Summary Gas exchange and water relations were investi-gated in Nothofagus solandri var. cliffortioides (Hook. f.) Poole (mountain beech) and Nothofagus menziesii (Hook. f.) Oerst (silver beech) seedlings in response to water stress and water-logging. At soil matric potentials (Ψsoil ) above −0.005 MPa, N. solandri had significantly higher photosynthetic rates (A), and stomatal and residual conductances (gsw and grc), and lower predawn xylem water potentials (Ψpredawn ) than N. menziesii. The relative tolerance of plants to water stress was defined in terms of critical soil matric potential (Ψcri ) and lethal xylem water potential (Ψlethal ). The estimated values of Ψcri and Ψlethal were −1.2 and −7 MPa, respectively, for N. solandri, and −0.7 and −4 MPa, respectively, for N. menziesii. Photosynthesis was sustained to a xylem water potential (Ψxylem ) of −7 MPa in N. solandri compared with −4 MPa in N. menziesii.
Following rewatering, both A and Ψxylem recovered quickly in N. solandri, whereas the two variables recovered more slowly in N. menziesii. During the development of water stress, nonstomatal inhibition significantly affected A in both N. so-landri and N. menziesii. Nothofagus menziesii was more sus-ceptible to inhibition of A by waterlogging than N. solandri. However, the tolerance of N. solandri to severe waterlogging was also limited as a result of a failure to form adventitious roots, suggesting a lack of adaptation to these conditions. The differences in tolerance to water stress and waterlogging be-tween the two species are consistent with the distribution patterns of N. solandri and N. menziesii in New Zealand. Keywords: gas exchange, geographical distribution, nonsto-matal inhibition, Nothofagus menziesii, Nothofagus solandri var. cliffortioides, water relations, water stress.
Introduction
Woody plants vary markedly in their response to and tolerance of water stress (e.g., Myers and Landsberg 1989, Ranney etal. 1990, Seiler and Cazell 1990, Ni and Pallardy 1991) and waterlogging (e.g., Pereira and Kozlowski 1977, Sena Gomes and Kozlowski 1980, Tang and Kozlowski 1982, Pezeshki and
Chambers 1985). Water stress influences metabolism, physiol-ogy and morpholphysiol-ogy in plants (Passioura 1982). Waterlogging causes inadequate aeration in soil, leading to rapid depletion of oxygen which induces many physiological and morphologi-cal changes (Kozlowski 1984), and affects mineralization and solubility of mineral substances, and leads to the formation of phytotoxic compounds (Janiesch 1991).
Nothofagus solandri var. cliffortioides (Hook. f.) Poole and Nothofagus menziesii (Hook. f.) Oerst are two of the five taxa in the genus Nothofagus (Southern beech) that are native to New Zealand. They both have a wide ecological and geo-graphical distribution, occurring from sea level to the upper timberlines at comparable latitudes. Taxonomically, however, the two species belong to two subgroups with distinct pollen types (Hanks and Fairbrothers 1976, Philipson and Philipson 1988, Hill and Read 1991). The genetic divergence of the two species may be dated back to the upper Cretaceous (Wardle 1984), approximately 70 million years ago, before New Zea-land was separated from Australia and Antarctica (Mildenhall 1980, McGlone 1985). From the present distribution, it ap-pears that soil water availability and drainage may play a prominent role in differentiating the geographical occurrence of N. solandri and N. menziesii (Wardle 1984). Drought has been suggested as an important factor in forest mortality that has led to the present vegetation pattern in New Zealand (Jane and Green 1986, Innes and Kelly 1992). Susceptibility of trees to waterlogging followed by drought has also been linked to forest decline (Jane and Green 1986). Plants that have been waterlogged may be more sensitive to drought than plants growing in aerated soils as a consequence of reductions in size of the root systems in response to inundated soil conditions (Jane and Green 1985, 1986). Nothofagus solandri is usually more dominant than N. menziesii in habitats with a dry climate (Wardle 1984), whereas on waterlogged soils, N. menziesii is stunted and often replaced by species such as N. solandri and Podocarpus dacrydioides A. Rich. (Wardle 1967). Despite many studies of the ecology and ecosystem dynamics of Noth-ofagus-dominated forests in New Zealand, a detailed knowl-edge of water relations of Nothofagus under conditions of
Physiological responses to water stress and waterlogging in
Nothofagus
species
OSBERT J. SUN,
1,2GEOFFREY B. SWEET,
1DAVID WHITEHEAD
3and GRAEME D.
BUCHAN
41
School of Forestry, University of Canterbury, Private Bag 4800, Christchurch, New Zealand
2 Present address: New Zealand Forest Research Institute, Private Bag 4800, Christchurch, New Zealand 3 Manaaki Whenua--Landcare Research, P.O. Box 69, Lincoln, Canterbury, New Zealand
4 Soil Science Department, Lincoln University, Canterbury, New Zealand
Received December 13, 1994
water stress and waterlogging is lacking. To investigate the role of water relations in determining the geographical distribution of N. solandri and N. menziesii in New Zealand, we tested (1) the responses of photosynthesis and xylem water potential to soil water availability, (2) tolerance of seedlings to progres-sively increased soil drying, and (3) tolerance to waterlogging in the two species.
