Pengembangan senyawa aktif dari ekstrak etil asetat rimpang Acorus calamus
L.
sebagai antidiabetes
Sri Hartati, Rizna T. Dewi, A. Darmawan and Megawati
Research Center for Chemistry - LIPI Kawasan PUSPIPTEK Serpong Tangerang Selatan
ABSTRAK
Penelitian sebelumnya menunjukkan bahwa ekstrak etil asetat rimpang Acorus calamus L. memiliki aktivitas biologis dalam diferensiasi pra-adiposit dan potensial dalam pengobatan diabetes tipe 2. Komponen aktif da-lam ekstrak tersebut adalah 3-β, 22α-trihidroksiolean-30-metoksikarbonil-12 ene-22-O-α-L-rhamnosida dan β-sitosterol-3-O-β-D-glukosidase. Penelitian ini bertujuan untuk mengisolasi komponen ekstrak etil asetat rimpang Acorus calamus L yang memiliki aktivitas penghambatan enzim α-glukosidase serta melakukan modi -fikasi struktur komponen aktif (SAR: Structure Activity Relationship). Hasil penelitian menunjukkan terdapat 4 fraksi semi polar dengan profil KLT yang mirip (fraksi 4, 5, 6, dan 7). Keempat fraksi tersebut menghambat aktivitas enzim α-glukosidase dengan IC50 berturut-turut sebesar 13.54; 13.34; dan 18.92 µg/ml. Selain itu,
terdapat pula 3 fraksi polar (fraksi 20, 21, dan 22) yang aktif dengan IC50 berturut-turut sebesar 13.55; 3.08; dan 6.85 µg/ml. Studi SAR menunjukkan bahwa β-sitosterol-3-O-β-D-glukosidase diduga memiliki struktur yang mirip dengan acarbose, suatu obat antidiabetes. Penelitian ini sebaiknya dilanjutkan untuk memperoleh komponen aktif yang dapat didesain sebagai obat antidiabetes.
Kata kunci: antidiabetes, α-glukosidase, Acorus calamus L.
ABSTRACT
In search development herbal medicine of selected plant for antidiabetic, ethyl acetate extract of Acorus
cala-mus L. plays on biological role in differentiation of preadiposites and posses powerful in diabetes (type 2) and
have known active compounds are 3-β, 22 α-trihiroksiolean-30-metoksikarbonil–12 ene-22-O-α-L-rhamnoside and β-sitosterol-3-O-β-D-glukosidase. The proposed of this search to isolate active compound which as inhibitor
α-glucosidase activity from ethyl acetate extract and modify of active compounds or (SAR = Structure Activity Re-lationship). The results of fraction of ethyl acetate extracts were four semi-polar fractions which similar TLC spot
(4, 5, 6 and 7 fractions) were active inhibition of α-glucosidase activity which IC50 22.86; 13.54; 13.34 and 18.92
ug/ml respectively and three polar fractions (20, 21 and 22 fractions) were active inhibition of α-glucosidase activity which IC50 13.55; 3.08 and 6.85 ug/ml respectively. SAR studied showed β-sitosterol-3-O-β-D-glukosidase with suspicion active compound have similarity with acarbose antidiabetic drug. This research should be contin-ued to establish of active compounds for further on antidiabetic drug design.
INTRODUCTION
Diabetes mellitus is a disease in which lev-els of glucose (simple sugar) in the blood is high because the body can not release or use insulin normally. Insulin is a hormone secreted by the pancreas, which is responsible in maintaining normal blood sugar levels. Insulin incorporate sugar into cells so that it can produce energy or stored as energy reserves. The number of diabet-ics worldwide currently is estimated at 150 mil-lion people and this figure is expected to increase to reach 220 million by 2010 and with the current rate of increase as in 2025 will be 300 million. Di-abetes mellitus of these type 2 are 90% (Collene
et al., 2005; So et al., 2000). According to WHO data, Indonesia ranks 4th largest in the number of people with diabetes mellitus in the World. In the year 2000, there are around 5.6 million peo-ple in Indonesia have diabetes. However, in 2006 the estimated number of diabetics in Indonesia increase sharply to 14 million people.
