i
LAPORAN AKHIR
PENELITIAN HIGH IMPACT
DANA ITS 2020
IMOBILISASI JAMUR PELAPUK COKLAT (Gloeophyllum trabeum)
DALAM METAL ORGANIC FRAMEWORKS UiO-66 DAN APLIKASINYA
DALAM BIODEGRADASI LIMBAH PEWARNA BATIK
REACTIVE BLACK 5
Tim Peneliti :
Adi Setyo Purnomo, M.Sc., Ph.D. (Departemen Kimia/Fakultas Sains dan Analitika Data) Dra. Ratna Ediati, M.Si., Ph.D. (Departemen Kimia/Fakultas Sains dan Analitika Data)
Prof. Dr. Taslim Ersam, MS. (Departemen Kimia/Fakultas Sains dan Analitika Data) Drs. Refdinal Nawfa, MS. (Departemen Kimia/Fakultas Sains dan Analitika Data)
DIREKTORAT RISET DAN PENGABDIAN KEPADA MASYARAKAT
INSTITUT TEKNOLOGI SEPULUH NOPEMBER
SURABAYA
2020
i
Daftar Isi
Daftar Isi ... i
Daftar Tabel ... ii
Daftar Gambar ... iii
BAB I RINGKASAN ... 1
BAB II HASIL PENELITIAN ... 3
BAB III STATUS LUARAN ... 12
BAB IV KENDALA PELAKSANAAN PENELITIAN ... 13
ii
Daftar Tabel
iii
Daftar Gambar
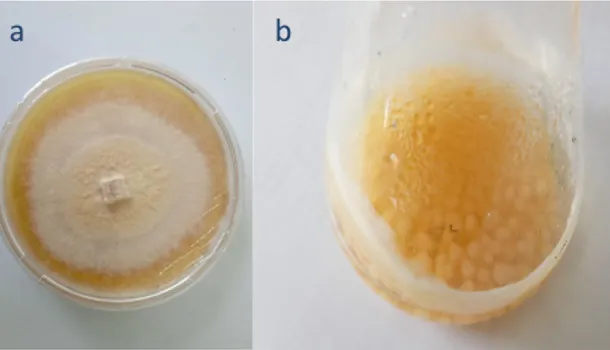
Gambar 2.1 a. kultur jamur G. trabeum di PDA; b. biokomposit Gt/UiO-66 ... 3
Gambar 2.2 Spektra UV-Vis dekolorisasi RB5 dengan kultur jamur G. trabeum murni ... 6
Gambar 2.3 Grafik Persen Dekolorisasi Vs Suhu ... 6
Gambar 2.4 Grafik Persen Dekolorisasi Vs Konsentrasi Pewarna ... 7
Gambar 2.5 Grafik Persen Dekolorisasi Vs pH ... 8
Gambar 2.6 Gabungan Difraktogram XRD dari UiO-66 dan Gt/UiO-66 ... 9
Gambar 2.7 Spektra FTIR UiO-66 dan Gt/UiO-66 ... 9
Gambar 2.8 Hasil SEM dari Gt/UiO-66 a. Perbesaran 1500x; b. Perbesaran 10.000x ... 10
Gambar 2.9 Spektra LC-Tof-MS metabolit produk dekolorisasi RB5 dengan Gt/UiO-66 ... 11
Gambar 2.10 Struktur molekul Reactive Black 5 ... 11
1
BAB I
RINGKASAN
Berdasarkan data dari Kementrian Perindustrian Republik Indonesia tahun 2019 pada triwulan III, Industri tekstil dan pakaian jadi merupakan sektor manufaktur yang mencatatkan pertumbuhan paling tinggi sebesar 15,08 persen. Hal ini diperkuat oleh data dari Badan Pusat Statistik (BPS) menunjukkan produksi industri pakaian jadi mengalami pertumbuhan signifikan sebesar 15,29 persen. Salah satu andalan industri tekstil di Indonesia yaitu produk batik, tekstil jenis ini telah lama dikerjakan mulai dari skala rumah tangga hingga skala pabrik. Namun di balik perkembangan industri batik ternyata menyisakan masalah lingkungan dengan adanya limbah dari proses pembuatannya terutama limbah pewarna. Banyak limbah yang memiliki tingkat toksisitas yang tinggi yang terdiri dari senyawa-senyawa yang sulit didegradasi dimana limbah pewarna ini dapat menimbulkan efek merugikan bagi lingkungan dan makhluk hidup. Salah satu pewarna azo yang sering terdapat pada limbah pewarna tekstil adalah reactive black 5 (RB5). Selama proses pewarnaan sekitar 15-50% pewarna tidak terikat dalam serat kain dan dibuang bersama limbah tekstil. Mengingat dampak negatif dari pencemaran limbah tekstil ini maka perlu dicari solusinya, dimana salah satu metode yang ramah lingkungan yaitu bioremediasi. Salah satu jamur yang dapat digunakan untuk biodegradasi pewarna adalah jamur pelapuk coklat Gloeophyllum trabeum. Biodegradasi menggunakan jamur pelapuk coklat ini masih menjanjikan untuk diteliti, dimana kemampuannya akan dioptimasi pada material Metal Organic Frameworks (MOF) UiO-66 dimana MOF ini memiliki kemampuan yang baik sebagai adsorben zat pewarna. Kombinasi keduanya diharapkan bisa menghasilkan material yang dapat digunakan dalam pengolahan limbah tekstil. Sistem jamur/MOF menawarkan kecepatan adsorpsi pewarna yang cepat dan fotokatalis oleh MOF, didukung oleh proses bioregenerasi oleh jamur sehingga diharapkan sistem ini dapat mendegradasi pewarna lebih efisien. Berdasarkan hal tersebut, tujuan penelitian ini adalah mensintesis dan mengkarakterisasi material jamur pelapuk coklat G. trabeum yang terimobilisasi dalam MOF UiO-66 serta menguji kemampuan degradasinya pada pewarna batik RB5. Metode penyisipan material MOF UiO-66 ke dalam kultur jamur G. trabeum yaitu dengan menambahkan UiO-66 pada proses pertumbuhan jamur pada media cair PDB serta diberikan perlakuan agitasi secara perlahan. Hasil yang diperoleh berupa granula-granula berbentuk bulat yang kemudian dicuci dengan aqua DM sebelum diaplikasikan ke larutan pewarna RB5. Hasil aplikasi komposit terhadap larutan pewarna
2
RB5 (konsentrasi 100 mg/L) diperoleh % dekolorisasi hingga 57,82% dibandingkan dengan kultur jamur G. trabeum murni yang hanya 44,94%. Selain itu juga diteliti pengaruh konsentrasi pewarna, suhu, dan pH dalam proses degradasi dengan komposit Gt/UiO-66, kondisi proses dekolorisasi optimum yang diperoleh yaitu pada konsentrasi 100 mg/L, suhu 37ºC, dan pH 10. Karakterisasi dari struktur padatan (XRD) komposit berubah dibanding UiO-66 murni ditemukan tingkat kristalinitas menurun. Morfologi dan bentuk dari komposit diketahui dengan insturmen SEM bentuknya hampir bulat dan partikel UiO-66 tersebar dalam granula yang terbentuk, sementara ukuran luas permukaan UiO-66 yaitu 605,976 m2/g, sedangkan ukuran rata-rata dari porinya 7,586
nm. Metabolit produk hasil degradasi yang dianalisis dengan instrumen LCMS QTOF, maka diusulkan senyawanya yaitu Asam 5-amino-3,4-dihidroksi-6-(fenildiazenil)naftalena-2-sulfonat. Target luaran dari penelitian ini adalah artikel terpublikasi dalam jurnal internasional terindeks Scopus yaitu di jurnal “Biodegradation” dengan Impact factor 2.805 (Q1). Penelitian ini diharapkan menghasilkan metode penanganan masalah limbah zat warna dan dapat bermanfaat bagi ITS serta pelaku industri batik khususnya dan dapat dijadikan sebagai referensi dalam upaya penanganan masalah limbah pewarna batik.