Materials and methods
Response to water stress
Plant material, experimental design and treatment In the spring of the first growing season, seedlings were raised from seed and grown in pots containing a mix of peat (60%), soils collected from a Nothofagus-dominated forest (20%), ground pine bark (10%), coarse sand (10%) and slow-release fertilizer (2 kg m−3, Osmocote® Plus, Grace-Sierra, Heerlen, The Neth-erlands). On March 20 (autumn) of the second growing season, 15 seedlings of each species were each transplanted to a 4-liter pot containing nursery top soil (loam-textured) packed at a bulk density (ρb) of approximately 1100 kg m−3. Leaf area (one-sided) was estimated as 0.06 m2 for N. solandri and 0.05 m2 for N. menziesii seedlings. The seedlings were arranged on a bench in a greenhouse, and a dilute complete nutrient solution (Ingestad 1971) containing 10 ppm nitrogen was applied regu-larly until treatments began. The photoperiod in the greenhouse was extended to 16 h with 400 W sodium vapor lamps. Maxi-mum photosynthetic photon flux density (Q) varied between 1000 µmol m−2 s−1 in midsummer and 600 µmol m−2 s−1 in winter. Day/night temperatures (25/15 °C) were controlled thermostatically.
Treatments began 4 weeks after transplanting. The experi-ment comprised three water stress treatexperi-ments in a completely randomized single-tree plot design, and each treatment con-tained five seedlings of each species. Seedlings in Treatment 1 were well watered throughout the experiment (control), to maintain the soil matric potential (Ψsoil ) above −0.005 MPa. Seedlings in Treatment 2 were rewatered on Day 30 when Ψsoil reached a critical level (see below). Seedlings in Treatment 3
were left unwatered for 52 days until the end of the experiment. All pots were placed randomly on the bench and rearranged every second day.
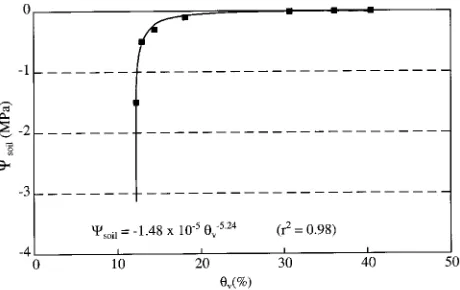
Determination of soil matric potential Soil matric potential (Ψsoil ) was estimated from volumetric soil water content (θv) based on an equation describing the soil water characteristic (Figure 1) determined using a tension table and pressure plate apparatus (Buchan and Grewal 1990). Measurements of θv were made with a time domain reflectometry (TDR) soil mois-ture meter (SoilMoismois-ture Equipment Corp., Santa Barbara, CA, USA).
Assessment of critical soil matric and plant water potentials Tolerance to water stress was defined in terms of critical soil matric potential (Ψcri ) and lethal xylem water potential (Ψlethal ). The value of Ψsoil at which Ψpredawn equals Ψmidday (of the preceding day) is defined as Ψcri , i.e., the minimum Ψsoil for adequate water uptake. When a plant reaches Ψcri , the hydrau-lic conductivity from the soil to leaves decreases abruptly (Tyree etal. 1992), so that continuing dehydration causes Ψxylem to decrease until leaf desiccation occurs. Survival and recovery of the plant following rewatering may then depend on the plant’s ability to tolerate Ψxylem that induces leaf desiccation. The values of Ψxylem below which leaf desiccation occurred were defined as Ψlethal .
Values of Ψcri for the two species were determined graphi-cally from measurements of Ψpredawn and Ψmidday , and values of
Ψlethal were taken as the minimum Ψxylem before leaf desicca-tion became visible.
Measurements of photosynthesis and leaf conductance Stomatal conductance to diffusion of water vapor (gsw), rates of net photosynthesis (A) and intercellular CO2 concentration (Ci) were measured at ambient CO2 concentration (about 380
µmol mol−1) in a controlled environment cabinet (Temperzone, Auckland, New Zealand) between 1100 and 1400 h (NZ ST) with a portable photosynthesis system (LI-6200, Li-Cor Inc., Lincoln, NE, USA). Measurements were made on twigs with 5--10 fully expanded leaves.
Seedlings were transferred from the greenhouse to the con-trolled environment cabinet at least 2 h before measurements were made. Photosynthetic photon flux density (Q) in the cabinet was 650 µmol m−2 s−1, and temperature and relative humidity were maintained at 20 °C and 55%, respectively. After each measurement, twigs were detached and immedi-ately used for Ψmidday measurements. Leaf areas (one-sided) were measured with an image area meter (Delta-T Devices, Burwell, Cambridge, U.K.).