Acorus calamus L. have been used in the was found to enhance adipocyte differentiation as did roglitazone. A. calamus has potentially to be useful for the treatment of diabetes and car-diovascular complication without body weight gain. The proposed of this search to isolate ac-tive compound which as inhibitor α-glucosidase activity from ethyl acetate extract and modify of active compounds 3-β, 22 α
-trihiroksiolean-30-metoksikarbonil-12ene-22-O-α-L-rhamnoside and β-sitosterol-3-O-β-D-glukosidase or (SAR =
Structure Activity Relationship).
materials and metHods
Methods of isolation
Rhizome material dried in oven at a tem-perature of 50oC, dry sampel then ground using
a grinder with a certain subtlety, then macerated with technical methanol for 2 x 24 hours as much as 3x. Concentrated and the residue was separat-ed and then the concentrate was concentratseparat-ed by rotary vacuum evaporator to aford methanol ex-tract. Methanol extract partitioned with solvent mixture n-hexane: water (1:1) as much as 3x. The results of partition obtained hexane and water fractions. On the water fraction partitioned with ethyl acetate: water (1:1) obtained ethyl acetate extracts and water fractions. In the same way it is partitioned with butanol, to afford butanol ex -tract and water fraction was dried to obtained water extract. Ethyl acetate extracts was washed again with n-hexane to reduce contained of α and
β asasron. Ethyl acetate extract was subjected to silica gel G60 of gravity column chromatography and using gradient mixture of n-hexane–ethyl acetate and methanol as mobile phases. Frac-tions obtained is evaporated then collected and analyzed by thin layer chromatography (TLC) aluminum plates SiGF254 sheet with eluent ad-justed. Results are grouped by suitability TLC spots. The fractions were tested to the activity of
α-glucosidase test methods
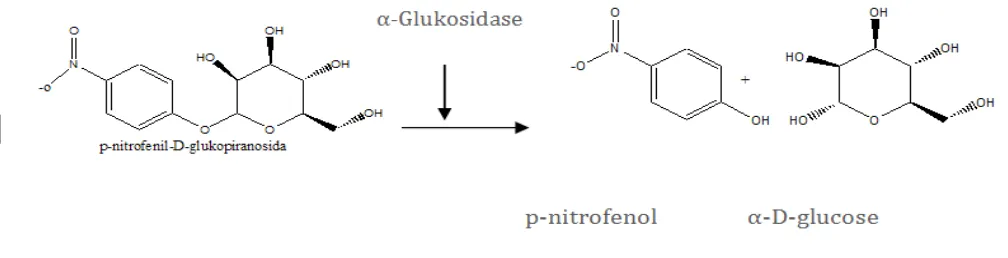
α-Glucosidase reaction mechanism is to catalyze the reaction of substrate to p- nitrofenol and glucose. p-Nitrophenyl- α-D-glukopiranosida was used as substrate, at temperature of 37oC.
The enzyme activity was measured by uptake of p-nitrofenol generated. If the sample has the ability to inhibit the activity of the α-glucosidase, p-nitrofenol generated will be reduced (Artanti
et al., 2002).
Figure 1. Reaction mechanism decomposition of the substrate p-nitrophenyl α-D- glukopiranosida.
Enzymatic α-glucosidase activity is an in vitro method for testing the ability of alternative that is cheaper and faster for initial screening.
test inhibition of α-glucosidase activity (in vitro)
α-glucosidase activity inhibitor performed according to Kim Yong-Mu et al. (2005) (kit Waco Chemical Ltd.). In test tube containing 5 µl test so-lution added 250 µl PNP and 495 µl of phosphate buffer solution, then pre incubation for 5 minutes at the temperature 37oC. 250 µl of α-glucosidase
enzyme solution was added and incubation was continued for 15 minutes at 37oC. The reaction
was stopped by the addition of 1000 µl solution of 0.2 µM Na2CO3.The number of p-nitrofenol re-leased measured with spectrophotometer at λ 400 nm.
