, Paramedis, karyawan / karyawati, serta para pasien di
Departemen Obstetri dan Ginekologi FK USU / RSUP. H. Adam Malik – RSU. Dr.
Pirngadi Medan, RS Tembakau Deli, RS Haji Mina, Rumkit Kesdam, RS Sundari,
RS HKBP Balige yang daripadanya saya banyak memperoleh pengetahuan, terima
kasih atas kerja sama dan saling pengertian yang diberikan kepada saya sehingga
dapat sampai pada akhir program adaptasi ini.
Sembah sujud, hormat dan terima kasih yang tidak terhingga saya sampaikan kepada
kedua Orang Tua saya yang tersayang ayahanda,
Tan A Heng (Alm)
dan Ibunda,
Tan Sie
Moy
yang telah membesarkan, membimbing, mendoakan, serta mendidik saya dengan
vi
menjalani hidup serta memberikan motivasi dan semangat kepada saya selama mengikuti
pendidikan ini.
Buat mertua saya yang tercinta
dr. H. Raja Imran Ritonga, MsC
dan
dr. Rosa Dalima,
terima kasih yang tidak terhingga saya sampaikan atas dukungan baik dari segi moril
maupun materiil kepada saya sehingga saya dapat menyelesaikan program adaptasi ini.
Buat istriku yang tercinta dan kukasihi
dr. Imelda Liana Ritonga, SkP, MPD, MN
, tiada
kata lain yang bisa saya sampaikan selain terima kasih yang sebesar-besarnya atas
pengertian, kesabaran, dorongan semangat, pengorbanan dan doa yang diberikan kepada
saya sehingga saya dapat menyelesaikan program adaptasi ini.
Anak-anak ku yang tercinta dan kukasihi
Nathasya Veronica Winardi, Josephine
Lidwina Winardi, Gabriella Valentina Winardi
, tiada kata lain yang bisa papa
sampaikan selain terima kasih yang sebesar-besarnya atas cinta kasih, pengertian,
kesabaran, dorongan semangat, pengorbanan dan doa yang diberikan kepada papa
sehingga papa dapat menyelesaikan program adaptasi ini. Semoga apa yang telah papa
lakukan dapat menjadi teladan dan semangat bagi ananda untuk mencapai cita-cita yang
lebih baik lagi
Kepada seluruh keluarga yang tidak dapat saya sebutkan satu persatu, yang secara langsung
maupun tidak langsung telah banyak memberikan bantuan, baik moril maupun materiil,
saya ucapkan terima kasih yang sebesar-besarnya.
Semoga Tuhan Yang Maha Esa senantiasa melimpahkan rahmat dan berkahNya serta
dibukakan pintu ilmu kepada kita semua.
Amin
Medan, Oktober 2011
vii
DAFTAR ISI
Halaman
KATA PENGANTAR……… i
DAFTAR ISI……….. vii
DAFTAR GAMBAR……….. x
DAFTAR TABEL……… xi
ABSTRAK………... xii
BAB I PENDAHULUAN
1.1
Latar Belakang………
1
1.2
Rumusan Masalah………...………
3
1.3
Hipotesis………...……….. 3
1.4
Tujuan Penelitian…...……….
3
1.4.1
Tujuan Umum………...
3
1.4.2
Tujuan Khusus………...
3
1.5
Manfaat Penelitian……….
4
BAB II TINJAUAN KEPUSTAKAAN
2.1
Polycystic Ovary Syndrome ……….
5
2.1.1
Definisi ……….. 5
2.1.2
Prevalensi ……….. 7
2.1.3
Etiologi ……….. 7
2.1.4
Gambaran Klinik ……… 8
2.1.5
Patofisiologi ……… 8
2.2
Resistensi Insulin ………..
11
2.2.1
Mekanisme Resistensi Insulin pada PCOS ……… 13
viii
2.2.3
Hiperinsulinemia dan Induksi Ovulasi ...
14
2.3
Metformin ……….
15
2.3.1
Metformin dan PCOS ……….
16
2.3.2
Ovulasi Spontan setelah Pengobatan dengan Metformin ………
16
2.3.3
Metformin dan Induksi Ovulasi dengan Clomiphene Citrate (CC) ……
17
2.4
Dosis dan Jangka Waktu Pemberian Metformin pada PCOS ………..
18
2.5
Efek Samping Metformin ……….………
19
2.6
Metformin XR (Extended Released) ………
19
BAB III METODE PENELITIAN
3.1
Desain Penelitian ……….
23
3.2
Tempat dan Waktu ………...
23
3.3
Populasi dan Sampel Penelitian ………
23
3.3.1
Populasi Penelitian ……….
23
3.3.2
Sampel Penelitian ………..
23
3.4
Kriteria Penelitian ………
23
3.4.1
Kriteria Inklusi ………...
24
3.4.2
Kriteria Ekslusi ………..
24
3.5
Variabel Penelitian ………..
24
3.5.1
Variabel Independen ………
24
3.5.2
Variabel Dependen ………..
24
3.6
Kerangka Konsep Penelitian ……….
25
3.7
Cara Kerja ……….
25
3.8
Batasan Operasional ……….
28
3.9
Pengolahan Data ………..
28
ix
BAB IV HASIL DAN PEMBAHASAN
4.1
Sebaran Karakteristik ………..
29
4.2
Efek Samping ………..
30
4.3
Luaran ………..
32
BAB V KESIMPULAN DAN SARAN
5.1
Kesimpulan ………..