Leaf conductance was partitioned into stomatal (gsc) and nonstomatal or residual conductance to diffusion of CO2 (grc) according to Farquhar and Sharkey (1982), where the term grc includes both mesophyll conductance (gmc) and carboxylation efficiency (k).
Measurements of xylem water potential Measurements of
Ψxylem were made with a pressure chamber (PMS Instrument Co., Corvallis, OR, USA). Measurements of Ψmidday were made Figure 1. Relationship between matric potential (Ψsoil ) and volumetric
immediately after gas exchange measurements in the control-led environment cabinet between 1100 and 1400 h, and Ψpredawn was measured in the greenhouse on the following day, about 14 h later. Twigs for measuring Ψxylem were obtained from a second- or third-order branch in the middle of the main stem. Data analysis Estimates of A, gsw, grc, Ψmidday and Ψpredawn in Treatment 1 were made over a 52-day period. Results were pooled for each species and compared by the Student’s t-test. Values of Ψcri and Ψlethal were well separated between species and were not compared statistically. Data for Treatments 1 and 3, and data from the early part of Treatment 2 (before soils were rewatered) were used to determine the relationship between
Ψpredawn and Ψsoil , responses of A, gsw and grc to Ψpredawn , and stomatal and nonstomatal (residual) components of photosyn-thesis. The data were evaluated by regression analysis and analysis of variance.
Response to waterlogging
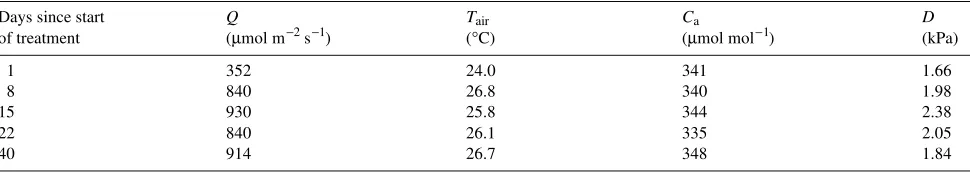
Fifteen seedlings of each species were grown in pots contain-ing pottcontain-ing mix as described previously. Soil water content of the control group was maintained near field capacity through-out the experiment. The water table in the second group of seedlings was raised 50 to 60 mm by placing pots in saucers containing tap water (partial waterlogging). Seedlings in the third group were totally flooded by submerging the pots indi-vidually in water containers (severe waterlogging). The water in the containers was replaced daily with fresh tap water. All pots were placed randomly on a bench in the greenhouse and rearranged weekly. The air-filled porosity (εa) for the partial waterlogging treatment was estimated to be only half of that of the control (Table 1).
Sequential measurements of A and gsw were conducted on twigs at ambient CO2 concentration between 1200 and 1400 h in the greenhouse, and effects were evaluated by analysis of variance. Means were compared by Duncan’s multiple-range
test with a confidence level of P≤ 0.05. Conditions in the greenhouse on the measurement days are given in Table 2.
Results
Response to water stress
When Ψsoil was maintained above −0.005 MPa, A, gsw and grc were significantly (P≤ 0.001) greater by 30, 25 and 30%, respectively, and Ψpredawn significantly lower by 30% for N. so-landri than for N. menziesii (Table 3). However, the values for
Ψmidday did not differ between the species.
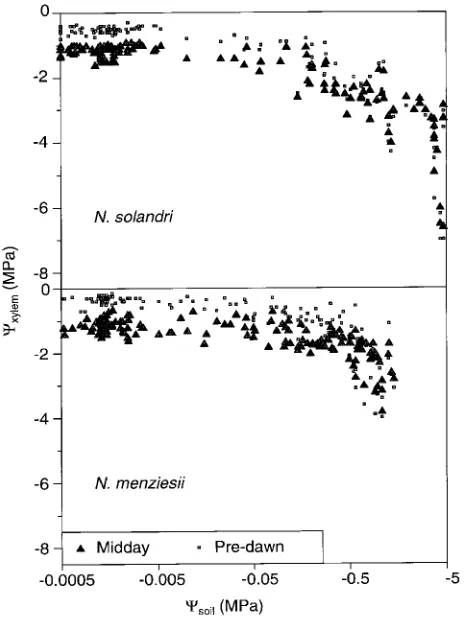
The effect of decreasing Ψsoil on Ψxylem differed according to species (Figure 2). In N. solandri, decreases in both Ψmidday and Ψpredawn occurred gradually in response to declining Ψsoil , whereas in N. menziesii, Ψmidday and Ψpredawn initially de-creased slowly with decreasing Ψsoil but declined abruptly when Ψsoil fell below −0.5 MPa. Values of Ψcri were estimated as −1.2 and −0.7 MPa, and values of Ψlethal were estimated as
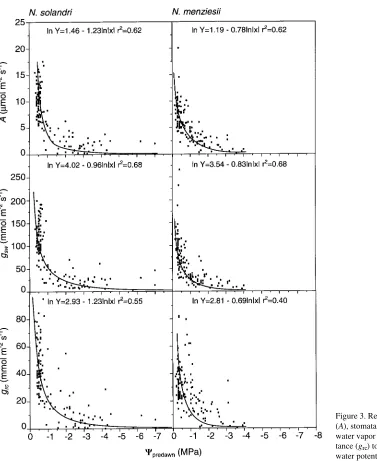
−7 and −4 MPa for N. solandri and N. menziesii, respectively. In both species, values of A, gsw and grc declined with decreasing Ψpredawn (Figure 3) following a relationship of the form:
ln(y)=a−b ln|Ψpredawn|, (1) where b defines the sensitivity of the response. Values for parameters a and b are summarized in Table 4. Both A and grc were more sensitive to decreasing Ψpredawn (P ≤ 0.001) in N. solandri than in N. menziesii, but there was no significant difference for gsw.