Presentation inhibitory activity was measured by using the equation:
(C - S) x 100
C
% Inhibition =
C = absorbance of blank (DMSO)
S = absorbance of sample (difference ab
-sorbance with and without enzyme)
IC50 values (concentration that inhibits 50% of the working enzyme) obtained from the curve equation between% inhibition (Y) as the ordinate axis with a concentration ug / mL (x) as ordinate. From the linear graph by entering the 50% inhi-bition (Y) will be calculated concentration from the regression formula. Y = ax + b
SAR (Structure Activyti Relationships)
Materials required
3D, proteins or enzymes that act as receptors (α-glucosidase enzyme), and ligand (active com -pound or com-pounds isolated and target synthe-sis).
Method
The method used the of data processing and analysis using a computer program, by first describing ligand compounds using Chem-Office 2D program, to further sought the position of the most stable structure of the ligand using Chem-3D Office. Chem-3D structure of α-glucosidase enzyme obtained from http://www.pdb.org. Value log P (lipofilisitas) obtained through the calculation of ligand 3D structures using the program Hy-perChem. Then the enzyme α-glucosidase as a receptor ligand compound using docking with MVD program to learn the value of the bond en-ergy between them for later comparison with the value of the docking was conducted on a positive standard (acarbose), so based on that value can be known correspondence between receptor and ligand with whether the alleged activities of li-gands will have better or equivalent to a positive standar.
RESULTS AND DISCUSSION
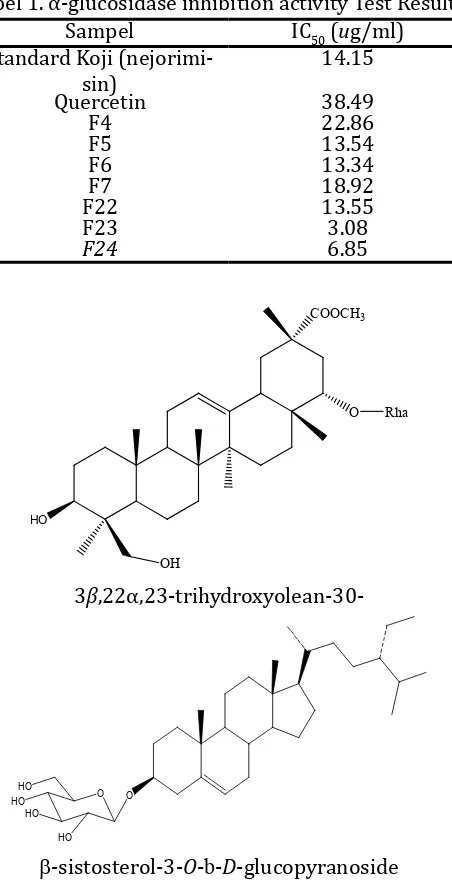
Results of fractination of 311 g of ethyl ac-etate extract of A. calamus are afforded 26 frac -tions is the result of combining frac-tions base on similarity spotting TLC results. From the results of fractionation was carried out testing of barriers work activities α-glucosidase enzyme that can be seen in Table 1. Where in Table 1 these data show that the active fractions compared with standard positive blank koji fraction MTC (methylene chloride) with IC50 was 14.15 ug/ml and IC50 is
comparison quercetin with 38.49 ug/ml. Where test results ethyl acetate fraction obtained four active fractions that have similar spots semipo-lar TLC are fraction number 4, 5, 6, 7 and three polar fractions of fractions number 22, 23, 24. IC50 value of each fraction to 4, 5, 6, 7 are 22.86, 13.54, 13.34 and 18.92 ug/ml, while the IC50 frac-tions no 22. 23, 24 is 13.55, 3.08 and 6.85 ug/mL. Those fraction 5, 6, 22, 23 and 24 showed more actively inhibit the activity of α-glucosidase than the standard koji and quercetin, because these factions have IC50 values less than blank. While fractions 4 and 7 activity slightly below the stan-dard koji but more active than quercetin blank.