Kata kunci : Biodegradasi, Jamur Pelapuk Coklat, Gloeophyllum trabeum. MOF, UiO-66,
3
BAB II
HASIL PENELITIAN
2.1 Kemajuan Pelaksanaan Penelitian
Berdasarkan prosedur penelitian yang telah disusun sebelumnya, maka beberapa kemajuan yang telah dapat dilaksanakan yaitu sebagai berikut:
1. Regenerasi Kultur Jamur G. trabeum
2. Pembuatan Biokomposit Gt/UiO-66 15, 30, dan 45 mg
3. Dekolorisasi Pewarna Reactive Black 5 (RB5) dengan Kultur Jamur Cair dan Gt/UiO-66 4. Karakterisasi Gt/UiO-66 dengan XRD, SEM, FTIR, dan Gas Sorption Instrument
(Adsorpsi desorpsi nitrogen)
5. Analisis LC-Tof-MS Produk metabolit (hasil degradasi)
2.2 Hasil Penelitian Yang Telah Diperoleh 2.2.1 Pembuatan Biokomposit Gt/UiO-66
Hasil regenerasi kultur jamur G. trabeum di media padat PDA diblender dengan aquades demineralisasi (aqua DM) dengan cup blender yang telah disterilisasi dengan autoklaf sebelumnya. Homogenat yang telah diperoleh kemudian dimasukkan dalam masing-masing erlenmeyer yang telah berisi media PDB dan UiO-66. Sampel diinkubasi pada suhu ±35 ºC selama 3 hari dengan perlakuan digoyang pelan dengan shaker horizontal. Hasilnya dapat dilihat pada Gambar 2.1:
Gambar 2.1 a. Kultur jamur G. trabeum di PDA; b. Biokomposit Gt/UiO-66
Selanjutnya biokomposit yang telah jadi dicuci dengan aqua DM 10 mL sebanyak 2-3 kali pada tiap-tiap erlenmeyer. Setelah dicuci biokomposit siap diaplikasikan padaa larutan pewarna RB5 dengan konsentrasi 100 mg/L sebanyak 20 mL.
5
2.2.3 Dekolorisasi Pewarna Reactive Black 5 dengan Biokomposit Gt/UiO-66
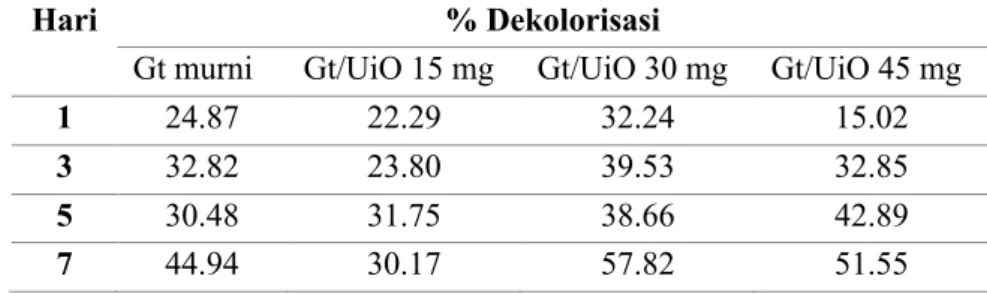
Uji dekolorisasi terhadap pewarna Batik RB5 atau Remazol Black B dilakukan dengan Gt/UiO-66 dengan variasi massa 15, 30, dan 45 mg. Hasil absorbansi diukur pada sampel dengan waktu inkubasi 1, 3, 5, dan 7 hari, sampel difiltrasi dan disentrifugasi sebelum diukur. Sentrifugasi dilakukan pada kecepatan 3000 rpm selama 10 menit, supernatan yang diperoleh selanjutnya diukur absorbansinya dengan spektrofotometer UV-Vis. Hasil perhitungan persen dekolorisasi ditunjukkan pada table 2.1.
Tabel 2.1 Persen dekolorisasi jamur G. trabeum dan biokomposit Gt/UiO-66
Hari % Dekolorisasi
Gt murni Gt/UiO 15 mg Gt/UiO 30 mg Gt/UiO 45 mg
1 24.87 22.29 32.24 15.02
3 32.82 23.80 39.53 32.85
5 30.48 31.75 38.66 42.89
7 44.94 30.17 57.82 51.55
Hasil dekolorisasi dengan kultur jamur murni G. trabeum mengalami perbedaan dengan penelitian sebelumnya (84,35% pada hari ke-7), sedangkan pada percobaan ini diperoleh 44,94%. Grafik profil absorbansi UV-Vis dari dekolorisasi dengan kultur jamur G. trabeum yang terakhir dapat dilihat pada Gambar 2.2. Perbedaan yang terjadi dapat dikarenakan pada percobaan sebelumnya kultur jamur masih dikondisikan dalam media PDB sehingga pasokan nutrisi dan keberadaan enzim masih berlimpah sedangkan pada percobaan terakhir dilakukan modifikasi tanpa ada media (kultur dicuci sebelum didekolorisasi). Perbedaan ini menunjukkan pengaruh yang signifikan akan keberadaan nutrisi dalam proses dekolorisasi. Selaras dengan hasil tersebut ditemukan juga oleh Adnan et al. [1] bahwa pemberian glukosa sebagai sumber karbon pada jamur
Trichoderma atroviride F03 menghasilkan persentase biodegradasi paling besar terhadap pewarna
RB5 [1].
Selain itu, penambahan UiO-66 ternyata memiliki 2 pengaruh berbeda, yaitu dapat menaikkan dan menurunkan proses degradasi/dekolorisasi dari RB5. Pada penelitian ini diperlihatkan dengan penambahan 30 mg UiO-66 maka komposit yang terbentuk dapat mencapai persen dekolorisasi 57,82%, sementara konsentrasi lain menunjukkan nilai lebih rendah. UiO-66 dalam penelitian ini dapat dikatakan sebagai zat aditif pada kultur jamur, sehingga memiliki 2 kemungkinan kontribusinya: positif ataupun negatif. Senthilkumar et al. [2] juga telah meneliti penambahan aditif berupa logam mangan dalam proses dekolorisasi pewarna Amido Black 10B,
6
dengan konsentrasi 0,1% menunjukan nilai terbaik sementara penambahan di atas 0,1% akan menurunkan persen dekolorisasi [2]. Bahkan Hadibrata [3] melaporkan penambahan Cu2+ dapat
menghasilkan persen dekolorisasi hingga 100% pada pewarna Amaranth [3].