Rapid reductions in A, gsw and grc occurred as Ψpredawn fell from −0.4 to −0.8 MPa in N. solandri, and from −0.2 to −1.0 MPa in N. menziesii. In N. solandri, the reductions in A, gsw and grc continued until Ψpredawn fell to −7 MPa, whereas in N. menziesii, A, gsw and grc initially declined slowly in response
Table 1. Soil bulk density (ρb), total porosity (ε), volumetric soil water content (θv), and air-filled porosity (εa) for three waterlogging treatments. The values shown are means ± standard errors, n = 6.
Treatment ρb (kg m−3) ε (%) θv (%) εa (%)
Control 50.2 ± 1.9 20.5 ± 0.5
Partial waterlogging 776 ± 11 70.7 ± 0.04 59.0 ± 0.3 11.7 ± 0.7
Severe waterlogging 70.7 0
Table 2. Mean photosynthetic photon flux density (Q), mean air temperature (Tair ), mean ambient CO2 concentration (Ca), and mean vapor pressure deficit (D) in the greenhouse during the experimental period.
Days since start Q Tair Ca D
of treatment (µmol m−2 s−1) (°C) (µmol mol−1) (kPa)
1 352 24.0 341 1.66
8 840 26.8 340 1.98
15 930 25.8 344 2.38
22 840 26.1 335 2.05
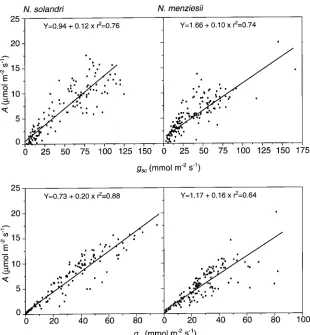
to decreasing Ψpredawn but approached zero when Ψpredawn reached about −4 MPa, which coincided with leaf desiccation. Regression analyses showed that, in N. solandri and N. menziesii, A was highly correlated with both the stomatal component, gsc, and the nonstomatal component, grc, of leaf conductance (Figure 4). However, the two species differed significantly in the response of A to gsc (P≤ 0.05) and of A to grc (P≤ 0.01). The slope b of the response curves for N. solan-dri was 20% greater than for N. menziesii (Table 5). In both
species, b for the response of A to grc was more than 60% greater than for the response of A to gsc.
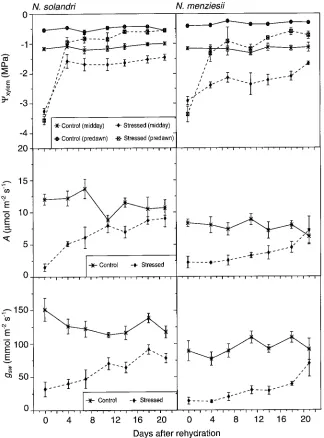
When seedlings were rewatered after being subjected to severe water stress (Ψcri ≤−1.2 and −0.7 MPa for N. solandri and N. menziesii, respectively), Ψxylem , A and gsw increased more rapidly for N. solandri seedlings than for N. menziesii seedlings (Figure 5). Three days after rewatering, both Ψmidday and Ψpredawn had recovered by more than 2 MPa in N. solandri, and Ψpredawn recovered to the control values after 14 days. However, in rewatered seedlings that had been water stressed previously, Ψmidday remained consistently lower than in control seedlings throughout the experiment. In N. menziesii, the re-covery of Ψxylem following rewatering was slower than in N. solandri, especially for Ψmidday , which had increased less than 1 MPa seven days after rewatering. Neither Ψmidday nor
Ψpredawn of previously water stressed N. menziesii seedlings reached control values by 21 days after rewatering.
The responses of A and gsw to rewatering were slower than that of Ψxylem . In N. solandri, there was a rapid recovery in A, whereas in N. menziesii, the recovery was slow. In both spe-cies, the recovery in gsw was slow, although it was slightly faster in N. solandri than in N. menziesii. Throughout the 21-day period after rewatering, gsw of previously water stressed seedlings of N. solandri did not reach control values.