34
5.2
Saran ………
34
x
DAFTAR GAMBAR
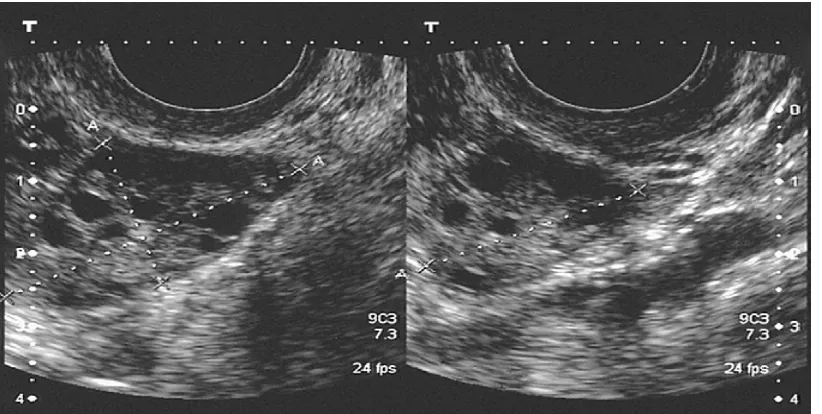
Gambar 1. Pengukuran diameter tiga dimensi dari ovarium untuk menghitung volume
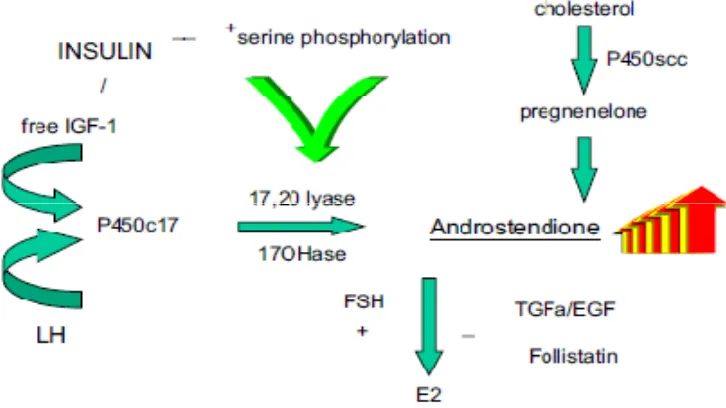
Gambar 2. Kunci utama dari produksi androgen yang berlebihan pada polycystic ovary
Gambar 3. Mekanisme dari produksi androgen yang berlebihan pada polycystic ovary
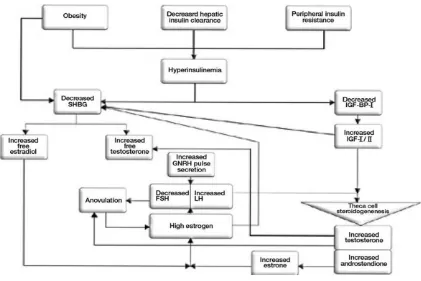
Gambar 4. Peranan hperinsulinemia dalam patogenesa anovulasi dan hperandrogenisme
Gambar 5. Potensial mekanisme dari resistensi insulin pada polycystic ovary syndrome
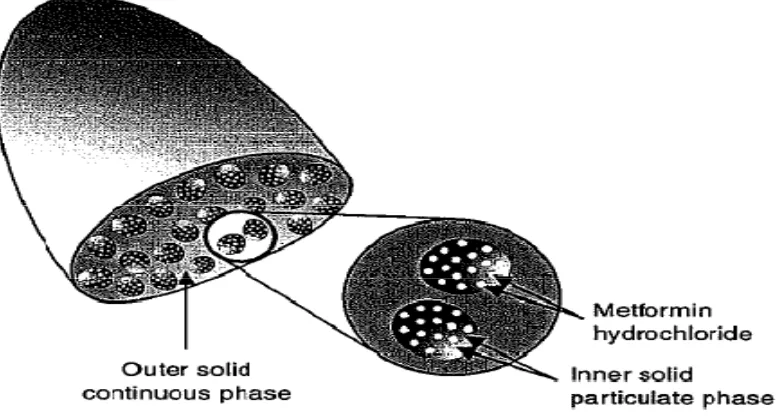
Gambar 6. Metformin Extended Release
Gambar 7. Rerata kadar plasma berbanding waktu pada pemberian metformin IR dan
metformin XR
xi
DAFTAR TABEL
Tabel 1. Sebaran Karakteristik Subjek menurut Kelompok Penelitian
Tabel 2. Efek Samping Minggu Pertama
Tabel 3. Efek Samping Minggu Kedua
Tabel 4. Efek Samping setelah Minggu Kedua
xii
Comparison of the Effectiveness and Side Effects of Metformin XR and
Metformin IR in the Management of Clomiphene Citrate Resistant
PCOS
Perbandingan Efektifitas dan Efek Samping Metformin XR dan Metformin IR dalam
Pengobatan PCOS yang Resisten terhadap Clomiphene Citrate
Hedy Tan, Binarwan Halim, Yostoto B Kaban
Deparment of Obstetrics and Gynecology Medical Faculty of North Sumatera
University / Haji Adam Malik Central General Hospital / Pirngadi District General
Hospital / Halim Fertility Centre Medan
Abstract
Objective :
To assess the effectiveness and side effects of metformin XR once daily and
metformin IR three times daily in the management of clomiphene citrate resistant
(CC-resistant) PCOS.
Method :
This is a prospective randomized controlled study conducted at Halim Fertility
Centre Medan. Fifty nine CC-resistant PCOS women who met the inclusion criterias
were randomly allotted into metformin XR and metformin IR groups. Twenty nine
women in the metformin XR group were given 500 mg metformin XR once a day and 30
women in the metformin IR group were given 500 mg metformin IR once a day for the
first week, twice a day for the second week and three times a day after the second week.
All women in both group were given 10 days of 10 mg norethisterone for withdrawal
bleeding and 150 mg clomiphene citrate on day 2 to day 6 of withdrawal bleeding for
ovulation induction. TVS were carried out to determine the growth of follicles, ovulation
and pregnancy. All women were enquired regarding the side effects of treatment at the
end of the week.
Results :
The baseline characteristics were not significantly different between the two
xiii
side effects of the treatment in the first week were also not significantly different between
the two groups with all the
p value
> 0.05. However there was significant difference
statistically between the two groups regarding side effects of the treatment in the second
and after the second week with the average
p value
< 0.05 which favoured the metformin
XR group. There were 2 patients dropped out from the study because of the side effects.
Each in the second and after the second week. Both of them were from metformin IR
group, however there weren’t statistically significant difference with the
p value
of 1.
The ovulation rate (65.5% vs 53.6%) and pregnancy rate (24.1% vs 17.9%) for
metformin XR group vs metformin IR group respectively. Even though there were higher
achievements in the ovulation and pregnancy rate for metformin XR group, there weren’t
significant differences after analyzed by statistic between the two groups with the
p value
of 0.358 and 0.561 respectively for ovulation and pregnancy rate.
Conclusion :
Metformin XR has better side effect profile and achieved higher ovulation
and pregnancy rate as compared to metformin IR in the management of CC-resistance
PCOS patients. More over metformin XR can be given once daily which can improve
patients compliance with the treatment.
Keywords :
Clomiphene citrate resistance PCOS, metformin XR, metformin IR
Abstrak
Tujuan : Untuk membandingkan efektifitas dan efek samping metformin XR sekali sehari
dan metformin IR 3 kali sehari dalam pengobatan PCOS yang resisten terhadap
clomiphene citrate.
Metode : Penelitian prospektif acak terkendali yang dilakukan di Halim Fertility Centre
Medan. Lima puluh sembilan wanita PCOS yang resisten terhadap clomiphene citrate
yang memenuhi kriteria inklusi di kelompokan secara acak ke dalam kelompok metformin
XR dan metformin IR. Dua puluh Sembilan wanita pada kelompok metformin XR
diberikan metformin XR 500 mg sekali sehari dan 30 wanita pada kelompok metformin
xiv
pada minggu ke dua dan tiga kali sehari setelah minggu ke dua. Semua wanita pada
kedua kelompok diberikan norethisterone 10 mg selama 10 hari untuk withdrawal
bleeding dan clomiphene citrate 150 mg pada hari ke dua sampai hari ke enam
withdrawal bleeding untuk induksi ovulasi. TVS dilakukan untuk memantau pertumbuhan
folikel, ovulasi dan kehamilan. Semua wanita tersebut di tanyakan tentang efek samping
pengobatan pada akhir minggu pengobatan.