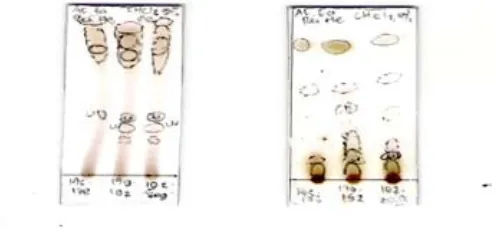
Figure 1. Thin layer chromatography (TLC), the result of semi polar active fractions eluted with n-hexane and ethyl acetate (9: 1).
Figure 2. Thin layer chromatography (TLC) results of the polar active fraction F21(f175-f178), F22 (f179 -f191) and, F22 (f192-203). a. eluted with CHCl2: Methanol 5%, b. eluted with CHCl2.
Tabel 1. α-glucosidase inhibition activity Test Results
β-sistosterol-3-O-b-D-glucopyranoside
Figure 3. Estimated active structure antidiabetes
Study Results SAR (structure Modification Study Results)
Modification of the structure is the de -velopment of the markers compounds of known biological activity to produce derivatives/ana-logues. It aims to produce new compounds that more effectively and safely used. This is based on
the assumption, that in general compounds that have similar chemical structure would have or show similar biological activity the same time, it is a basic principle of the SAR.Compounds guide is not intended specifically as a clinical agent, but it is a starting point to develop compounds that function in the clinic. Thus, to increase its activ-ity then studied the relationship of structure and biological activity clusters by changing the substituents in lead compounds. To predict the activity of synthesized compounds that would be predicted by using software Molegro Virtual Docking (MVD), HyperChem Pro-6.0, or compare the lead with a compound/ drug (native ligand) glucosidase enzyme, (acarbose, deoxynojirimi-cin, and miglitol) of drugs such as MIMS Catalog Indonesia 2006 (http://www/mims.com) (Fig-ure 3) also with a few isolated compounds of natural materials that have proved active as an inhibitor of-glucosidase, accessible base on line of site (http://pubchem.ncbi.nlm.nih.gov) (Fig-ure 4).
N N
OH
N N
OH HO
Vasicine
Vasicinol
S
O3S O
HO
HO
OH
OH
Salacinol
Figure 4. Some of the structure of α-glucosidase inhibitors isolated from plants.
.
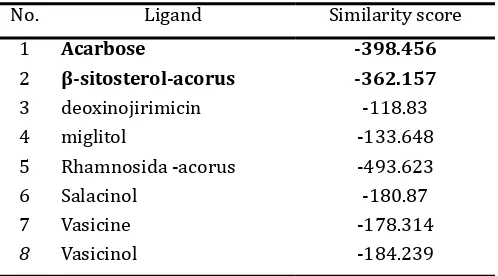
With the software we can determine phar-macopore of compounds that have been known active against α-glucosidase enzyme. The result of calculation parameters can be known whe-ther the compound or our compound has some similarities (similarity) with comparison com-pound (deoxynojirimisin/ gliset) and likelihood (posibility) to synthesize an analog or derivative compound of the isolates. In phase I of this ac-tivity, we have performed calculations using the software to look for similarities sulochrin MVD with the active compounds are α-Gis deoxynoji -rimisin or miglitol (mimic sugar), vasacine and vasacinol (isolated from Adthoda vasica Ness), and salasinol (results isolated from Salacia ob-longa). Doxynojirimisin used as reference com-pounds because these comcom-pounds are active as compounds Glyset (oral drug α-glucosidase inhi -bitor) with the mechanism of action as a compe-titive inhibitor. From the calculation of similarity of data obtained as in Table 2.