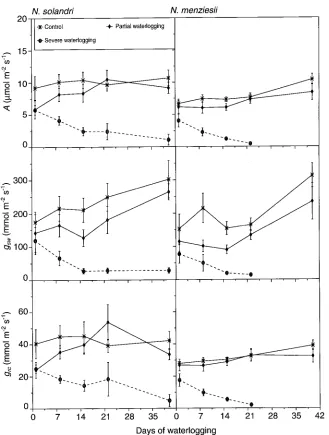
Response to waterlogging
One day after waterlogging treatments were imposed, there were slight declines in A, gsw and grc in seedlings of both species (Figure 6), but the effects were not significant until after 8 days of severe waterlogging. Both A and grc were about 60% less in severely waterlogged seedlings than in control seedlings of N. solandri (average decreases in A and grc were from 10.4 to 4.1 µmol m−2 s−1, and from 44.8 to 18.4 mmol m−2 s−1, respectively). The difference was more than 65% for N. menziesii (7.4 to 2.3 µmol m−2 s−1 for A and 29.3 to 9.7 mmol m−2 s−1 for grc). In response to waterlogging, g
sw de-clined by 70% in N. solandri (from 216 to 66 mmol m−2 s−1) and by almost 80% in N. menziesii (from 215 to 50 mmol m−2 s−1).
After 22 days of severe waterlogging, A, gsw and grc fell to nearly zero in N. menziesii, but severely waterlogged N. solan-dri seedlings retained some photosynthetic activity for 40 days. In N. menziesii, partial waterlogging resulted in signifi-cant (P≤ 0.05) reductions in gsw until Day 15 and in A on Day 15, but both gsw and A started to recover thereafter. Partial waterlogging did not alter the water relations of N. solandri.
Under severely waterlogged conditions, N. menziesii seed-lings suffered lethal leaf desiccation after 22 days, whereas leaf desiccation did not occur in N. solandri even after 40 days. No adventitious roots were observed in seedlings of either species in the severe waterlogging treatment.
Discussion
Although plants clearly differ in their response to and tolerance of water stress, it is difficult to define water stress tolerance quantitatively. The permanent wilting point used in conven-Table 3. Net photosynthetic rates (A), stomatal conductance to
diffu-sion of water vapor (gsw), residual conductance to diffusion of CO2 (grc), and midday and predawn xylem water potentials (Ψmidday and
Ψpredawn ) of seedlings at Ψsoil ≥−0.005 MPa. The values shown are means ± standard errors.
Variable N. solandri N. menziesii
A (µmol m−2 s−1) 10.61 ± 0.34*1 8.17 ± 0.36 gsw (mmol m−2 s−1) 123.8 ± 4.0* 99.1 ± 5.2 grc (mmol m−2 s−1) 46.5 ± 2.0* 34.5 ± 1.7
Ψmidday (MPa) −1.15 ± 0.02 −1.14 ± 0.03
Ψpredawn (MPa) −0.52 ± 0.01* −0.35 ± 0.02 1 An asterisk indicates that the difference between species is
signifi-cant at P≤ 0.001.
tional studies of water relations is difficult to define precisely, and it may not be applicable to many woody plants, such as N. solandri and N. menziesii, that do not show visible wilting even under severe water stress because of rigid leaf structures. We have quantified tolerance of both soil and plant water stress in N. solandri and N. menziesii from estimates of Ψcri and Ψlethal , and using these estimates, we have been able to differentiate between the two species in the water stress tolerance of seed-lings grown in confined soil volumes. Although extension of our results to field-grown trees is not straightforward, differ-ences in water stress tolerance between N. solandri and N. menziesii determined using Ψcri and Ψlethal appear to be consistent with ecological observations (Wardle 1970, 1984).
We observed large differences in water stress tolerance between N. solandri and N. menziesii. Nothofagus solandri had Ψcri and Ψlethal values of −1.2 and −7 MPa, respectively,
whereas N. menziesii had values of only −0.7 and −4 MPa, respectively. Nothofagus solandri has abundant sclerenchyma associated with highly developed vascular bundles and ligni-fied epidermal cells (Sun 1993), which may explain why it is more water stress tolerant than N. menziesii, which has struc-tures typical of mesophytic species.