Hasil : Tidak dijumpai perbedaan yang bermakna dalam karakteristik dasar antara
kedua kelompok dengan nilai p = 0,999 untuk umur dan 0,554 untuk BMI. Secara
statistik, tidak dijumpai perbedaan bermakna dalam efek samping pengobatan pada
minggu pertama antara kedua kelompok dengan semua nilai p > 0,05. Akan tetapi
dijumpai perbedaan yang bermakna secara statistik antara kedua kelompok dalam efek
samping pengobatan pada minggu kedua dan setelah minggu kedua dengan rerata nilai p
< 0,05 yang lebih baik pada kelompok metformin XR. Terdapat 2 wanita yang drop out
dari penelitian ini yang disebabkan oleh efek samping pengobatan. Satu wanita pada
minggu kedua dan 1 wanita setelah minggu kedua. Keduanya berasal dari kelompok
metformin IR, akan tetapi secara statistik tidak dijumpai perbedaan bermakna dengan
nilai p = 1. Rerata ovulasi adalah 65,5% dan kehamilan adalah 24,1% untuk kelompok
metformin XR. Rerata ovulasi adalah 53,6% dan kehamilan adalah 17,9% untuk
kelompok metformin IR. Walaupun terdapat rerata ovulasi dan kehamilan yang lebih
tinggi pada kelompok metformin XR, setelah di analisa secara statistik tidak dijumpai
perbedaan yang bermakna dengan nilai p = 0,358 untuk ovulasi dan 0,561 untuk
kehamilan.
Kesimpulan : Metformin XR mempunyai efek samping yang lebih baik dan mencapai
rerata ovulasi dan kehamilan yang lebih tinggi dibandingkan metformin IR dalam
pengobatan wanita PCOS yang resisten terhadap clomiphene citrate. Lagi pula
metformin XR dapat diberikan hanya satu kali sehari sehingga dapat meningkatan
kepatuhan pasien dalam pengobatannya.
Kata kunci : PCOS yang resisten terhadap clomiphene citrate, metformin XR, metformin
xv
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies
affecting 5%–10% of reproductive age women
1. This syndrome consist of combination
between clinical, ultrasonographic and laboratory features such as oligo/amenorrhoea,
oligo/anovulation, hirsutism, hyperandrogenaemia, specific ovarian morphology,
hyperinsulinaemia and insulin resistance. An internationally accepted definition was been
adopted in 2003 by the European Society for Human Reproduction and Embryology and
the American Society for Reproductive Medicine, known as the ESHRE/ASRM
Rotterdam consensus
2. It required the presence of two of the following three diagnostic
criteria: [1] oligoamenorrhea or anovulation; [2] clinical or biochemical evidence of
hyperandrogenism; and [3] the presence of polycystic ovarian morphology.
The exact aetiology of PCOS is unknown. However, insulin resistance with
compensatory hyperinsulinemia is a prominent feature of the syndrome and appears to
have a pathophysiologic role in the hyperandrogenism of the disorder, especially in those
with CC resistance. Both lean and obese women with PCOS show evidence of decreased
insulin sensitivity
3, but insulin resistance, accompanied by compensatory
hyperinsulinemia, is most marked when there is an interaction between obesity and the
syndrome
4,5. There is ample evidence that hyperinsulinemia results in increased ovarian
androgen biosynthesis in vivo and in vitro
5,6and decreased sex hormone–binding
globulin (SHBG) synthesis from the liver
7,8, leading to increased bioavailability of free
androgens. This excess in local ovarian androgen production augmented by
hyperinsulinemia causes premature follicular atresia and anovulation
9,10. Although this
idea remains controversial, hyperinsulinemia may have a direct effect on the
hypothalamus and/or pituitary to increase serum luteinizing hormone (LH) concentrations
and therefore indirectly increase LH-dependent ovarian androgen biosynthesis
5,8,
possibly resulting in abnormal LH and follicle-stimulating hormone (FSH) release and
subsequent oligoamenorrhea. Hyperinsulinemia may also directly affect folliculogenesis
and may arrest growth of antral follicles after they have reached a diameter between 5
xvi
Given the importance of hyperinsulinemia in the development of hyperandrogenism and
disrupted folliculogenesis, it seems likely that medications that act as insulin-sensitizing
agents may be useful in restoration of normal endocrinologic and clinical parameters of
this condition. Therapeutic measures directed at lowering insulin secretion in women
with PCOS should theoretically ameliorate their hyperandrogenism and restore normal
follicular growth, thus facilitating ovulation
11. The most extensively studied
insulin-sensitizing drug in the treatment of PCOS is metformin
12,13. Metformin
(dimethylbiguanide) is an orally administered drug used to lower blood glucose
concentrations in patients with noninsulin- dependent diabetes mellitus (NIDDM)
14. It is
antihyperglycemic in action and does not cause hypoglycemia. Metformin enhances
insulin sensitivity in both the liver, where it inhibits hepatic glucose production, and the
peripheral tissue, where it increases glucose uptake and utilization in muscle tissue. By
increasing insulin sensitivity, metformin reduces insulin resistance, insulin secretion, and
hyperinsulinemia. Hence, metformin seems to be a perfect drug to treat patients with
PCOS, including those with CC resistance
12,13. It was reported that metformin treatment
for patients with PCOS improves a patient’s menstrual cycle and increases the sensitivity
for the ovulation induction drug reaction, especially in women with CC-resistant
PCOS
11,12,13.
Even though the use of metformin in PCOS patients so popular, until recently there was
no consensus regarding the doses, when and how long the drug should been given. Many
studies has been done, however the regimens been use were very wide in variety. The
conventional metformin used in many studies was metformin IR, this tandard metformin
suffers from the limitations of having to be administered two or three times a day and
with the attendant risk of triggering gastrointestinal symptoms such as nausea, vomit,
bloated, epigastric pain and diarrhea. This event making dose optimization problematic
and reduced patients compliances. Some studies showed the dropout rate in the
metformin group was 30% owing to side effects
15.
To overcome the side effects and improved patients compliances of metformin
xvii
(ADA) and the European Society for the study of Diabetes (EASD) give advice on how
to minimize poor compliance with standard metformin. In the 5 point plan for introducing
metformin, the ADA/EASD draw attention to the recently introduced extended release
metformin
16. Many studies showed this extended release metformin had similar
efficacies, lower side effects as compared to standard immediate release metformin. It
also improved patients compliances due to the simple once daily dosing
17-20.
This study aimed to assessed the effectiveness and side effects of metformin XR once
daily and metformin IR three times daily in the management of CC-resistant PCOS.