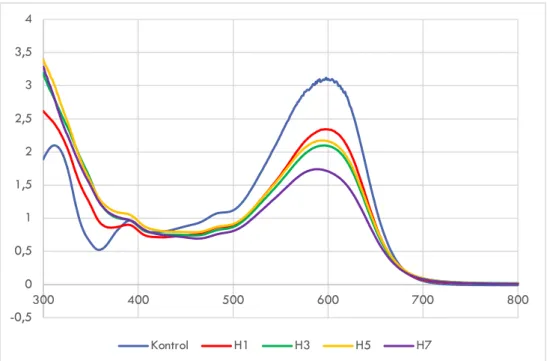
Gambar 2.2 Spektra UV-Vis dekolorisasi RB5 dengan kultur jamur G. trabeum murni (H1: hari ke-1, H3: hari ke-3; H5: hari ke-5; H7: hari ke-7)
2.2.4 Efek Beberapa Parameter terhadap Dekolorisasi Reactive Black 5 2.2.4.1 Pengaruh Suhu terhadap Dekolorisasi RB5
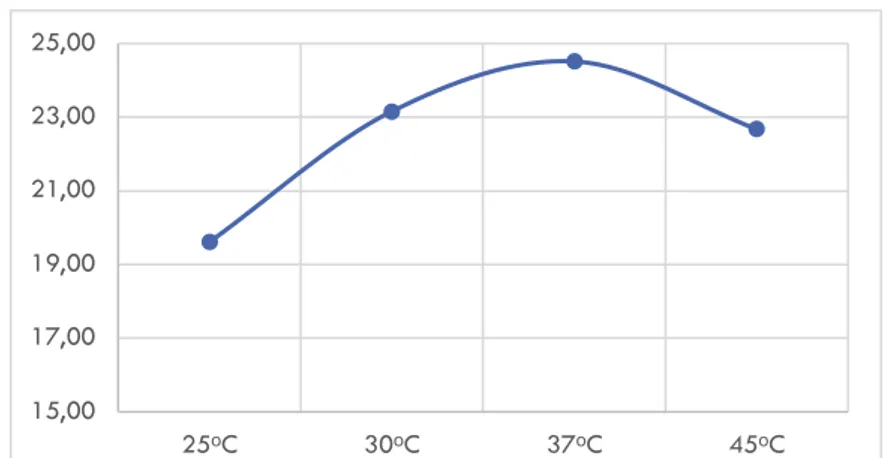
Variasi suhu dalam proses dekolorisasi pewarna RB5 memiliki pengaruh penting, terbukti proses dekolorisasi mengalami nilai optimum pada suhu 37º C setelah 3 hari masa inkubasi. Hal yang hampir mirip juga dilaporkan Kalpana et al. [4] dengan jamur Irpex lacteus menunjukkan dekolorisasi pewarna Dark blue 2S-GL01 nilai maksimum dekolorisasi pada suhu 35º C [4]. Suhu merupakan parameter yang spesifik bagi setiap jenis makhluk hidup, metabolisme jamur untuk menghasilkan enzim juga terpengaruh dengan suhu lingkungannya. Enzim-enzim biologis yang terlibat dalam jalur degradasi memiliki suatu temperatur optimum dan tidak memiliki pergantian metabolik yang sama pada setiap suhu [5].
-0,5 0 0,5 1 1,5 2 2,5 3 3,5 4 300 400 500 600 700 800 Kontrol H1 H3 H5 H7
7
Gambar 2.3 Grafik Persen Dekolorisasi Vs Suhu
2.2.4.2 Pengaruh Konsentrasi Pewarna terhadap Dekolorisasi RB5
Pewarna sintetis atau buatan dalam proses pembuatan Batik jelas memiliki resiko akan meracuni lingkungan. Demikian halnya dengan hasil penelitian yang berikut ini, dengan konsentrasi RB5 yang semakin tinggi komposit Gt/UiO-66 tidak dapat mendekolorisasi dengan baik. Kultur jamur G. trabeum yang ada tidak dapat mendekolorisasi lagi dengan baik karena sifat toksik yang meningkat, konsentrasi di atas 200 mg/L jamur sudah tidak efektif lagi mendekolorisasi seperti pada Gambar 2.4.
Gambar 2.4 Persen Dekolorisasi Vs Konsentrasi Pewarna
Hal yang mirip juga diperoleh Daassi et al. [6], jamur terimobilisasi dari jenis Coriolopsis
gallica, Trametes versicolor, Trametes trogii, dan Bjerkandera adusta persen dekolorisasi
15,00 17,00 19,00 21,00 23,00 25,00 25ᵒC 30ᵒC 37ᵒC 45ᵒC 0,00 10,00 20,00 30,00 40,00 0 100 200 300 400 500 600 700 800 900 1000
8
maksimum pada konsentrasi pewarna Lanaset Grey 20 mg/L, sedangkan semakin tinggi konsentrasi pewarna maka persen dekolorisasi semakin menurun [6]. Demikian juga yang terjadi pada bakteri
Pseudomonas aeruginosa HS-DY012, kemampuan degradasi bakteri ini terhadap pewarna Direct
Blue 6 menjadi lebih kecil dari 50% apabila konsentrasi pewarna lebih dari 90 mg/L [7].
2.2.4.3 Pengaruh pH terhadap Dekolorisasi RB5
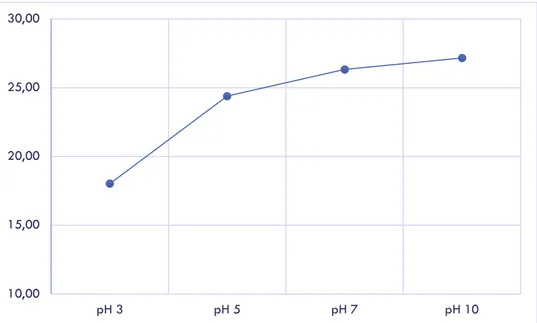
Parameter lain yang penting juga dalam pertumbuhan dan aktivitas organisme yaitu pH, dalam kasus ini dekolorisasi RB5 dengan komposit Gt/UiO-66 mencapai maksimum (27,17%) pada pH 10 setelah 3 hari masa inkubasi. Hasil pengukuran dekolorisasinya dapat dilihat pada Gambar 2.5 di bawah ini:
Gambar 2.5 Grafik Persen Dekolorisasi Vs pH
Kemampuan dekolorisasi setiap jamur terhadap pewarna akan berbeda-beda kondisi optimumnya, berdasarkan penelitian ini komposit jamur G. trabeum/UiO-66 memiliki pH optimum pada pH= 10. Sementara Sharma et al. [8] menemukan jamur Phanerochaete chrysosporium kemampuan degradasi maksimumnya terhadap pewarna Orange II pada kondisi pH sedikit asam (pH= 5).
2.2.5 Karakterisasi UiO-66 dan Gt/UiO-66
Karakterisasi yang telah dilakukan dengan material yang ada yaitu morfologi dari UiO-66 dan. Gt/UiO-66 menggunakan instrumen X-Ray Diffraction (XRD), Spektrofotometer FTIR, SEM, dan Gas Sorption Instrument. Karakterisasi UiO-66 yang digunakan dengan Gas Sorption
10,00 15,00 20,00 25,00 30,00 pH 3 pH 5 pH 7 pH 10
9
Instrument diperoleh data luas permukaan MOF yaitu 634,886 m2/g, sedangkan ukuran rata-rata
dari porinya 6,387 nm.