Values of A declined with decreasing Ψxylem in both N. so-landri and N. menziesii, but the slope of the relationship differed significantly. Nothofagus solandri showed a rapid decrease in A in response to an initial decrease in Ψpredawn , but subsequently maintained a low but positive value of A as
Ψpredawn fell to −7 MPa. The responses of A and gsw in N. menzi-esii were initially less steep than in N. solandri, but as Ψpredawn fell below −4 MPa, the values for both A and gsw approached zero.
with reductions in both stomatal and residual leaf conductance in both N. solandri and N. menziesii. The impact of stomatal and nonstomatal inhibition on A depends on species, the sever-ity of the water stress, and the rate of water stress development. Although stomatal closure may account for the reduction in A under moderate water stress (Chaves 1991), the significance of
nonstomatal inhibition in water-stressed plants has been shown in many studies (Mooney etal. 1977, Comstock and Ehleringer 1984, Ni and Pallardy 1991). Farquhar and Sharkey (1982) suggested that, although stomatal closure reduces water loss, it reduces CO2 fixation only marginally and indirectly, because the latter is limited by the same factors that cause stomatal closure. Teskey etal. (1986) concluded that internal limitations are the major cause of reduction of CO2 assimila-tion in Pinus taeda L., although stomatal response of the species to several environmental variables is closely correlated with changes in photosynthetic rate. In water-stressed N. so-landri and N. menziesii seedlings, we found that A was more closely correlated with grc than with gsc.
The rapid response of A to water stress in N. solandri may have resulted from an adaptation to drought. As Ψpredawn fell from −0.4 to about −0.8 MPa, there was a sharp decline in gsw and A in N. solandri seedlings. Such a response may minimize water loss and possibly increase water stress tolerance. The high water stress tolerance of N. solandri seedlings was dem-onstrated by their sustained photosynthetic activity at values of
Ψpredawn as low as −7 MPa, a level that has been observed to kill many woody plants native to New Zealand (Innes and Kelly 1992). Photosynthesis and gsw in N. menziesii were less responsive to water stress than in N. solandri at Ψpredawn values between −0.2 and −1.0 MPa. Subsequently, however, A de-clined linearly with decreasing Ψpredawn in both species. Sensi-Table 4. Parameters obtained from regression analyses using
Equa-tion 1 for net photosynthesis (A), stomatal conductance to diffusion of water vapor (gsw), and residual conductance to diffusion of CO2 (grc) in response to predawn xylem water potential (ln|Ψpredawn |). The values shown are means ± standard errors.
Variable Parameter N. solandri N. menziesii
ln(A) a 1.46 ± 0.07 1.19 ± 0.05 b 1.23 ± 0.08*1 0.78 ± 0.05
r2 0.62 0.62
ln(gsw) a 4.02 ± 0.05 3.54 ± 0.04 b 0.96 ± 0.06 0.83 ± 0.05
r2 0.68 0.68
ln(grc) a 2.93 ± 0.08 2.81 ± 0.06 b 1.26 ± 0.10* 0.69 ± 0.07
r2 0.55 0.40
1 An asterisk indicates that the difference between species is signifi-cant at P≤ 0.001.
tivity of the response of gas exchange characteristics to water stress may thus reflect adaptation to drought.
Rewatering severely water-stressed seedlings led to rapid
recovery of both A and Ψxylem in N. solandri, whereas recovery occurred more slowly in N. menziesii. In both species, the recovery in A and gsw lagged behind the recovery in Ψxylem , Table 5. Linear regressions and correlations between net photosynthesis (A, µmol m−2 s−1) and stomatal conductance to diffusion of CO2 (gsc, mmol m−2 s−1) and residual conductance to diffusion of CO2 (grc, mmol m−2 s−1). The values shown are means ± standard errors.
Dependent variable Independent variable Parameter N. solandri N. menziesii
A gsc a 0.94 ± 0.36 1.66 ± 0.24
b 0.12 ± 0.006*1 0.10 ± 0.005
r2 0.76 0.74
A grc a 0.73 ± 0.25 1.17 ± 0.33
b 0.20 ± 0.006** 0.16 ± 0.01
r2 0.88 0.64
1 An asterisk indicates that the difference between species is significant at P≤ 0.05, and two asterisks indicate that the difference is significant at P≤ 0.01.
indicating that some injury might have occurred to the photo-synthetic apparatus during the water stress treatments, possi-bly increasing susceptibility to photoinhibition (Björkman et al. 1981, Araus and Hogan 1994). Lack of complete recovery in Ψmidday was probably due to physical changes such as dying of root tips, cavitation and embolism of xylem vessels that adversely affect the movement of water from soil to leaves (Schulze and Hall 1982, Zimmermann and Milburn 1982, Tyree and Sperry 1989). Nothofagus menziesii was damaged more than N. solandri under conditions of severe water stress, as indicated by the poor recovery of Ψpredawn , despite the increased soil water availability. The response of Ψxylem to decreasing Ψsoil in N. solandri and its tolerance to water stress suggests that the species may be classified as dehydration tolerant, whereas N. menziesii exhibited dehydration post-ponement; that is, this species is capable of maintaining rela-tively high water potential under conditions of moderate water stress.