Method
This is a prospective randomized, controlled study conducted at Halim Fertility Centre
Medan. The study protocol was approved by Health Research Ethical Committee of
North Sumatera c/o Medical School, Universitas Sumatera Utara. A total of 59 women
with CC-resistant PCOS were recruited. The diagnosis of PCOS was based on
ESHRE/ASRM
criteria, which included at least two of three criteria of the following: [1]
chronic anovulation; [2] clinical or biochemical signs of hyperandrogenism; and [3]
polycystic ovary (PCO) morphology, shown on ultrasound scan, defined as the presence
of 12 or more follicles (with one ovary being sufficient for diagnosis) measuring 2 - 9
mm in diameter or increase in ovarian volume of more than 10 mL. Clomiphene
resistance was defined as failure of follicular development after CC treatment up to 150
mg daily for 5 days for two cycles. Informed consent was obtained and all baseline
evaluations were carried out before entry to study. The body mass index (BMI, weight in
kilograms/the square of the height in meters) was calculated. Women who were eligible
and consented were randomly allotted to the metformin XR group (A) or metformin IR
group (B). Randomizations were done by picking an envelope labeled AB or BA. If the
AB labeled envelope was picked out, the first woman was assigned to group A and the
second woman was assigned to group B. Vice versa was apply if the BA labeled envelope
xviii
Women in group A were given 500 mg metformin XR once a day and women in the
group B were given 500 mg metformin IR once a day for the first week, twice a day for
the second week and three times a day after the second week. All women in both groups
were given 10 days of 10 mg norethisterone for withdrawal bleeding and 150 mg
clomiphene citrate on day 2 to day 6 of withdrawal bleeding for ovulation induction. At
the end of the week, all women will be enquired regarding the side effects of the
treatment. A transvaginal ultrasound (TVS) were carried out to determine the growth of
follicles on day 8, 12 and 16 of withdrawal bleeding. If there was follicle with diameter
≥
18 mm (dominant follicle), TVS was carried out daily to determined ovulation. Women
were asked to have sexual intercourse after 34-36 hours every 2 day for 5 consecutive
times. If there was no dominant follicle, the treatment was considered failed. Urinary
pregnancy test was carried out after a week of missing period, and TVS was carried out
to confirmed pregnancy. Pregnancy was defined as the presence of a gestational sac seen
on TVS. All the side effects will be recorded and if the women were unable to tolerate the
treatment, they will be discharged from the study.
Statistical analysis was performed using the Statistical Package for Social Sciences
(SPSS) software version 17.0 for Windows. Comparisons of baseline values, side effects,
ovulation rates and pregnancy rates in the two groups were made by using the chi-square
test and t-test. A
p
value of less than 0.05 was considered statistically significant.
Results
A total of 59 women with CC-resistant PCOS were randomized with 29 women in group
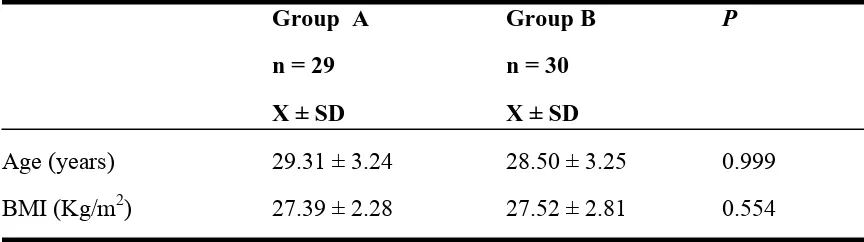
A and 30 women in group B. The baseline characteristics were not significantly different
between the two groups with
p
value
of 0.999 and 0.554 for the age and BMI
respectively (Table 1). We did not analyzed the baseline characteristic of parity as all the
xix
Table 1. Baseline Characteristic
Group A
Group B
P
n = 29
n = 30
X ± SD
X ± SD
Age (years)
29.31 ± 3.24
28.50 ± 3.25
0.999
BMI (Kg/m
2)
27.39 ± 2.28
27.52 ± 2.81
0.554
p = t-Test
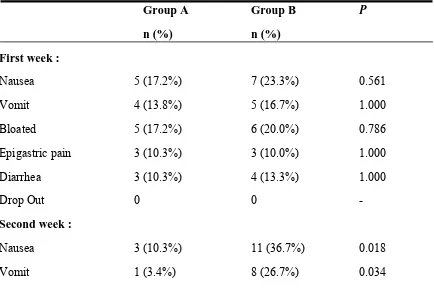
Statistically, side effects of the treatment in the first week were also not significantly
different between the two groups with all the
p value
> 0.05. However there was
significant difference statistically between the two groups regarding side effects of the
treatment in the second and after the second week with the average
p value
< 0.05 which
favored group A. There were 2 patients dropped out from the study because of the side
effects. Each in the second and after the second week. Both of them were from group B,
however there weren’t statistically significant difference with the
p value
of 1 (Table 2).
Table 2. Side Effects of Treatment
Group
A
Group
B
P
n
(%)
n
(%)
First week :
Nausea
5 (17.2%)
7 (23.3%)
0.561
Vomit
4 (13.8%)
5 (16.7%)
1.000
Bloated
5 (17.2%)
6 (20.0%)
0.786
Epigastric pain
3 (10.3%)
3 (10.0%)
1.000
Diarrhea
3 (10.3%)
4 (13.3%)
1.000
Drop Out
0
0
-
Second week :
Nausea
3 (10.3%)
11 (36.7%)
0.018
xx
Bloated
0
11 (36.7%)
0.000
Epigastric pain
1 (3.4%)
11 (36.7%)
0.002
Diarrhea
1 (3.4%)
7 (23.3%)
0.064
Drop Out
0
1 (3.3%)
1.000
After second week :
Nausea
2 (6.9%)
14 (48.3%)
0.000
Vomit
0
10 (34.5%)
0.001
Bloated
1 (3.4%)
14 (48.3%)
0.000
Epigastric pain
0
9 (31.0%)
0.004
Diarrhea 0
9
(31.0%)
0.004
Drop Out
0
1 (3.3%)
1.000
p = chi Square
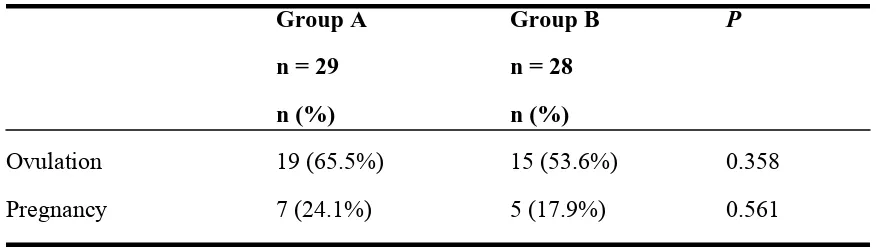
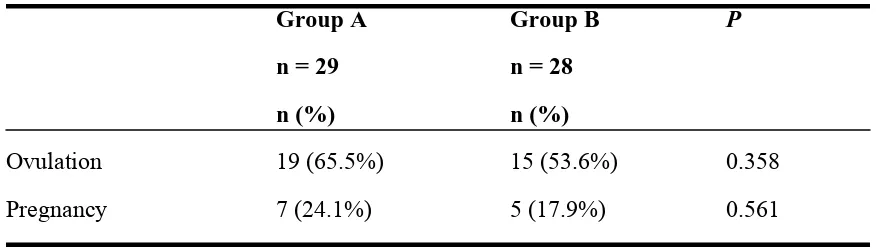
The ovulation rate (65.5% vs 53.6%) and pregnancy rate (24.1% vs 17.9%) for group A
vs group B respectively. Even though there were higher achievements in the ovulation
and pregnancy rate for group A, there weren’t significant differences after analyzed by
statistic between the two groups with the
p value
of 0.358 and 0.561 respectively for
ovulation and pregnancy rate (Table 3).