Table 2. Calculation results with MVD ligand simila-rity scores
No. Ligand Similarity score
1 Acarbose -398.456
2 β-sitosterol-acorus -362.157
3 deoxinojirimicin -118.83
4 miglitol -133.648
5 Rhamnosida -acorus -493.623
6 Salacinol -180.87
7 Vasicine -178.314
8 Vasicinol -184.239
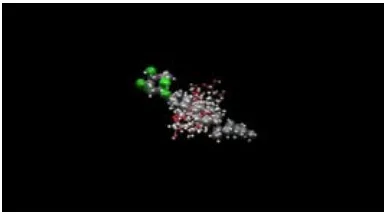
Based on these results compound acar-bose, has the lowest similarity value and close to the value of β-sitosterol-acorus, or it can be said instead that the compound β-sitosterol-acorus has some similarities with the compound / drug acarbose (Figure 5). The next step is to place (docking), compound ligand on the target enzyme (α-glucosidase crystallographic results that can be downloaded on line: http// www. rbcs.pdb), this stage is to determine whether the compound has ligand affinity towards the target (Figure 7).
Table 3. Docking calculation results with the MVD score
Ligand MolDock Score Rerank Score
ACG_989 [Acarbose] -181.76 -165.093
b-sitosterol acorus.MOL -134.266 -115.66
deoksinojirimisin.MOL -60.0821 -58.3225
rahamnosida acorus .MOL -110.567 -46.8888
Pose compounds (ligands) in the binding site can be viewed as shown in Figure 7.
Figure 7. Pose ligand binding sites on α-glucosidase enzyme (MVD docking). 1, Amino acid residues on the side α-glucosidase enzyme bond; 2, native ligandacarbose); 3, didocking ligands (b-sitosterol, rham -nosida, and acarbose).
Figure 5. The result of alignment of the lead
compounds with comparable compounds Figure 6: X-ray crystallographic α-glucosidase enzyme
1
2
CONCLUSIONS
The results activity of (F4, F5, F6 and F7) are inhibited α-glucosidase activity which IC50 22.86; 13.54; 13.34 and 18.92 ug/ml respectively and three polar fractions (F20, F21 and F22) are in-hibited α-glucosidase activity which IC50 13.55; 3.08 and 6.85 ug/ml respectively. Those fractions F5, F6, F22; F23 and F24 showed more actively inhibit the activity of α-glucosidase than the stan -dard koji and quercetin, because these factions have IC50 values less than blank. While F4 and F7 activity slightly below the standard koji but more active than quercetin blank. SAR studied showed
β-sitosterol-3-O-β-D-glukosidase with suspicion active compound have similarity with acarbose antidiabetic drug. This research should be con-tinued to establish of active compounds for fur-ther on antidiabetic drug design.
REFERENCES
Artanti N., Hanafi M., and Kardono LBS. 2002. Ak-tivitas Penghambatan Ekstrak Gambir (Un-caria gambir Roxb.) dan Ekstrak Taxus su-matrana (Miquel) De Launbenfels terhadap enzym α-Glukosidase, Prosiding Seminar Nasional V Kimia Dalam Pembangunan, Yo-gyakarta, 483-488.
Collene LA., Steven R.H., Williams JA., and Bryan WW. 2005. Effects of a Nutritional Supple -ment Containing Salacia oblonga Extract and Insulinogenic Amino Acids on Post-prandial Glycemia, Insulinemia, and Breath Hydrogen Responses in Healthy Adults. Nu-trition, 21: 848-854.
Kim YM., Jeong YK., Wang MH., Lee WY., Rhee HI. 2005. Inhibitory Effect of Fine Extract on
α-Glucosidase Activity and Postprandial Hyperglikemia, Nutrition, 21: 756-761. So WY., Ng MC, Lee Sc., Sanke T., Lee HK., Chan JC.
2000. Genetics of type 2 diebetes mellitus.
Hongkong Med. J., 6:69-76
Wu HS., Li YY., Weng LJ., Zhou CX., He QJ., and Lou YJ. 2007. A Fraction of Acorus calamus L. Extract Devoid of β-asaron Enhances Adi-pocyte Differentiation in 3T4-Ll Cells, Phy-totherapy Research, 21: 262-264.
Wu SH., Zhu DF., Zhou CH., Feng CR., Lou YJ., Bo Y. and He QJ. 2009. Insulin Sensitizing activity of Ethyl Acetate fraction of Acorus calamus