Tolerance to waterlogging is correlated with changes in A, gsw and transpiration in many woody plants (Regehr et al. 1975, Pereira and Kozlowski 1977, Zaerr 1983, van der Moezel etal. 1989). Stomatal closure usually occurs within a few days after the soil is waterlogged, and in many species sensitive to waterlogging, the stomata remain closed for a long time (Kozlowski 1984). In some species tolerant to waterlog-ging, stomata may reopen after a critical period. In Fraxinus pennsylvanica Marsh. seedlings, stomata close rapidly follow-ing waterloggfollow-ing but reopen after about 2 weeks (Sena Gomes and Kozlowski 1980). Stomata of Eucalyptus camaldulensis Dehnh. reopen about 5 weeks after waterlogging (van der Moezel etal. 1989). Tang and Kozlowski (1982) reported that stomata of Quercus macrocarpa Michx. seedlings reopen about 30 days after flooding. Reopening of stomata did not occur in severely waterlogged N. solandri or N. menziesii seedlings during the experiment.
to waterlogging. Moderate waterlogging resulted in a reduc-tion of gas exchange properties in N. menziesii, whereas N. sol-andri seedlings were not affected. However, neither species was well adapted to severe or prolonged waterlogging. Severe waterlogging reduced A, gsw and grc in both N. solandri and N. menziesii, but the patterns of reduction differed between the two species. Regehr etal. (1975) observed a 50% reduction in A in waterlogging-tolerant Populus deltoides Bartr. ex Marsh. during complete inundation of the root system for 28 days. In our study, A was reduced by approximately 50% in N. solandri and by more than 65% in N. menziesii after only 8 days of waterlogging. In moderately waterlogging-tolerant Liquidam-bar styraciflua L. seedlings, A was reduced by 70% over a 9-day flooding period (Pezeshki and Chambers 1985). Zaerr (1983) showed that following flooding, A decreased to about half the control rate within 4 days for Pseudotsga menziesii (Mirb.) Franco and Picea abies (L.) Karst., but did not change in Pinus sylvestris L. In N. solandri and N. menziesii, the reduction in A of seedlings under waterlogged conditions was associated with decreased gsw and with a marked reduction in grc, indicating that waterlogging resulted in severe damage to the photosynthetic apparatus of the plants.
In New Zealand, N. solandri is adapted to a wider range of soil water conditions than N. menziesii. Nothofagus solandri is usually either the sole or the dominant species in forests of dry habitats or on poorly drained soils (Wardle 1970, 1984), and it codominates with N. menziesii at the wetter end of its ecologi-cal range. In areas where the climate is dry and where both species are present, N. solandri is most likely to occupy the exposed sites, whereas N. menziesii is more or less restricted to sheltered sites (Wardle 1984). Our results are consistent with these observations.
Acknowledgments
The authors thank Karl Schasching for his help in seed collection and for organizing research facilities. We also thank B. Bullsmith, D. Clark, D. Conder and P. Fuller for technical assistance, and T. Pearson for help with preparing graphs. O.J. Sun is grateful for the financial support provided by T.W. Adams Scholarship, BNZ Scholarship for Forestry Research, Robert C. Bruce Trust and Young Scientists’ Fund of the Royal Society of New Zealand, and support of NZ Forest Research Institute in preparing the manuscript. Comments from K.P. Hogan on the manuscript are greatly appreciated.
References
Araus, J.L. and K.P. Hogan. 1994. Leaf structure and patterns of photoinhibition in two neotropical palms in clearings and forest understory during the dry season. Am. J. Bot. 81:726--738. Björkman, O., S.B. Powles, D.C. Fork and G. Öquist. 1981.
Interac-tion between high irradiance and water stress on photosynthetic reactions. Year Book, Carnegie Inst., Washington, D.C. 80:57--59. Buchan, G.D. and K.S. Grewal. 1990. The power-function models for
the soil moisture characteristics. J. Soil Sci. 41:111--117. Chaves, M.M. 1991. Effects of water deficits on carbon assimilation.
J. Exp. Bot. 42:1--16.
Comstock, J. and J. Ehleringer. 1984. Photosynthetic response to slowly decreasing leaf water potential in Encelia frutescens. Oe-cologia 61:241--248.
Farquhar, G.D. and T.D. Sharkey. 1982. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33:317--345.
Hanks, S.L. and D.E. Fairbrothers. 1976. Palynotaxonomic investiga-tion of Fagus L. and Nothofagus BL. Light microscopy, scanning electron microscopy, and computer analysis. In Botanical Sys-tematics 1. Ed. V.H. Heywood. Academic Press, London, U.K., pp 11--141.
Hill, R.S. and J. Read. 1991. A revised infrageneric classification of Nothofagus (Fagaceae). Bot. J. Linn. Soc. 105:37--72.
Ingestad, T. 1971. A definition of optimum nutrient requirements in birch seedlings. II. Physiol. Plant. 24:118--125.
Innes, K.P. and D. Kelly. 1992. Water potentials in native woody vegetation during and after a drought in Canterbury. N.Z. J. Bot. 30:81--94.