Table 3. Ovulation and Pregnancy Rate
Group A
Group B
P
n = 29
n = 28
n (%)
n (%)
Ovulation
19 (65.5%)
15 (53.6%)
0.358
Pregnancy
7 (24.1%)
5 (17.9%)
0.561
xxi
Discussion
The beneficial effects of metformin in the management of PCOS are now well
established, particular in patients with CC-resistance. One of the limiting factors,
however, in the use of metformin has been its side effects, which have led to large
dropout rates in many studies. These side effects were well known not only in the PCOS
patients, many of NIDDM patients whose were on metformin treatment also suffered
from these side effects and led to reduction in the compliance with the treatment. To
overcome the side effects and improved patients compliances of metformin treatments,
the joint consensus statement from the American Diabetes Association (ADA) and the
European Society for the study of Diabetes (EASD) give advice on how to minimize poor
compliance with standard metformin. In the 5 point plan for introducing metformin, the
ADA/EASD draw attention to the recently introduced extended release metformin. Many
studies showed this extended release metformin had similar efficacies, lower side effects
as compared to standard immediate release metformin. It also improved patients
compliances due to the simple once daily dosing.
Our study showed that simple once daily dosing of 500 mg metformin XR achieved
higher ovulation and pregnancy rates as compared to 3 times daily of 500 mg metformin
IR (65.5% & 24.1% VS 53.6% & 17.9% respectively) even though after analyzed did
not showed any statistically significant with
p
value of 0.358 & 0.561 for ovulation and
pregnancy rate respectively. This findings are consistent with previous studies done by
Hwu et al
21and Khorram et al
22.
In contrast to ovulation and pregnancy rates, the side
effects of the treatment showed significantly differences between the two groups which
favored metformin XR group.
The primary outcome of our study is to see the effectiveness and side effects of low dose
simple once daily dosing metformin XR in the management of CC-resistance PCOS
patients as until recently there was no consensus regarding the used of this medication in
such patients. Our study showed it had the benefits as compared to standard dosing of
xxii
Even though this is a simple study, only based on the clinical outcomes without any
laboratories support to determine the effects of the treatment, hence it showed the
benefits. To further proved the beneficial of this simple metformin XR dosing, its
required more larger study with clinical and laboratories support to evaluate the effects of
this treatment.
Conclusion
Metformin XR has better side effect profile and achieved higher ovulation and pregnancy
rate as compared to metformin IR in the management of CC-resistance PCOS patients.
More over metformin XR can be given once daily which can improve patients
compliance with the treatment
References
1.
Murizah M Z, Ridzuan J, Adibah I et al. Comparison of clomiphene citrate,
metformin, or the combination of both for first-line ovulation induction,
achievement of pregnancy, and live birth in Asian women with polycystic ovary
syndrome: a randomized controlled trial. Fertility and Sterilit 2009;91(2):514-21.
2.
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group.
Revised 2003 consensus on diagnostic criteria and long-term health risks related
to polycystic ovary syndrome. Fertil Steril 2004;8:19-25.
3.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and
implications for pathogenesis. Endocr Rev 1997;18:774-800.
4.
Dunaif A, Segal K. R, Shelley D. R et al. Evidence for distinctive and intrinsic
defects in insulin action in polycystic ovary syndrome. Diabetes 1992;4:1257-66.
5.
Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin
action associated with an increased risk of non-insulin-dependent diabetes
mellitus. Am J Med 1995;98(1A):33-9.
6.
Homburg R. Polycystic ovary syndrome - from gynaecological curiosity to multi
system endocrinopathy. Hum Reprod 1996;1:29-39.
7.
Amato P & Simpson J. L. The genetics of polycystic ovary syndrome. Best Pract
xxiii
8.
Poretsky L. On the paradox of induced hyperandrogenism in
insulin-resistant states. Endocrinol Rev 199;12:3-13.
9.
Webber L. J, Stubbs S, Stark J et al. Formation and early development of follicles
in the polycystic ovary. Lancet 2003;362:1017-21.
10.
Willis D. S, Watson H, Mason H. D et al. Premature response to luteinizing
hormone of granulosa cells from anolulatory women with polycystic ovary
syndrome: relevance to mechanisrn of anovulation. J Clin Endocrinol Metab
1998;83:3984-91.
11.
Velazquez E. M, Mendoza S. G, Hamer T et al. Metformin therapy in Polycystic
ovary syndrome reduces hyperinsulinemia, insulin resistance,
hyperandrogenaemia and systolic blood pressure while facilitating normal menses
and pregnancy. Metab Clin Exp 1994;43:647-54.
12.
Lord J. M, Flight I. H. K, Norman R. I. Metformin in polycystic ovary syndrome:
systematic review and meta-analysis. BMJ 2003;327 (7421):951-60.
13.
Kolodziejczyk B, Duleba A. J, Spaczynski R. Z et al. Metformin therapy
decreases hyperandrogenism and hyperinsulinemia in women with polycystic
ovary syndrome. Fertil Steril 2000;73 :1149-54.
14.
American Diabetes Association. In: Consensus Development Conference on
lnsulin Resistance; Diab. Care 1998;21:310-14.
15.
Thomas I. Siebert M, Thinus F et al. Is the addition of metformin efficacious in
the treatment of clomiphene citrate-resistant patients with polycystic ovary
syndrome? A structured literature review. Fertility and Sterility
2006;86(5):1432-37.
16.
Bailey CJ, Turner RC
.
American Diabetes Association
, "
Standards of Medical
Care in Diabetes Mellitus 2009”
,
Diabetes Care
,
2009
;
32(1)113-61.
17.
Timmins P, Donahue S, Meeker J et al. Steady-state Pharmacokinetics of a Novel
Extended-Release Metformin Formulation. Clin Pharmacokinet 2005;
44(7):721-9.
18.
Levy J, Cobas R.A, Gomes M.B. Assessment of efficacy and tolerability of once
daily extended release metformin in patients with type 2 diabetes mellitus.
xxiv
19.
Davidson J, Howlett H. New prolonged-release metformin improves
gastrointestinal tolerability. British Journal of Diabetes and Vascular Disease
2004;4(4):273-77.
20.
Jabbour S, Ziring B. Advantages of extended-release metformin in patients with
type 2 diabetes mellitus. Postgraduate Medicine 2011;123(1):15-23
21.
Hwu Y. M, Lin S. Y, Huang W. Y et al. Ultra-short metformin pretreatment for
clomiphene citrate-resistant polycystic ovary syndrome. Int J Gynaecol Obstet
2005;90:39-43.
22.
Khorram O, Jason P, Helliwell et al. Two weeks of metformin improves
clomiphene citrate-induced ovulation and metabolic profiles in women with
xii
Comparison of the Effectiveness and Side Effects of Metformin XR and
Metformin IR in the Management of Clomiphene Citrate Resistant
PCOS
Perbandingan Efektifitas dan Efek Samping Metformin XR dan Metformin IR dalam
Pengobatan PCOS yang Resisten terhadap Clomiphene Citrate
Hedy Tan, Binarwan Halim, Yostoto B Kaban
Deparment of Obstetrics and Gynecology Medical Faculty of North Sumatera
University / Haji Adam Malik Central General Hospital / Pirngadi District General
Hospital / Halim Fertility Centre Medan
Abstract
Objective :
To assess the effectiveness and side effects of metformin XR once daily and
metformin IR three times daily in the management of clomiphene citrate resistant
(CC-resistant) PCOS.