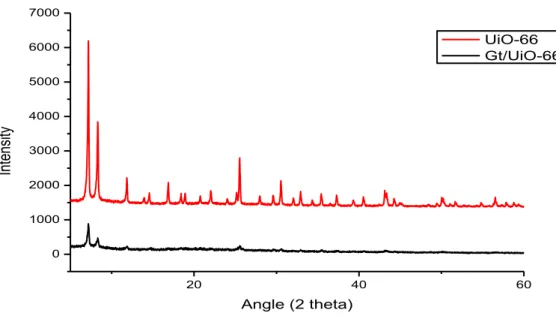
Berikut di bawah ini Gambar 2.6 yang menampilkan profil difraktogram XRD dari UiO-66 dan komposit Gt/UiO-66. UiO-66 memiliki beberapa sudut 2 theta yang spesifik diantaranya yaitu pada 7,1976º dan 8,3347º, pada komposit Gt/UiO-66 terjadi pergeseran menjadi 7,1921º dan 8,3376º.
Gambar 2.6 Gabungan Difraktogram XRD dari UiO-66 dan Gt/UiO-66
Karakter komposit Gt/UiO-66 dengan UiO-66 juga terlihat berbeda dilihat dari spektra FTIR yang digambarkan pada Gambar 2.7. Semisal puncak pada ±3300 cm-1 dari UiO-66 yang
menunjukkan uluran O-H yang relatif tajam, sedangkan pada komposit Gt/UiO-66 menjadi lebih lebar. Demikian pula pita stretching C=O dari gugus karboksilat pada ±1700 cm-1 juga terjadi
perubahan dari bentuk yang tajam menjadi melebar ketika telah menjadi komposit Gt/UiO-66.
Gambar 2.7 Spektra FTIR UiO-66 dan Gt/UiO-66
20 40 60 0 1000 2000 3000 4000 5000 6000 7000 Intensi ty Angle (2 theta) UiO-66 Gt/UiO-66 4000 3500 3000 2500 2000 1500 1000 500 10 20 30 % Transmitt ance Wavelenght (cm-1) UiO-66 Gt-UiO-66
10
Morfologi dari komposit Gt/UiO-66 dapat dilihat menggunakan Scanning Electron Microscope (SEM), dimana terlihat pada Gambar 2.8, bentuk yang dapat diidentifikasi seperti bola dengan UiO-66 yang tersebar dipermukaannya.
Gambar 2.8 Hasil SEM dari Gt/UiO-66: a. Perbesaran 1500x; b. Perbesaran 10.000x
2.2.6 Identifikasi Metabolit Produk
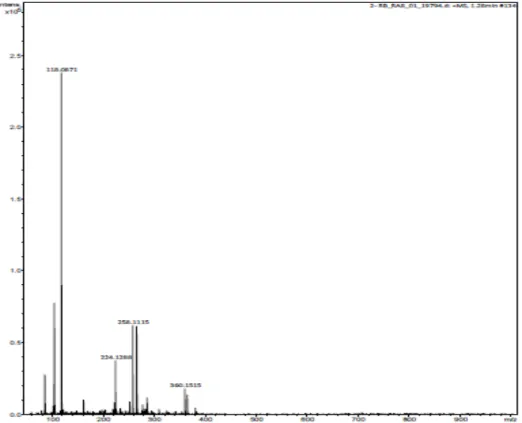
Metabolit produk diidentifikasi dari supernatan hasil dekolorisasi RB5 oleh komposit Gt/UiO-66 dengan menggunakan instrumen LC-Tof-MS dan diperoleh 1 spektra seperti pada Gambar 2.9.
Gambar 2.9 Spektra LC-Tof-MS metabolit produk dekolorisasi RB5 dengan Gt/UiO-66
11
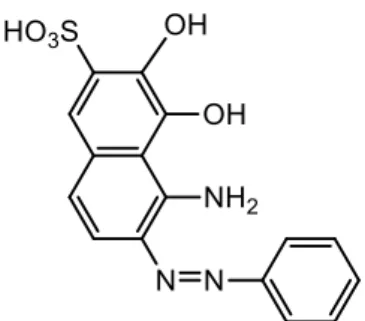
Struktur molekul dari pewarna RP seperti pada Gambar 2.10 berikut:
Gambar 2.10 Struktur molekul Reactive Black 5
Sementara puncak-puncak yang terdapat pada spektra MS pada Gambar 2.9 diantaranya memiliki m/z = 360,1515; 258,1115; 224,1288; dan 118,0871. Nilai m/z 360 pernah pula diperoleh oleh Guaraldo et al. [9] pada dekolorisasi RB5 dengan metode oksidasi fotoelektrokatalitik dengan
photoanode TiO2/WO3 akan tetapi struktur metabolit ini tidak dapat diidentifikasi [9]. Disini penulis
memprediksikan senyawa metabolit produk yang memiliki m/z = 360 adalah senyawa Asam 5-amino-3,4-dihidroksi-6-(fenildiazenil)naftalena-2-sulfonat yang rumus strukturnya pada gambar 2.11.
12
BAB III
STATUS LUARAN
Luaran pada penelitan High Impact ini yaitu satu artikel terpublikasi di jurnal internasional terindeks scopus yang masuk dalam Quartile 1 (Q1). Target jurnal yang dipilih yaitu Biodegradation yang Impact Factor-nya 2.805. Draft artikel telah disubmit dan sedang menunggu hasil review/balasan dari tim redaksi jurnal tersebut.
13
BAB IV
KENDALA PELAKSANAAN PENELITIAN
Kendala yang dihadapi peneliti dalam eksperimennya diantaranya:
1. Kondisi alat dan lingkungan yang belum steril menimbulkan kontaminasi pada kultur jamur dan dalam pembuatan komposit Gt/UiO-66
2. Adanya kerusakan pada alat inkubator shaker sehingga digunakan kombinasi alat shaker yang dimasukkan dalam inkubator
3. Analisa kimia yang dilakukan di instansi/laboratorium lain pada saat pandemik ini memerlukan waktu lebih lama.
14
BAB V
DAFTAR PUSTAKA
[1] L. Adnan, P. Sathishkumar, A. Yusoff and T. Hadibrata, "Metabolites characterisation of laccase mediated Reactive Black 5 biodegradation by fast growing ascomycete fungus Trichoderma atroviride F03," International Biodeterioration & Biodegradation, 104: 274-282 (2015).
[2] S. Senthilkumar, M. Perumalsamy and H. Prabhu, "Decolourization potential of white-rot fungus
Phanerochaete chrysosporium on synthetic dye bath effluent containing Amido black 10B," Journal of Saudi Chemical Society, 18: 845-853 (2014).
[3] T. Hadibrata, "Effect of Metals on Amaranth Decolorization by White-Rot Fungus Pleurotus eryngii F019," Journal of Biological Sciences, 13(6): 550-554 (2013).
[4] D. Kalpana, J. Shim, B.-T. Oh, K. Senthil and Y. Lee, "Bioremediation of the heavy metal complex dye Isolan Dark Blue 2SGL-01 by white rot fungus Irpex lacteus" Journal of Hazardous Materials, 198: 198-205 (2011).
[5] N. Joutey, W. Bahafid, H. Sayel and E. Ghachtouli, "Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms," in Biodegradation - Life of Science, InTechOpen, 289-320 (2013).
[6] D. Daassi, T. Mechichi, M. Nasri and S. Rodriguez-Couto, "Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi," Journal of Environmental Management, 129: 324-332 (2013).
[7] W. Jing, W. Cong, P. Nanmei, X. Huan, Y. Yue, X. Biling and Z. Yongliang, "Decolorization and Degradation of Direct Blue 6 from textile wastewaters by Pseudomonas aeruginosa HS-DY012," Proceedings of the 2017 3rd International Forum on Energy, Environment Science and Materials (IFEESM 2017).