Jane, G.T. and T.G.A. Green. 1985. Patterns of stomatal conductance in six evergreen tree species from a New Zealand cloud forest. Bot. Gaz. 146:413--420.
Jane, G.T. and T.G.A. Green. 1986. Etiology of forest dieback areas within the Kaimai Range, North Island, New Zealand. N.Z. J. Bot. 24:513--527.
Janiesch, P. 1991. Ecophysiological adaptations of higher plants in natural communities to waterlogging. In Ecological Responses to Environmental Stresses. Eds. J. Rozema and J.A.C. Verkleij. Klu-wer Academic Publishers, The Netherlands, pp 50--60.
Kozlowski, T.T. 1984. Plant responses to flooding of soil. BioScience 34:162--167.
McGlone, M.S. 1985. Plant biogeography and the late Cenozoic his-tory of New Zealand. N.Z. J. Bot. 23:723--749.
Mildenhall, D.C. 1980. New Zealand late Cretaceous and Cenozoic plant biogeography: a contribution. Palaeogeogr. Palaeoclimatol. Palaeoecol. 31:197--233.
Mooney, H.A., O. Björkman and G.J. Collatz. 1977. Photosynthetic acclimation to temperature and drought in the desert shrub Larrea divarcata. Year Book, Carnegie Inst., Washington, D.C. 76:328--335. Myers, B.J. and J.J. Landsberg. 1989. Drought and seedling growth of two eucalypt species from contrasting habitats. Tree Physiol. 5:207--218.
Ni, B.-R. and S.G. Pallardy. 1991. Response of gas exchange to water stress in seedlings of woody angiosperms. Tree Physiol. 8:1--9. Passioura, J.B. 1982. Water in the soil--plant--atmosphere continuum.
In Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Encyclopedia of Plant Physiology, New Series, Vol. 12B. Eds. O.L. Lange, P.S. Nobel, C.B. Osmond and H. Ziegler. Springer-Verlag, Berlin, Heidelberg, pp 5--33.
Pereira, J.S. and T.T. Kozlowski. 1977. Variations among woody angiosperms in relation to flooding. Physiol. Plant. 41:184--192. Pezeshki, S.R. and J.L. Chambers. 1985. Stomatal and photosynthesis
response of sweet gum Liquidambar styraciflua to flooding. Can. J. For. Res. 15:371--375.
Philipson, W.R. and M.N. Philipson. 1988. A classification of the genus Nothofagus (Fagaceae). Bot. J. Linn. Soc. 98:27--36. Ranney, T.G., T.H. Whitlow and N.L. Bassuk. 1990. Response of five
temperate deciduous tree species to water stress. Tree Physiol. 6:439--448.
Regehr, D.L., F.A. Bazzaz and W.R. Boggess. 1975. Photosynthesis, transpiration, and leaf conductance of Populus deltoides in relation to flooding and drought. Photosynthetica 9:52--61.
Seiler, J.R. and B.H. Cazell. 1990. Influence of water stress on the physiology and growth of red spruce seedlings. Tree Physiol. 6:69--77.
Sena Gomes, A.R. and T.T. Kozlowski. 1980. Growth response and adaptation of Fraxinus pennsylvania seedlings to flooding. Plant Physiol. 66:267--271.
Sun, O.J. 1993. Genetics and physiology of Nothofagus solandri var. cliffortioides and N. menziesii----a comparative study. Ph.D. Thesis. University of Canterbury, Christchurch, New Zealand, 279 p. Tang, Z.C. and T.T. Kozlowski. 1982. Some physiological and
mor-phological responses of Quercus macrocarpa seedlings to flooding. Can. J. For. Res. 12:196--202.
Teskey, R.O., J.A. Fites, L.J. Samuelson and B.C. Bongarten. 1986. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol. 2:131--142.
Tyree, M.T., J. Alexander and J.-L. Machado. 1992. Loss of hydraulic conductivity due to drought in intact juveniles of Quercus rubra and Populus deltoides. Tree Physiol. 10:411--415.
Tyree, M.T. and J.S. Sperry. 1989. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Mol. Biol. 40:19--38. van der Moezel, P.G., L.E. Watson and D.T. Bell. 1989. Gas exchange
responses of two Eucalyptus species to salinity and waterlogging. Tree Physiol. 5:251--257.
Wardle, J. 1970. The ecology of Nothofagus solandri. 1. The distribu-tion and reladistribu-tionship with other major forest and shrub species. N.Z. J. Bot. 8:494--531.
Wardle, J. 1984. The New Zealand beeches. Ecology, utilisation and management. New Zealand Forest Service, Christchurch, New Zea-land, 447 p.
Wardle, P. 1967. Biological flora of New Zealand. 2. Nothofagus menziesii (Hook. f.) Oerst. (Fagaceae). N.Z. J. Bot. 5:276--302. Zaerr, J.B. 1983. Short-term flooding and net photosynthesis in
seed-lings of three conifers. For. Sci. 29:71--78.