Method :
This is a prospective randomized controlled study conducted at Halim Fertility
Centre Medan. Fifty nine CC-resistant PCOS women who met the inclusion criterias
were randomly allotted into metformin XR and metformin IR groups. Twenty nine
women in the metformin XR group were given 500 mg metformin XR once a day and 30
women in the metformin IR group were given 500 mg metformin IR once a day for the
first week, twice a day for the second week and three times a day after the second week.
All women in both group were given 10 days of 10 mg norethisterone for withdrawal
bleeding and 150 mg clomiphene citrate on day 2 to day 6 of withdrawal bleeding for
ovulation induction. TVS were carried out to determine the growth of follicles, ovulation
and pregnancy. All women were enquired regarding the side effects of treatment at the
end of the week.
Results :
The baseline characteristics were not significantly different between the two
xiii
side effects of the treatment in the first week were also not significantly different between
the two groups with all the
p value
> 0.05. However there was significant difference
statistically between the two groups regarding side effects of the treatment in the second
and after the second week with the average
p value
< 0.05 which favoured the metformin
XR group. There were 2 patients dropped out from the study because of the side effects.
Each in the second and after the second week. Both of them were from metformin IR
group, however there weren’t statistically significant difference with the
p value
of 1.
The ovulation rate (65.5% vs 53.6%) and pregnancy rate (24.1% vs 17.9%) for
metformin XR group vs metformin IR group respectively. Even though there were higher
achievements in the ovulation and pregnancy rate for metformin XR group, there weren’t
significant differences after analyzed by statistic between the two groups with the
p value
of 0.358 and 0.561 respectively for ovulation and pregnancy rate.
Conclusion :
Metformin XR has better side effect profile and achieved higher ovulation
and pregnancy rate as compared to metformin IR in the management of CC-resistance
PCOS patients. More over metformin XR can be given once daily which can improve
patients compliance with the treatment.
Keywords :
Clomiphene citrate resistance PCOS, metformin XR, metformin IR
Abstrak
Tujuan : Untuk membandingkan efektifitas dan efek samping metformin XR sekali sehari
dan metformin IR 3 kali sehari dalam pengobatan PCOS yang resisten terhadap
clomiphene citrate.
Metode : Penelitian prospektif acak terkendali yang dilakukan di Halim Fertility Centre
Medan. Lima puluh sembilan wanita PCOS yang resisten terhadap clomiphene citrate
yang memenuhi kriteria inklusi di kelompokan secara acak ke dalam kelompok metformin
XR dan metformin IR. Dua puluh Sembilan wanita pada kelompok metformin XR
diberikan metformin XR 500 mg sekali sehari dan 30 wanita pada kelompok metformin
xiv
pada minggu ke dua dan tiga kali sehari setelah minggu ke dua. Semua wanita pada
kedua kelompok diberikan norethisterone 10 mg selama 10 hari untuk withdrawal
bleeding dan clomiphene citrate 150 mg pada hari ke dua sampai hari ke enam
withdrawal bleeding untuk induksi ovulasi. TVS dilakukan untuk memantau pertumbuhan
folikel, ovulasi dan kehamilan. Semua wanita tersebut di tanyakan tentang efek samping
pengobatan pada akhir minggu pengobatan.
Hasil : Tidak dijumpai perbedaan yang bermakna dalam karakteristik dasar antara
kedua kelompok dengan nilai p = 0,999 untuk umur dan 0,554 untuk BMI. Secara
statistik, tidak dijumpai perbedaan bermakna dalam efek samping pengobatan pada
minggu pertama antara kedua kelompok dengan semua nilai p > 0,05. Akan tetapi
dijumpai perbedaan yang bermakna secara statistik antara kedua kelompok dalam efek
samping pengobatan pada minggu kedua dan setelah minggu kedua dengan rerata nilai p
< 0,05 yang lebih baik pada kelompok metformin XR. Terdapat 2 wanita yang drop out
dari penelitian ini yang disebabkan oleh efek samping pengobatan. Satu wanita pada
minggu kedua dan 1 wanita setelah minggu kedua. Keduanya berasal dari kelompok
metformin IR, akan tetapi secara statistik tidak dijumpai perbedaan bermakna dengan
nilai p = 1. Rerata ovulasi adalah 65,5% dan kehamilan adalah 24,1% untuk kelompok
metformin XR. Rerata ovulasi adalah 53,6% dan kehamilan adalah 17,9% untuk
kelompok metformin IR. Walaupun terdapat rerata ovulasi dan kehamilan yang lebih
tinggi pada kelompok metformin XR, setelah di analisa secara statistik tidak dijumpai
perbedaan yang bermakna dengan nilai p = 0,358 untuk ovulasi dan 0,561 untuk
kehamilan.
Kesimpulan : Metformin XR mempunyai efek samping yang lebih baik dan mencapai
rerata ovulasi dan kehamilan yang lebih tinggi dibandingkan metformin IR dalam
pengobatan wanita PCOS yang resisten terhadap clomiphene citrate. Lagi pula
metformin XR dapat diberikan hanya satu kali sehari sehingga dapat meningkatan
kepatuhan pasien dalam pengobatannya.
Kata kunci : PCOS yang resisten terhadap clomiphene citrate, metformin XR, metformin
xv
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies
affecting 5%–10% of reproductive age women
1. This syndrome consist of combination
between clinical, ultrasonographic and laboratory features such as oligo/amenorrhoea,
oligo/anovulation, hirsutism, hyperandrogenaemia, specific ovarian morphology,
hyperinsulinaemia and insulin resistance. An internationally accepted definition was been
adopted in 2003 by the European Society for Human Reproduction and Embryology and
the American Society for Reproductive Medicine, known as the ESHRE/ASRM
Rotterdam consensus
2. It required the presence of two of the following three diagnostic
criteria: [1] oligoamenorrhea or anovulation; [2] clinical or biochemical evidence of
hyperandrogenism; and [3] the presence of polycystic ovarian morphology.