[8]
[9]
P. Sharma, L. Singh1, N. Dilbaghi, “Biodegradation of Orange II dye by Phanerochaete chrysosporium insimulated wastewater”, Journal of Scientific & Industrial Research, 68: 157-161 (2009).
T. Guaraldo, V. Goncales, B. Silva, S. de Torresi and M. Zanoni, "Hydrogen production and simultaneous photoelectrocatalytic pollutant oxidation using a TiO2/WO3 nanostructured photoanode under visible light irradiation," Journal of Electroanalytical Chemistry, 765: 188-196 (2016).
15
Lampiran 1
Program : High Impact
Nama Ketua Tim : Adi Setyo Purnomo, M.Sc., Ph.D
Judul : Imobilisasi Jamur Pelapuk Coklat (Gloeophyllum trabeum) Dalam Metal Organic Frameworks UiO-66 dan Aplikasinya dalam Biodegradasi Limbah Pewarna Batik Reactive Black 5
1.Artikel Jurnal
No Judul Artikel Nama Jurnal Status Kemajuan*)
1 Brown-rot fungus Gloeophyllum trabeum and UiO-66 composite application for decolorizing the synthetic dye Reactive Black 5
Biodegradation Persiapan
*) Status kemajuan: Persiapan, submitted, under review, accepted, published 2. Artikel Konferensi
No Judul Artikel Nama Konferensi (Nama Penyelenggara, Tempat,
Tanggal)
Status Kemajuan*)
- - -
*) Status kemajuan: Persiapan, submitted, under review, accepted, presented 3. Paten
No Judul Usulan Paten Status Kemajuan
- -
*) Status kemajuan: Persiapan, submitted, under review 4. Buku
No Judul Buku (Rencana) Penerbit Status Kemajuan*)
- - -
*) Status kemajuan: Persiapan, under review, published 5. Hasil Lain
No Nama Output Detail Output Status Kemajuan*)
- - -
16
6. Disertasi/Tesis/Tugas Akhir/PKM yang dihasilkan
No Nama Mahasiswa NRP Judul Status*) 1 Taufiq Rinda Alkas 01211860012001 Komposit
JPC/UiO-66 yang Terimobilisasi dalam PVA-Alginat untuk Proses Biodegradasi Pewarna Sintetik In progress
17
Lampiran 2. Draft Paper
BROWN-ROT FUNGUS Gloeophyllum trabeum AND UIO-66 COMPOSITE
APPLICATION FOR DECOLORIZING THE SYNTHETIC DYE REACTIVE BLACK 5
Adi Setyo Purnomo*, Taufiq Rinda Alkas, Ratna Ediati, Taslim Ersam, Refdinal Nawfa Department of Chemistry, Faculty of Science and Data Analytical, Institut Teknologi Sepuluh Nopember (ITS), Kampus ITS Sukolilo, Surabaya 60111, Indonesia
*Corresponding author
E-mail: adi_setyo@chem.its.ac.id; adi.spurnomo@yahoo.com (A.S. Purnomo) Tel/Fax: +62-31-5943353/ +62-31-5928314
Abstract
Biodegradation of Reactive Black 5 (RB5) dye was conducted by using a combination of brown-rot fungus Gloeophyllum trabeum and metal-organic framework UiO-66. This composite G.
trabeum and UiO-66 (Gt/UiO-66) was designed to be new material that has enzymatic activities,
Fenton mechanism, and adsorption ability. These two materials have different character but in this study, we tried to synthesize them by mixing them into the growing phase of fungi. The initial application of this composite was to be a decolorizer of RB5 dye which is always used in Batik painting. Pure UiO-66 almost decolorized RB5 dye completely (99.29%), while pure G.
trabeum culture only gained 44.94% in 7 days. UiO-66 addition was proved to increase the RB5
decolorization ability of Gt/UiO-66 composite slightly up to 57.82% after 7 days incubation period. The optimum RB5 decolorization condition by the Gt/UiO-66 composite occurred in pH 10 and at
18
temperature 37ºC. 5-amino-3,4-dihydroxy-6-(phenyldiazenyl)naphathalene-2-sulfonic acid was the proposed product metabolite of this decolorization by analyzing the LC-MS QTOF spectrum.
Keywords: Brown-rot fungus, Metal organic framework, Gloeophyllum trabeum, Decolorization
Introduction
In recent years, the production of synthetic dyes has been increasing rapidly due to the demand for colored textiles, foods, cosmetics, printing materials, etc. As a consequence of that development, it stimulated the significant rise of dye wastewater in most countries. Maas and Chaudhari (2005) estimated there were 280,000 tons of textile dyes every year which were removed as an industrial effluent globally. One of the reasons why that was occurred, about 15-50% of dyes were not bound in the textile fibers on the coloring process and released away (Rehman, et al. 2018). While Chowdhury et al. (2020) stated there is a huge portion of unused chemicals and dyestuffs during the textile coloration process and the excess amount of dye liquor is discharged into the environment. The dye wastewater has several negative impacts on the environment such as the dye’s toxicity and the recalcitrant properties (Lellis et al. 2019). Therefore, the wastewater must be carried out by some treatments before discharged into the water bodies. Various methods have been developed for treating the dye wastewater, one of the effective and inexpensive methods is biodegradation. By using microorganisms as a degrader, this developed technology used aerobic/anaerobic microbial degradation and pure enzymatic systems (Pereira and Alves 2012). In another term of decolorization, it has been implemented in two ways for fungal application. Firstly, decolorization of the effluents can be conducted by the fungal culture directly and the second is by using an enzymatic liquid culture medium (Chaurasia and Bharati 2019).
19
Meanwhile, another point of view defined that the dye removal is gained by biodegradation process (mineralization or biotransformation) and/or adsorption on biomass (Przystas et al. 2018). Dead or living microbial biomasses have been studied for the removal of reactive dyes in the effluent. They can absorb dye molecules or the enzymes secreted by them can degrade chromophores of dye molecules causing their decolorization (Hassan and Carr 2018). White rot fungi were the familiar species to be subjected in the research of dyes biodegradation or decolorization ( (Enayatizamir et al. 2011; Bibi and Bhatti 2012; Adnan et al. 2014). Brown rot fungi are still rare to be explored in this field and one of the species which we focused in this study is a brown-rot fungus Gloeophyllum
trabeum. This species has been reported to have some important enzymes namely laccase (D'Souza
et al. 1996), xylanase (Ritschkoff et al. 1994), and alcohol oxidase (Daniel et al. 2007). Besides, this fungus also has been reported to have a Fenton reaction mechanism which produces hydroxyl radical that plays an important role in the degradation process (Kerem et al. 1999).
Previous research showed that Gloeophyllum trabeum had an interesting ability to degrading several pollutants. This fungus had been tested to decompose some organic pollutants such as pesticides (Purnomo et al. 2008), dyes (Hartikainen et al. 2016; Purnomo et al. 2019), and biomass decomposer ( (Presley et al. 2018; Schilling et al. 2012). Meanwhile, UiO-66 with high thermal stability also has been reported to have a good adsorption ability of dyes (Jiang et al. 2019; Azhar et al. 2017) and micropollutants in water (Schelling et al. 2018). In this research, we combined those materials to make a new composite with a good adsorption and degradation ability to decolorize Reactive Black 5 dye.