The exact aetiology of PCOS is unknown. However, insulin resistance with
compensatory hyperinsulinemia is a prominent feature of the syndrome and appears to
have a pathophysiologic role in the hyperandrogenism of the disorder, especially in those
with CC resistance. Both lean and obese women with PCOS show evidence of decreased
insulin sensitivity
3, but insulin resistance, accompanied by compensatory
hyperinsulinemia, is most marked when there is an interaction between obesity and the
syndrome
4,5. There is ample evidence that hyperinsulinemia results in increased ovarian
androgen biosynthesis in vivo and in vitro
5,6and decreased sex hormone–binding
globulin (SHBG) synthesis from the liver
7,8, leading to increased bioavailability of free
androgens. This excess in local ovarian androgen production augmented by
hyperinsulinemia causes premature follicular atresia and anovulation
9,10. Although this
idea remains controversial, hyperinsulinemia may have a direct effect on the
hypothalamus and/or pituitary to increase serum luteinizing hormone (LH) concentrations
and therefore indirectly increase LH-dependent ovarian androgen biosynthesis
5,8,
possibly resulting in abnormal LH and follicle-stimulating hormone (FSH) release and
subsequent oligoamenorrhea. Hyperinsulinemia may also directly affect folliculogenesis
and may arrest growth of antral follicles after they have reached a diameter between 5
xvi
Given the importance of hyperinsulinemia in the development of hyperandrogenism and
disrupted folliculogenesis, it seems likely that medications that act as insulin-sensitizing
agents may be useful in restoration of normal endocrinologic and clinical parameters of
this condition. Therapeutic measures directed at lowering insulin secretion in women
with PCOS should theoretically ameliorate their hyperandrogenism and restore normal
follicular growth, thus facilitating ovulation
11. The most extensively studied
insulin-sensitizing drug in the treatment of PCOS is metformin
12,13. Metformin
(dimethylbiguanide) is an orally administered drug used to lower blood glucose
concentrations in patients with noninsulin- dependent diabetes mellitus (NIDDM)
14. It is
antihyperglycemic in action and does not cause hypoglycemia. Metformin enhances
insulin sensitivity in both the liver, where it inhibits hepatic glucose production, and the
peripheral tissue, where it increases glucose uptake and utilization in muscle tissue. By
increasing insulin sensitivity, metformin reduces insulin resistance, insulin secretion, and
hyperinsulinemia. Hence, metformin seems to be a perfect drug to treat patients with
PCOS, including those with CC resistance
12,13. It was reported that metformin treatment
for patients with PCOS improves a patient’s menstrual cycle and increases the sensitivity
for the ovulation induction drug reaction, especially in women with CC-resistant
PCOS
11,12,13.
Even though the use of metformin in PCOS patients so popular, until recently there was
no consensus regarding the doses, when and how long the drug should been given. Many
studies has been done, however the regimens been use were very wide in variety. The
conventional metformin used in many studies was metformin IR, this tandard metformin
suffers from the limitations of having to be administered two or three times a day and
with the attendant risk of triggering gastrointestinal symptoms such as nausea, vomit,
bloated, epigastric pain and diarrhea. This event making dose optimization problematic
and reduced patients compliances. Some studies showed the dropout rate in the
metformin group was 30% owing to side effects
15.
To overcome the side effects and improved patients compliances of metformin
xvii
(ADA) and the European Society for the study of Diabetes (EASD) give advice on how
to minimize poor compliance with standard metformin. In the 5 point plan for introducing
metformin, the ADA/EASD draw attention to the recently introduced extended release
metformin
16. Many studies showed this extended release metformin had similar
efficacies, lower side effects as compared to standard immediate release metformin. It
also improved patients compliances due to the simple once daily dosing
17-20.
This study aimed to assessed the effectiveness and side effects of metformin XR once
daily and metformin IR three times daily in the management of CC-resistant PCOS.
Method
This is a prospective randomized, controlled study conducted at Halim Fertility Centre
Medan. The study protocol was approved by Health Research Ethical Committee of
North Sumatera c/o Medical School, Universitas Sumatera Utara. A total of 59 women
with CC-resistant PCOS were recruited. The diagnosis of PCOS was based on
ESHRE/ASRM
criteria, which included at least two of three criteria of the following: [1]
chronic anovulation; [2] clinical or biochemical signs of hyperandrogenism; and [3]
polycystic ovary (PCO) morphology, shown on ultrasound scan, defined as the presence
of 12 or more follicles (with one ovary being sufficient for diagnosis) measuring 2 - 9
mm in diameter or increase in ovarian volume of more than 10 mL. Clomiphene
resistance was defined as failure of follicular development after CC treatment up to 150
mg daily for 5 days for two cycles. Informed consent was obtained and all baseline
evaluations were carried out before entry to study. The body mass index (BMI, weight in
kilograms/the square of the height in meters) was calculated. Women who were eligible
and consented were randomly allotted to the metformin XR group (A) or metformin IR
group (B). Randomizations were done by picking an envelope labeled AB or BA. If the
AB labeled envelope was picked out, the first woman was assigned to group A and the
second woman was assigned to group B. Vice versa was apply if the BA labeled envelope
xviii
Women in group A were given 500 mg metformin XR once a day and women in the
group B were given 500 mg metformin IR once a day for the first week, twice a day for
the second week and three times a day after the second week. All women in both groups
were given 10 days of 10 mg norethisterone for withdrawal bleeding and 150 mg
clomiphene citrate on day 2 to day 6 of withdrawal bleeding for ovulation induction. At
the end of the week, all women will be enquired regarding the side effects of the
treatment. A transvaginal ultrasound (TVS) were carried out to determine the growth of
follicles on day 8, 12 and 16 of withdrawal bleeding. If there was follicle with diameter
≥
18 mm (dominant follicle), TVS was carried out daily to determined ovulation. Women
were asked to have sexual intercourse after 34-36 hours every 2 day for 5 consecutive
times. If there was no dominant follicle, the treatment was considered failed. Urinary
pregnancy test was carried out after a week of missing period, and TVS was carried out
to confirmed pregnancy. Pregnancy was defined as the presence of a gestational sac seen
on TVS. All the side effects will be recorded and if the women were unable to tolerate the
treatment, they will be discharged from the study.
Statistical analysis was performed using the Statistical Package for Social Sciences
(SPSS) software version 17.0 for Windows. Comparisons of baseline values, side effects,
ovulation rates and pregnancy rates in the two groups were made by using the chi-square
test and t-test. A
p
value of less than 0.05 was considered statistically significant.
Results
A total of 59 women with CC-resistant PCOS were randomized with 29 women in group
A and 30 women in group B. The baseline characteristics were not significantly different
between the two groups with
p
value
of 0.999 and 0.554 for the age and BMI
respectively (Table 1). We did not analyzed the baseline characteristic of parity as all the
xix
Table 1. Baseline Characteristic
Group A
Group B
P
n = 29
n = 30
X ± SD
X ± SD
Age (years)
29.31 ± 3.24
28.50 ± 3.25
0.999
BMI (Kg/m
2)
27.39 ± 2.28
27.52 ± 2.81
0.554
p = t-Test
Statistically, side effects of the treatment in the first week were also not significantly
different between the two groups with all the
p value
> 0.05. However there was
significant difference statistically between the two groups regarding side effects of the
treatment in the second and after the second week with the average
p value
< 0.05 which
favored group A. There were 2 patients dropped out from the study because of the side
effects. Each in the second and after the second week. Both of them were from group B,
however there weren’t statistically significant difference with the
p value
of 1 (Table 2).