Materials and Methods
Fungal Cultures and Materials
Gloeophyllum trabeum NBRC (NITE Biological Resource Center, NBRC; Chiba, Japan) was a
20
Indonesia. The stock was regenerated on the solid media PDA (Potato Dextrose Agar; Merck, Germany) and the liquid media PDB (Potato Dextrose Broth; HiMedia, India). Reactive Black 5 (RB5) was purchased from Sigma Aldrich (Germany). UiO-66 was synthesized in our laboratory by analytical grade chemicals by the solvothermal method (Ediati, et al. 2018).
Preparation of G. trabeum and UiO-66 Composite
One plate of G. trabeum culture (in PDA media) and 25 mL demineralized water were homogenized by blender on a sterilized cup. One milliliter mixture was inoculated in flasks with 20 mL PDB media which contained a variation mass of UiO-66 (15, 30, and 45 mg) and incubated at 35º C for 3 days in horizontal slowly shaking. After 3 days, granules were appeared and washed aseptically twice with demineralized water (10 mL) to dismiss the liquid media. Biocomposites were all ready to use in the dye decolorization. Biocomposite was also characterized by various instruments such as SEM, XRD, Gas Sorption Instrument, and FTIR spectrophotometer.
Decolorization Studies
The decolorization of RB5 dye was conducted by adding 20 mL of RB5 solution (100 mg L-1) into the flasks which were contained with biocomposite and then incubated in static condition. Decolorization of the samples was observed and measured after 1, 3, 5, and 7 days. The supernatant of those samples was then centrifuged at 3000 rpm for 10 min and analyzed by spectrophotometer UV-Vis (Thermo Genesys-10S) at a range of 400-800 nm. Decolorization percentage was calculated from the absorbances of the samples at 598 nm (λ max), based on the equation:
% decolorization (PD) = !"#!$!"#"
!"#! 𝑥100%
21
Effect of different parameters on the dye decolorization
The effect of various parameters was studied in this research including the initial pH, temperature, and dye initial concentration. Various pH addition was evaluated by giving the composite with the different pH (3,5,7, and 10) by using HCl 0.1 M and NaOH 0.1 M. Various temperature (25-45 ºC) and dye concentration (100, 200, 500, and 1000 mg.L-1) were also examined by incubating the granules with those conditions separately. After the pre-incubated period, the composite was washed twice by demineralized water and treated by adding the dye solution with those different conditions. After three days, the supernatants were collected after filtering and centrifuging the samples, then it was measured by spectrophotometer UV-Vis.
Identification of metabolic products
Metabolic products analyzed by LCMS-QTOF using column C18 (4.6 mm x 250 mm). the mobile phase was methanol: water (50:50 v/v). flow rate (0.6 ml/min) and UV detector at 254 nm were used. The injection volume of the dye and its degraded product was 10 µl. mass spectra were obtained using an ion-trap mass spectrometer fitted with an electron spray (ESI) interface operated in negative ionization mode with a spray voltage of 4.5 kV, at a capillary temperature at 275 ºC, sheath gas at 40 AU (arbitrary unit), and auxiliary gas at 26 AU.
Results
Synthesized UiO-66 and Gt/UiO-66 Biocomposite Characterization
Fig. 1 shows the appearance of G.trabeum and UiO-66 (Gt/UiO-66) composite, UiO-66, and the magnified image from the Scanning Electron Microscope instrument.
22
Fig. 1. (a) Biocomposite Gt/UiO-66; (b) Syntesized UiO-66; SEM micrographs of Gt/UiO-66 (c) 1500 magnified; (d) 10000 magnified
Those pictures described that composites were not formed as one size, it can be varied one and other. But, the granules have been contained with some UiO-66 in their body which is depicted in Fig 1(d). The materials are shaped almost spheric where the UiO-66 can be spread out on all sides. According to Ediati et al (2018), UiO-66 has a large surface area, high porosity, and stable in water. From the characterization, by the adsorption-desorption instrument was recorded that the synthesized UiO-66 have 605.976 m2/g surface area and 7.586 nm average pore size.
Fig. 2 (a) illustrated the differences of UiO-66 and Gt/UiO-66 in FTIR spectrum and (b) XRD diffractogram of UiO-66 and Gt/UiO-66. In the FTIR spectrum, there is a broad O-H peak at ± 3300 cm-1 from the carboxylic groups contained in UiO-66 and also the peaks of an associated carbonyl group in 1730-1680 cm-1. But in the Gt/UiO-66 band, those peaks became broader maybe because of the physical/chemical interactions between those materials. While the XRD diffractogram can
c
d
23
be interpreted that the metal-organic frameworks crystallinity of UiO-66 was decreased after the formation of Gt/UiO-66 composite.
Fig. 2 a. FTIR spectrum and b. XRD Difractogram of UiO-66 and Gt/UiO-66
Reactive Black 5 (RB5) Decolorization
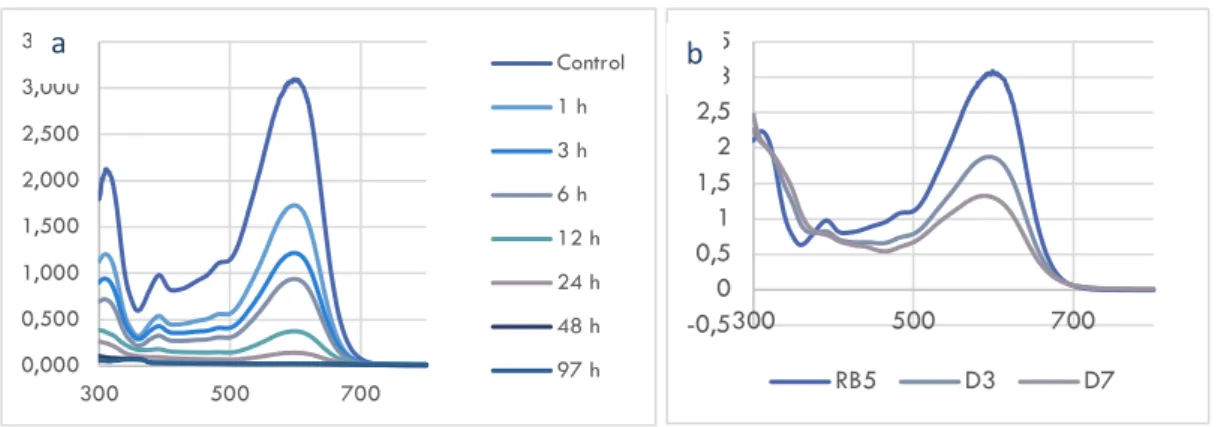
Decolorization of RB5 by the metal-organic frameworks (synthesized UiO-66) and Gt/UiO-66 composite were shown in Fig. 3. Decolorization percentage by UiO-66 (100 mg.L-1) achieved maximum value 99.29% after 48 hours incubation (data not shown). Adsorption is the main mechanism of UiO-66 in this decolorization process, the degradation did not occur since there were no significant change of the UV-Vis profiles from time to time (Fig 3. a).
Fig. 3 UV-Vis spectra of RB5 decolorization by: a. UiO-66; b. Gt/UiO-66 (30 mg) 4000 3500 3000 2500 2000 1500 1000 500 10 20 30 % Transmitt ance Wavelenght (cm-1) UiO-66 Gt-UiO-66 20 40 60 0 1000 2000 3000 4000 5000 6000 7000 Intensi ty Angle (2 theta) UiO-66 Gt/UiO-66 0,000 0,500 1,000 1,500 2,000 2,500 3,000 3,500 300 500 700 Control 1 h 3 h 6 h 12 h 24 h 48 h 97 h -0,5 0 0,5 1 1,5 2 2,5 3 3,5 300 500 700 RB5 D3 D7 a b a b
24
On the other hand, decolorization results of the biocomposites were shown any different shape from the RB5 curve (blue line) particularly at 300 nm in Fig 4.b. Besides, the decolorization percentages of both of the materials were given in Table 1. and illustrated in Fig.4.
Table 1. RB5 dye decolorization percentages by pure G. trabeum and Gt/UiO-66 composites
Day % Decolorization Pure Gt Gt/UiO 15 mg Gt/UiO 30 mg Gt/UiO 45 mg 1 24.87 22.29 32.24 15.02 3 32.82 23.80 39.53 32.85 5 30.48 31.75 38.66 42.89 7 44.94 30.17 57.82 51.55
Fig 4. RB5 decolorization by pure G. trabeum and Gt/UiO-66 composites
Effect of initial concentration on the dye decolorization
The effect of initial concentration was evaluated by giving a variation of RB5 concentration from 100 to 1000 mg.L-1 to the Gt/UiO-66 composite. After incubating the samples in statically condition, a significant decrease occurred as Fig. 5. It was shown that the samples with 100
mg.L-0,00 10,00 20,00 30,00 40,00 50,00 60,00 70,00 1 3 5 7 % D ec ol or iz a tio n Days
25
1 concentration (RB5) had a maximum decolorization percentage of 37.13% after 3 days incubation. While the decolorization dropped to the minimum level at 1000 mg.L-1 (0.37%).
Fig 5. Effect of different initial concentrations for RB5 decolorization
Effect of pH on the dye decolorization
In this case, different pH has shown a different result of RB5 decolorization. Maximum decolorization percentage by Gt/UiO-66 composite was achieved at pH 10 while pH 3 of the dye solution resulting in the minimum values. After 3 days of incubation, Gt/UiO-66 at pH 10 could reach up to 27.17% decolorization percentage.
0,00 10,00 20,00 30,00 40,00 0 100 200 300 400 500 600 700 800 9001000
26
Fig. 6 Effect of pH variation for RB5 decolorization
Effect of temperature on the dye decolorization
The temperature was an important factor for microorganism to grow normally, and also in the fungal metabolism. In this study, the degradation process by G. trabeum composite obtained an optimum condition at 37ºC. In the meantime, the composite only showed a lower degradation at 25ºC which because of the inactivated enzyme at that temperature.
Fig 7. Effect of various temperature for RB5 decolorization
10,00 15,00 20,00 25,00 30,00 pH 3 pH 5 pH 7 pH 10 15,00 17,00 19,00 21,00 23,00 25,00 25ᵒC 30ᵒC 37ᵒC 45ᵒC
27
LC-MS Analysis
Metabolite product which was identified in this study, indicating there was a degradation process of RB5 by the Gt/UiO-66 composite. Some peaks were different from the control, and we proposed the compound that found was 5-amino-3,4-dihydroxy-6-(phenyldiazenyl)naphathalene-2-sulfonic acid with molecular weight 360.1515 at retention time 1.28 min.
Fig 8. MS spectrum of product metabolite of RB5 decolorization
28
Fig 9. Proposed product metabolite of RB5 decolorization by Gt/UiO-66
Discussion
Fungi in wastewater treatment have been recognized for their role, it became a suitable and effective player for dye degradation/decolorization and the removal of colorants from the textile effluents (Singh 2017). In this treatment, fungi or other microbes adapt themselves to the toxic wastes and then naturally they reproduce new resistant strains, which then transform various toxic chemicals into less harmful forms (Saratale, et al. 2011). This approach makes the biodegradation method more eco-friendly, less sludge, and provides reliable results (Hefnawy, et al. 2017).
In this research, brown-rot fungus Gloeophyllum trabeum was evaluated as a dye degrader of Reactive Black 5. This fungus was grown with the metal-organic frameworks UiO-66 to become a new composite. Another brown-rot fungus Daedalea dickinsii also tested (not reported) to combine with UiO-66 but it did not work. The reason why it may happen because of the toxicity of UiO-66. Some fungi may tolerate the toxicity but some will not, besides that UiO-66-NH2 also have shown antibacterial activity (Lv, et al. 2020). The toxicity of UiO-66 has affected the fungal culture whether it can combine or not. G. trabeum was proved to be resistant in this study and it could create a new composite and degrade the RB5 dye.
Various parameters were also assessed in this study such as initial concentration, pH, and temperature. Those variables are important factors in dye decolorization by microorganisms. Many
29
previous studies have evaluated some parameters in the dyes decolorization processes using microbes. Sen et al. (2016) have been described that dyes biodecolorization or biodegradation was affected by several factors such as nutrients, oxygen, pH, temperature, enzyme, dyes concentration, redox mediators, and dye structure.
Our study reported a higher concentration than 100 mg.L-1 decrease the RB5 decolorization percentage. The toxicity of RB5 caused the G. trabeum culture both in pure or composite form might be a terminated or inactive condition. A previous study by Chen and Ting (2015) also found the dye decolorization efficiency was better at lower initial dye concentration (50, 100 mg.L-1) compared to the higher concentration (200 mg/L). The higher concentration required a longer period up to 14 days than the lower concentration. Same with that, Daassi et al. (2013) have been found the dye decolorization by immobilized fungi such as Coriolopsis gallica, Trametes
versicolor, Trametes trogii, and Bjerkandera adusta achieved maximum decolorization at lower
concentration (20 mg.L-1). They concluded that the higher concentration will decrease the dye decolorization rate by inhibiting the fungal growth and cellular metabolic activities because of their toxicity.
In this present study, the range of pH for dye decolorization was examined between 3-10, and the optimum pH for RB5 decolorization was 10. Maximum decolorization of Reactive Brilliant Blue R (RBBR) was achieved after 3 days of incubation in medium pH 5.5 and laccase activity also gained the maximum value (222.23 IU/mL) at this pH for Trametes hirsuta. pH is a very important parameter for the proper growth of the fungi and the functioning of enzymes involved in various degradation processes (Bibi 2012).
The result from the range of temperature (25-45ºC) showed the best temperature for Gt/UiO-66 composite to decolorize RB5 was 37ºC. The percentage of dye decolorization was decreased at a lower and higher temperature for these composites. The other study which used Aspergillus
30
Direct blue decolorization respectively (Hefnawy, et al. 2017). While Afreen et al. (2018) claimed the effect of temperature was very significant for enzyme activity, they revealed that the maximum activity of laccase from Arthospira maxima (cyanobacteria) was on the fourth day at 30ºC, a regular decrease in laccase production occurred in incubation temperature above 35ºC.
In summary, the composite of G. trabeum and UiO-66 was created in this study and proved that it could decolorize Reactive Black 5 (57.82%) better than the pure culture of G. trabeum. The optimum condition for Gt/UiO-66 (30 mg) composite to decolorize RB5 was on 100 mg.L-1 concentration, pH 10, and at 37ºC. And the proposed product metabolite from LCMS analysis was 5-amino-3,4-dihydroxy-6-(phenyldiazenyl)naphathalene-2-sulfonic acid.