Table 2. Side Effects of Treatment
Group
A
Group
B
P
n
(%)
n
(%)
First week :
Nausea
5 (17.2%)
7 (23.3%)
0.561
Vomit
4 (13.8%)
5 (16.7%)
1.000
Bloated
5 (17.2%)
6 (20.0%)
0.786
Epigastric pain
3 (10.3%)
3 (10.0%)
1.000
Diarrhea
3 (10.3%)
4 (13.3%)
1.000
Drop Out
0
0
-
Second week :
Nausea
3 (10.3%)
11 (36.7%)
0.018
[image:35.612.91.525.438.726.2]xx
Bloated
0
11 (36.7%)
0.000
Epigastric pain
1 (3.4%)
11 (36.7%)
0.002
Diarrhea
1 (3.4%)
7 (23.3%)
0.064
Drop Out
0
1 (3.3%)
1.000
After second week :
Nausea
2 (6.9%)
14 (48.3%)
0.000
Vomit
0
10 (34.5%)
0.001
Bloated
1 (3.4%)
14 (48.3%)
0.000
Epigastric pain
0
9 (31.0%)
0.004
Diarrhea 0
9
(31.0%)
0.004
Drop Out
0
1 (3.3%)
1.000
p = chi Square
The ovulation rate (65.5% vs 53.6%) and pregnancy rate (24.1% vs 17.9%) for group A
vs group B respectively. Even though there were higher achievements in the ovulation
and pregnancy rate for group A, there weren’t significant differences after analyzed by
statistic between the two groups with the
p value
of 0.358 and 0.561 respectively for
[image:36.612.87.531.523.647.2]ovulation and pregnancy rate (Table 3).
Table 3. Ovulation and Pregnancy Rate
Group A
Group B
P
n = 29
n = 28
n (%)
n (%)
Ovulation
19 (65.5%)
15 (53.6%)
0.358
Pregnancy
7 (24.1%)
5 (17.9%)
0.561
xxi
Discussion
The beneficial effects of metformin in the management of PCOS are now well
established, particular in patients with CC-resistance. One of the limiting factors,
however, in the use of metformin has been its side effects, which have led to large
dropout rates in many studies. These side effects were well known not only in the PCOS
patients, many of NIDDM patients whose were on metformin treatment also suffered
from these side effects and led to reduction in the compliance with the treatment. To
overcome the side effects and improved patients compliances of metformin treatments,
the joint consensus statement from the American Diabetes Association (ADA) and the
European Society for the study of Diabetes (EASD) give advice on how to minimize poor
compliance with standard metformin. In the 5 point plan for introducing metformin, the
ADA/EASD draw attention to the recently introduced extended release metformin. Many
studies showed this extended release metformin had similar efficacies, lower side effects
as compared to standard immediate release metformin. It also improved patients
compliances due to the simple once daily dosing.
Our study showed that simple once daily dosing of 500 mg metformin XR achieved
higher ovulation and pregnancy rates as compared to 3 times daily of 500 mg metformin
IR (65.5% & 24.1% VS 53.6% & 17.9% respectively) even though after analyzed did
not showed any statistically significant with
p
value of 0.358 & 0.561 for ovulation and
pregnancy rate respectively. This findings are consistent with previous studies done by
Hwu et al
21and Khorram et al
22.
In contrast to ovulation and pregnancy rates, the side
effects of the treatment showed significantly differences between the two groups which
favored metformin XR group.
The primary outcome of our study is to see the effectiveness and side effects of low dose
simple once daily dosing metformin XR in the management of CC-resistance PCOS
patients as until recently there was no consensus regarding the used of this medication in
such patients. Our study showed it had the benefits as compared to standard dosing of
xxii
Even though this is a simple study, only based on the clinical outcomes without any
laboratories support to determine the effects of the treatment, hence it showed the
benefits. To further proved the beneficial of this simple metformin XR dosing, its
required more larger study with clinical and laboratories support to evaluate the effects of
this treatment.
Conclusion
Metformin XR has better side effect profile and achieved higher ovulation and pregnancy
rate as compared to metformin IR in the management of CC-resistance PCOS patients.
More over metformin XR can be given once daily which can improve patients
compliance with the treatment
References
1.
Murizah M Z, Ridzuan J, Adibah I et al. Comparison of clomiphene citrate,
metformin, or the combination of both for first-line ovulation induction,
achievement of pregnancy, and live birth in Asian women with polycystic ovary
syndrome: a randomized controlled trial. Fertility and Sterilit 2009;91(2):514-21.
2.
The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group.
Revised 2003 consensus on diagnostic criteria and long-term health risks related
to polycystic ovary syndrome. Fertil Steril 2004;8:19-25.
3.
Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and
implications for pathogenesis. Endocr Rev 1997;18:774-800.
4.
Dunaif A, Segal K. R, Shelley D. R et al. Evidence for distinctive and intrinsic
defects in insulin action in polycystic ovary syndrome. Diabetes 1992;4:1257-66.
5.
Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin
action associated with an increased risk of non-insulin-dependent diabetes
mellitus. Am J Med 1995;98(1A):33-9.
6.
Homburg R. Polycystic ovary syndrome - from gynaecological curiosity to multi
system endocrinopathy. Hum Reprod 1996;1:29-39.
7.
Amato P & Simpson J. L. The genetics of polycystic ovary syndrome. Best Pract
xxiii
8.
Poretsky L. On the paradox of induced hyperandrogenism in
insulin-resistant states. Endocrinol Rev 199;12:3-13.
9.
Webber L. J, Stubbs S, Stark J et al. Formation and early development of follicles
in the polycystic ovary. Lancet 2003;362:1017-21.
10.
Willis D. S, Watson H, Mason H. D et al. Premature response to luteinizing
hormone of granulosa cells from anolulatory women with polycystic ovary
syndrome: relevance to mechanisrn of anovulation. J Clin Endocrinol Metab
1998;83:3984-91.
11.
Velazquez E. M, Mendoza S. G, Hamer T et al. Metformin therapy in Polycystic
ovary syndrome reduces hyperinsulinemia, insulin resistance,
hyperandrogenaemia and systolic blood pressure while facilitating normal menses
and pregnancy. Metab Clin Exp 1994;43:647-54.
12.
Lord J. M, Flight I. H. K, Norman R. I. Metformin in polycystic ovary syndrome:
systematic review and meta-analysis. BMJ 2003;327 (7421):951-60.
13.
Kolodziejczyk B, Duleba A. J, Spaczynski R. Z et al. Metformin therapy
decreases hyperandrogenism and hyperinsulinemia in women with polycystic
ovary syndrome. Fertil Steril 2000;73 :1149-54.
14.
American Diabetes Association. In: Consensus Development Conference on
lnsulin Resistance; Diab. Care 1998;21:310-14.
15.
Thomas I. Siebert M, Thinus F et al. Is the addition of metformin efficacious in
the treatment of clomiphene citrate-resistant patients with polycystic ovary
syndrome? A structured literature review. Fertility and Sterility
2006;86(5):1432-37.
16.
Bailey CJ, Turner RC
.
American Diabetes Association
, "
Standards of Medical
Care in Diabetes Mellitus 2009”
,
Diabetes Care
,
2009
;
32(1)113-61.
17.
Timmins P, Donahue S, Meeker J et al. Steady-state Pharmacokinetics of a Novel
Extended-Release Metformin Formulation. Clin Pharmacokinet 2005;
44(7):721-9.
18.
Levy J, Cobas R.A, Gomes M.B. Assessment of efficacy and tolerability of once
daily extended release metformin in patients with type 2 diabetes mellitus.
xxiv
19.
Davidson J, Howlett H. New prolonged-release metformin im