Acknowledgments
This work was supported by Institut Teknologi Sepuluh Nopember (High impact research, Grant number: 835/PKS/ITS/2020).
References
Adnan, L.A., A.R.M. Yusoff, T. Hadibrata, and A.B. Khudhair. 2014. "Biodegradation of bis-azo dye Reactive Black 5 by white-rot fungus Trametes gibbosa sp. WRF 3 and its metabolite characterization." Water Air Soil Pollution 225: 1-11.
Afreen, S., R. Anwer, R.K. Singh, and T. Fatma. 2018. "Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes." Saudi
Journal of Biological Sciences 1446-1453.
Azhar, M.R., Abid, H.R., Sun, H., Periasamy, V., Tade, M.O., Wang, S. 2017. "One-pot synthesis of binary metal organic frameworks (HKUST-1 and UiO-66) for enhanced adsorptive removal of water contaminants." Journal of Colloid and Interface Science 490: 685-694. Bibi, I., and HN. Bhatti. 2012. "Enhanced Biodecolorization of Reactive Dyes by Basidiomycetes
31
Chaurasia, PK., and SL. Bharati. 2019. "Recent Myco-Dye Decolorization Studies (Mini-review) ."
Journal of Biotechnology and Bioengineering 27-31.
Chen, S.H., and A.S.Y. Ting. 2015. "Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost ." Journal of
Environmental Management 150: 274-280.
Chowdhury, MF., S. Khandaker, F. Sarker, A. Islam, MT. Rahman, and MR. Awual. 2020. "Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review." Journal of Molecular Liquids 1-20.
Daassi, D., T. Mechichi, M. Nasri, and S. Rodriguez-Couto. 2013. "Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi." Journal of Environmental
Management 129: 324-332.
Daniel, G., J. Volc, L. Filonova, O. Plihal, E. Kubatova, and P. Halada. 2007. "Characteristics of Gloeophyllum trabeum Alcohol Oxidase, an Extracellular Source of H2O2 in Brown Rot Decay of Wood." Applied And Environmental Microbiology 73 (19): 6241-6253.
D'Souza, T.M., K. Boominathan, and C.A. Reddy. 1996. "Isolation of Laccase Gene-Specific Sequences from White Rot Fungi and Brown Rot Fungi by PCR." Applied and
Environmental Microbiology 3739-3744.
Ediati, R, L L Zulfa, K A Nugroho, Mukminin A, D O Sulistiono, M Nadjib, and Y Kusumawati. 2018. "One-pot solvothermal synthesis and characterization of UiO-66/HKUST-1 composites." Solo: IOP Publishing.
Enayatizamir, N., F. Tabandeh, S. Rodriguez-Couto, B. Yakhchali, H.A. Alikhani, and L. Mohammadi. 2011. "Biodegradation pathway and detoxification of the diazo dye Reactive Black 5 by Phanerochaete chrysosporium." Bioresource Technology 102: 10359-10362. Hartikainen, E.S., O. Miettinen, A. Hatakka, and M.A. Kahkonen. 2016. "Decolorization of six
synthetic dyes by fungi." American Journal of Environmental Sciences 12 (2): 77-85. Hassan, MH., and CM. Carr. 2018. "A critical review on recent advancements of the removal of
reactive dyes from dyehouse effluent by ion-exchange adsorbents." Chemosphere 201-219. Hefnawy, M.A., M.M. Gharieb, M.T. Shaaban, and A.M. Soliman. 2017. "Optimization of Culture Condition for Enhanced Decolorization of Direct Blue Dye by Aspergillus flavus and Penicillium canescens." Journal of Applied Pharmaceutical Science 083-092.
Jiang, D., M. Chen, H. Wang, G. Zeng, D. Huang, M. Cheng, Y. Liu, W. Xue, and Z.W. Wang. 2019. "The application of different typological and structural MOFs-based materials for the dyes adsorption." Coordination Chemistry Reviews 471-483.
Kerem, Z., K.A. Jensen, and K.E. Hammel. 1999. "Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven fenton reaction." FEBS Letters 446: 49-54.
Lellis, B., C.Z. Favaro-Polonio, J.A. Pamphile, and J.C. Polonio. 2019. "Effects of textile dyes on health and the environment and bioremediation potential of living organisms."
32
Lv, H., Y. Zhang, P. Chen, J. Xue, X. Jia, and J. Chen. 2020. "Enhanced Synergistic Antibacterial Activity through a Smart Platform Based on UiO-66 Combined with Photodynamic Therapy and Chemotherapy." Langmuir 4025-4032.
Maas, R., and S. Chaudhari. 2005. "Adsorption and biological decolorization of azo dye reactive red 2 in semicontinuous anaerobic reactors." Process Biochemistry 40 (2): 699-705.
Pereira, L., and M. Alves. 2012. "Dyes-Environmental Impact and Remediation." In Environmental
Protection Strategies for Sustainable Development, 111-162. Dordrecht: Springer.
Presley, G.N., B.K. Ndimba, and J.S. Schilling. 2018. "Brown Rot-Type Fungal Decomposition of Sorghum Bagasse: Variable Success and Mechanistic Implications." International Journal
of Microbiology 1-7.
Przystas, W., E. Zablocka-Godlewska, and E. Grabinska-Sota. 2018. "Efficiency of decolorization of different dyes using fungal biomass immobilized on different solid supports." Brazilian
Journal of Microbiology 49: 285-295.
Purnomo, A.S., V.T. Mauliddawati, M. Khoirudin, A.F. Yonda, R. Nawfa, and S.R. Putra. 2019. "Bio-decolorization and novel bio-transformation of methyl orange by brown-rot fungi."
International Journal of Environmental Science and Technology.
Purnomo, Adi Setyo, Ichiro Kamei, and Ryuichiro Kondo. 2008. "Degradation of 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane (DDT) by brown-rot fungi." Journal of Bioscience and
Bioengineering 105 (6): 614-621.
Rehman, K., T. Shahzad, A. Sahar, S. Hussain, F. Mahmood, M.H. Siddique, M.A. Siddique, and M.I. Rashid. 2018. "Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling." PeerJ 1-14.
Ritschkoff, Anne-Christine, Johanna Buchert, and Liisa Viikari. 1994. "Purification and characterization of a thermophilic xylanase from the brown-rot fungus Gloeophyllum trabeum." Journal of Biotechnology 32: 67-74.
Saratale, R.G., G.D. Saratale, J.S. Chang, and S.P. Govindwar. 2011. "Bacterial decolorization and degradation of azo dyes: A review." Journal of the Taiwan Institute of Chemical Engineers 138-157.
Schelling, M., M. Kim, E. Otal, and J. Hinestroza. 2018. "Decoration of cotton fibers with a water-stable metal-organic framework (UiO-66) for the decomposition and enhanced adsorption of micropollutants in water." Bioengineering 1-11.
Schilling, J.S., J. Ai, R.A. Blanchette, S.M. Duncan, and T.R. Filley. 2012. "Lignocellulose modifications by brown rot fungi and their effects, as pretreatments, on cellulolysis."
Bioresource Technology 116: 147-154.
Sen, S.K., S. Raut, P. Bandyopadhyay, and S. Raut. 2016. "Fungal decolouration and degradation of azo dyes: A review." Fungal Biology Reviews 112-133.
Singh, L. 2017. "Biodegradation of synthetic dyes: a mycoremediation approach for degradation/decolourization of textile dyes and effluents." Journal of Applied Biotechnology